
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wagner Luiz Batista | + 2156 word(s) | 2156 | 2021-03-03 08:41:53 | | | |
| 2 | Lily Guo | Meta information modification | 2156 | 2021-03-14 10:53:51 | | | | |
| 3 | Lily Guo | Meta information modification | 2156 | 2021-03-14 10:54:32 | | |
Video Upload Options
Paracoccidioidomycosis (PCM) is systemic mycosis caused by fungi of the genus Paracoccidioides. This disease is endemic in Latin America that mainly affects workers in rural areas and causes some degree of disability for working-age people. For people who develop symptoms, PCM usually affects the lungs, skin, mucous membranes, lymph nodes, and internal organs. Paracoccidioides spp. are thermally dimorphic fungi that present infective mycelia at 25 °C and differentiate into pathogenic yeast forms at 37 °C.
1. Introduction
Paracoccidioides spp. is the aetiologic agent of paracoccidioidomycosis (PCM), a systemic mycosis that mainly affects workers in rural areas and causes some degree of disability for working-age people. In 1908, Adolpho Lutz described the causative agent of PCM in the oral lesions and cervical lymph nodes of two patients. By that time, the disease and its agent received several names, until in 1930 Floriano Almeida finally proposed the name Paracoccidioides brasiliensis [1]. More recently, genomics analyses have identified the existence of cryptic species within the Paracoccidioides genus and new species have been proposed [2][3].
The disease is widely distributed in Latin American countries, such as Brazil [4], Argentina [5], Uruguay [6], Paraguay [7], Peru [8], Ecuador [9], Colombia [10], Venezuela [11], and Mexico [12]. However, there are reports of imported cases in such countries as Japan [13], the Netherlands [14], Germany [15], and Austria [16] (Figure 1). Due to the reduced lethality and restricted geographic distribution of the etiological agent of PCM, public health agencies have largely neglected the disease. PCM is geographically limited to Latin American countries and the highest number of reported cases are concentrated in Brazil (80%). Although Brazil reports more cases than any other country (Figure 1), these numbers are probably underestimated, given that until February 2020 PCM was not a notifiable disease, although there is currently a recommendation for reporting cases as proven or probable [17]. It is expected that with this change, the data regarding epidemiology will soon be more accurate.
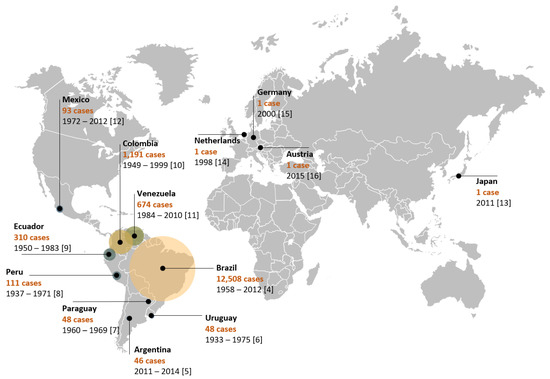
Figure 1. Epidemiological distribution of PCM cases throughout the world. The Netherlands, Germany, Austria, and Japan have imported cases. Legends show the name of the country and the number of cases identified through the period mentioned in each study.
2. Paracoccidioidomycosis: Disease, Diagnosis and Treatment
PCM is a granulomatous disease that usually presents in two distinct forms: acute or subacute and chronic forms. The acute or subacute form is responsible for 5–25% of cases and is characterized by rapid dissemination to the reticuloendothelial system. In the chronic form (74–96% of cases), the disease progresses slowly and may take months to years to become apparent, and pulmonary manifestations are present in more than 90% of cases [17][18]. In addition, the chronic form can manifest as unifocal or multifocal. In the unifocal form (25% of cases), the lungs and, rarely, other sites are the only organs involved [19]. In the multifocal form, along the lungs, the main sites involved are the oral mucosa, skin, lymph nodes, and adrenal glands and, to a lesser extent, the central nervous system, bones, genitals, and blood vessels. The residual form, observed less frequently, is expressed by sequelae left after treatment [17].
The clinical manifestations of PCM can be related both to characteristics inherent to the pathogen and to the host’s immune response profile. The analysis of the adaptive immune response in different clinical spectra of PCM has contributed to a better understanding of the disease evolution. The presence of the T helper 2 (Th2)/Th9 profile with a high production of cytokines IL-4, IL-5, IL-9, IL-10, TGF-β, IL-27; the polyclonal activation of B cells; the production of large amounts of specific IgG4, IgA, and IgE; and a low production of IFN-γ and TNF-α is related to the acute form of PCM with the fungus spreading to different organs and systems [20][21]. On the other hand, the prevalence of Th17 and Th22 with the production of the cytokines IFN-γ, TNF-α, and IL-2; varying amounts of IL-10 and IL-4; and the presence of high levels of specific IgG1 antibodies are immunological characteristics of the chronic form [20][21]. Asymptomatic and mild chronic forms have a Th1 profile, while the severe chronic form may have a predominance of Th2 response [21]. Interestingly, mice infected with different P. brasiliensis genotypes showed differences in their immune response profile, suggesting that factors of the pathogen may also contribute to the different activation profiles [22]. The review of innate and adaptive host immunity to Paracoccidioides was not the purpose of this work. These aspects are widely covered in another review by Calich et al., 2019 [23].
PCM is rarely observed in children and young people and is more common in men aged 30 years or more. The susceptibility of men and women does not differ notably; the ratio of men who develop the disease to women is 22:1 [17]. This difference has been attributed to the presence of high levels of endogenous estrogens in women that act as binding proteins in the fungal cytosol, inhibiting the transition to the pathogenic phase [24][25][26]. In fact, pre-adolescent girls, women in menopause, and women suffering from hormonal disorders may manifest the disease, given their decrease in 17β-estradiol [27]. Although the relationship between the presence of estrogens and the clinical manifestation of the disease can be explained by epidemiological data and by experiments inhibiting the mycelium–yeast transition, the understanding of the complex relationship between steroid hormones and the immune system is rapidly developing and may help to elucidate the differing disease responses between women and men [28]. Therefore, the mechanisms underlying estrogen protection against PCM warrant further clarification.
The clinical diagnosis of PMC remains a challenge, given the ability of the fungus to mimic the most diverse pathological conditions, such as tuberculosis, leprosy, histoplasmosis, coccidioidomycosis, blastomycosis, leishmaniasis, and syphilis, as well as non-infectious diseases, including bone tissue neoplasia, oral and pharyngeal cancer, cholangiocarcinoma, hypercalcemia, Crohn’s disease, Hodgkin’s lymphoma, icteric syndromes, sarcoidosis, and others [16][17][29][30][31][32]. Such variability of clinical presentations makes it necessary to include PCM among the differential diagnoses for patients who live in or have visited endemic areas, even for patients with symptoms that are not suggestive of PCM. Particular attention should be directed to patients with immunosuppression, such as patients with HIV, cancer, transplants, and autoimmune diseases. There are at least nine reported cases of renal transplant patients who developed PCM [33]. Data in this population remain scarce, and the differential diagnosis becomes even more important for implementing the correct treatment.
The diagnosis of PCM is based on clinical and laboratory findings. In the acute form of the disease, skin lesions are frequently observed. On the other hand, in the chronic form the lungs are more often affected. In these cases, in endemic areas chest radiography may present bilateral and symmetrical opacities in the midline of the lungs, a finding known as butterfly wings [34]. The laboratory diagnosis of PCM must be based on the direct mycological examination of biological materials, such as sputum, bronchial lavage, lesion scraping, lymph node aspiration, or biopsy samples, in which the typical morphology of the fungus can be visualized. Under a light microscope, small chains of blastoconidia or single budding cells can be observed. Histopathologically stained preparations can demonstrate multiple budding yeasts, preferably within granulomas. Although the isolation of the agent is not always possible, the biological material can be cultured at 25–30 °C to finalize the diagnosis [19]. Serological tests are utilized to facilitate the diagnosis, to monitor the evolution of the disease, and to measure the response of the patient to the treatment of this mycosis [35]. Radial immunodiffusion using yeast phase culture filtrate is the most frequently utilized method, presenting a high sensitivity and specificity [35]. Other serological methods, such as immunoblotting and ELISA [35], have been developed for patient monitoring. Molecular methods using PCR have also been proposed to facilitate the definitive diagnosis of the fungus but have not been adopted for use in routine laboratory tests [35]. The standardization of diagnostic techniques among clinical laboratories is still a challenge. The identification of new antigens and the development of better diagnostic methodologies can contribute to the correct identification and better monitoring of PCM patients.
Regarding the therapy of PCM, the first-line treatment is itraconazole, a drug of choice for the treatment of mild and moderate forms of PCM within a time period of 9–18 months. However, given the interactions between itraconazole and a wide range of drugs often used by patients with comorbidities, cotrimoxazole (18–24 months) can be used as a second-choice line of treatment. Sulfamethoxazole is indicated when associated with trimethoprim (an association known as cotrimoxazole). Among the advantages of this association are the low cost, good tolerance, safety for prolonged use [19], and good penetration in the central nervous system [36]. Cotrimoxazole is used in patients with mild to moderate forms of PCM and neuroparacoccidioidomycosis [36]. Although the side effects of itraconazole and cotrimoxazole (e.g., nausea, vomiting, and diarrhea) can make treatment difficult, overall both drugs are well tolerated by patients with PCM.
In severe and disseminated forms, the drug of choice is amphotericin B, and the patient needs to maintain the treatment with itraconazole or cotrimoxazole for an extended period [17]. Despite the effectiveness of amphotericin B, it produces several toxic effects in the host, including acute symptoms, such as nausea, vomiting, fever, hyper- and hypotension, and hypoxia, in addition to chronic nephrotoxicity. New formulations, such as amphotericin B lipid complex, liposomal amphotericin B, and amphotericin B colloidal dispersion, have yielded better tissue distribution and less toxicity [36].
3. Paracoccidioides Phylogeny and Ecology
PCM was initially recognized as being caused only by the fungus P. brasiliensis [37]. After 75 years, phylogenetic studies demonstrated the need to separate the etiological agent in at least three distinct phylogenetic species (PSs)—namely, S1 (38 isolates), PS2 (6 isolates), and PS3 (21 isolates) [2]. This was because the Paracoccidioides genus seems to contain several cryptic species. Cryptic species are those that cannot be easily distinguished based on morphology but which form distinct phylogenetic lineages based on molecular markers. Although researchers recognized the need for separating identified PSs, for years, the isolates continued to receive names related to P. brasiliensis, such as Pb01, Pb03, Pb18, and Pb339, among others. However, there remained a set of isolates, including Pb01, that differed from the proposed PSs. Soon, isolate Pb01 and others (an additional 16 isolates) were recognized as a new species, P. lutzii [3].
This set of changes in the classification of Paracoccidioides isolates occurs frequently among organisms recognized as cryptic species [38]. At present, it is believed that PCM is caused by fungi of the genus Paracoccidioides [39], which is composed of at least five species (P. brasiliensis, P. lutzii, P. americana, P. restrepiensis, and P. venezuelensis) [40]. Despite the great genetic variability among these species, there do not seem to be important differences in the clinical findings when comparing P. brasiliensis with P. americana [41] or P. lutzii [42], for instance. Currently, the genus Paracoccidioides is taxonomically classified as Eukaryota of the kingdom Fungi, phylum Ascomycota, subphylum Pezizomycotina, and family Ajellomycetaceae. Additionally, part of this family includes the fungi Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides immitis [1].
Although overlooking the fact that fungi of the genus Paracoccidioides are organized in cryptic species, the recognition of the existence of isolates with different degrees of virulence, as in the comparisons between Pb18 (considered virulent) and Pb265 (considered of lower virulence), enabled the establishment of an animal model to study the disease [43][44]. Today, however, it is known that even isolated Pb18, considered virulent, can become attenuated after successive culture passages [45]. This model of attenuation and virulence recovery through inoculation in animals enables the study of virulent and attenuated fungi of the same genetic background.
The Paracoccidioides fungi are found in soil, which may be the primary source of infection for both humans and wildlife, given the lack of evidence of transmission between hosts or reservoirs and hosts [4]. Natural infections have been described in some wild and domestic animals [46], and a Paracoccidioides fungus was isolated from two armadillo species, Dasypus novemcinctus L. and Cabassous centralis [47]. These findings suggest that the armadillo is an important natural reservoir of Paracoccidioides [48], and that the same animal can carry multiple species of the fungus [49]. Close contact with soil and animal digging habits enable the dissemination of fungal propagules [47]. Furthermore, the armadillo appears to be highly susceptible to infections [50]. Paracoccidioides fungi have been isolated from infected animals in various states, such as Pará, São Paulo, and Minas Gerais [39]. Approximately 75% to 100% of all animals caught in endemic areas are favorable for PCM [47]. The permissiveness of infectivity in several mammalian hosts can help to explain the spread of the Paracoccidioides fungi over extensive areas. In addition, the great variability observed among the various isolates may be due to environmental pressure and/or to immune responses from different animal species infected by fungi. More in-depth research on these topics may help to elucidate the genetic variability and ecology of Paracoccidioides.
In this entry, we aimed to summarize the major findings in the field of Paracocccidioides biology as well as its genetic aspects. Next, we describe and detail the findings related to dimorphism, stress response, infection, and evasion mechanisms and point out the difficulty and advances in the genetic manipulation of this fungus. Understanding these aspects is necessary to advance in identifying virulence factors, diagnostic markers, new pharmacological targets, and therapeutic strategies.
References
- Van Dyke, M.C.C.C.; Teixeira, M.M.; Barker, B.M. Fantastic yeasts and where to find them: The hidden diversity of dimorphic fungal pathogens. Curr. Opin. Microbiol. 2019, 52, 55–63.
- Matute, D.R.; McEwen, J.G.; Puccia, R.; Montes, B.A.; San-Blas, G.; Bagagli, E.; Rauscher, J.T.; Restrepo, A.; Morais, F.; Niño-Vega, G.; et al. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol. Biol. Evol. 2006, 23, 65–73.
- Teixeira, M.M.; Theodoro, R.C.; de Carvalho, M.J.A.; Fernandes, L.; Paes, H.C.; Hahn, R.C.; Mendoza, L.; Bagagli, E.; San-Blas, G.; Felipe, M.S.S. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol. Phylogenet. Evol. 2009, 52, 273–283.
- Martinez, R. New Trends in Paracoccidioidomycosis Epidemiology. J. Fungi (Basel Switz.) 2017, 3, 1.
- Tracogna, M.F.; Fernández Lugo, S.; Gariboglio Vázquez, M.L.; Fernández, M.S.; Andriani, M.E.; Presti, S.E.; Arce, V.; López, R.; Iliovich, E.; Marques, I.A.; et al. Características clínicas y epidemiológicas de pacientes con paracoccidioidomicosis diagnosticados en un hospital de Resistencia, Chaco. Rev. Argent. Microbiol. 2019, 51, 144–147.
- Conti-Díaz, I.A.; Calegari, L.F. [Paracoccidioidomycosis in Uruguay; its status and current problems]. Bol. Oficina Sanit. Panam. 1979, 86, 219–229.
- Rolon, P.A. [Paracoccidioidomycosis: An epidemic in the Republic of Paraguay, the center of South America]. Mycopathologia 1976, 59, 67–80.
- Alva, Z.B. Aspectos clínicos de la Blastomicosis sudamericana (Paracoccidioidomicosis) en el Perú. Rev. Peru. Med. Exp. Salud Publica 2002, 19, 43–47.
- Fernandez, T.; Lazo, R.F.; Mera, R. Prevalencia de la paracoccidioidomicosis e histoplasmosis en la cuenca del Rio Guayas. Rev. Ecuat. Hig. Med. Trop. 1987, 37, 15–35.
- Torrado, E.; Castañeda, E.; De la Hoz, F.; Restrepo, A. Paracoccidioidomicocis: Definición de las áreas endémicas de Colombia. Biomédica 2000, 20, 327.
- Salzer, H.J.F.J.F.; Burchard, G.; Cornely, O.A.A.; Lange, C.; Rolling, T.; Schmiedel, S.; Libman, M.; Capone, D.; Le, T.; Dalcolmo, M.P.P.; et al. Diagnosis and Management of Systemic Endemic Mycoses Causing Pulmonary Disease. Respiration 2018, 96, 283–301.
- López-Martínez, R.; Hernández-Hernández, F.; Méndez-Tovar, L.J.; Manzano-Gayosso, P.; Bonifaz, A.; Arenas, R.; del Padilla-Desgarennes, M.C.; Estrada, R.; Chávez, G. Paracoccidioidomycosis in Mexico: Clinical and epidemiological data from 93 new cases (1972–2012). Mycoses 2014, 57, 525–530.
- Onda, H.; Komine, M.; Murata, S.; Ohtsuki, M. Letter: Imported paracoccidioidomycosis in Japan. Dermatol. Online J. 2011, 17, 11.
- Van Damme, P.A.; Bierenbroodspot, F.; Telgt, D.S.C.; Kwakman, J.M.; De Wilde, P.C.M.; Meis, J.F.G.M. A case of imported paracoccidioidomycosis: An awkward infection in the Netherlands. Med. Mycol. 2006, 44, 13–18.
- Horré, R.; Schumacher, G.; Alpers, K.; Seitz, H.M.; Adler, S.; Lemmer, K.; de Hoog, G.S.; Schaal, K.P.; Tintelnot, K. A case of imported paracoccidioidomycosis in a German legionnaire. Med. Mycol. 2002, 40, 213–216.
- Wagner, G.; Moertl, D.; Eckhardt, A.; Sagel, U.; Wrba, F.; Dam, K.; Willinger, B. Chronic Paracoccidioidomycosis with adrenal involvement mimicking tuberculosis—A case report from Austria. Med. Mycol. Case Rep. 2016, 14, 12–16.
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; de Telles, F.Q.; Kono, A.; Paniago, A.M.M.; Nathan, A.; do Valle, A.C.F.; Bagagli, E.; Benard, G.; et al. II Consenso Brasileiro em Paracoccidioidomicose—2017. Epidemiol. Serviços Saúde 2018, 27, e0500001.
- Borges-Walmsley, M.I.I.; Chen, D.; Shu, X.; Walmsley, A.R. The pathobiology of Paracoccidioides brasiliensis. Trends Microbiol. 2002, 10, 80–87.
- Restrepo, A.; Benard, G.; de Castro, C.; Agudelo, C.; Tobón, A. Pulmonary Paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2008, 29, 182–197.
- Mamoni, R.L.; Blotta, M.H.S.L. Flow-cytometric analysis of cytokine production in human paracoccidioidomycosis. Cytokine 2006, 35, 207–216.
- de Castro, L.F.; Ferreira, M.C.; da Silva, R.M.; de Blotta, M.H.S.L.; Longhi, L.N.A.; Mamoni, R.L. Characterization of the immune response in human paracoccidioidomycosis. J. Infect. 2013, 67, 470–485.
- Felipe, M.S.S.; Andrade, R.V.; Arraes, F.B.M.M.; Nicola, A.M.; Maranhão, A.Q.; Torres, F.A.G.G.; Silva-Pereira, I.; Poças-Fonseca, M.J.; Campos, E.G.; Moraes, L.M.P.P.; et al. Transcriptional profiles of the human pathogenic fungus Paracoccidioides brasiliensis in mycelium and yeast cells. J. Biol. Chem. 2005, 280, 24706–24714.
- Calich, V.L.G.; Mamoni, R.L.; Loures, F.V. Regulatory T cells in paracoccidioidomycosis. Virulence 2019, 10, 810–821.
- Stover, E.P.; Schär, G.; Clemons, K.V.; Stevens, D.A.; Feldman, D. Estradiol-binding proteins from mycelial and yeast-form cultures of Paracoccidioides brasiliensis. Infect. Immun. 1986, 51, 199–203.
- Shankar, J.; Restrepo, A.; Clemons, K.V.; Stevens, D.A. Hormones and the resistance of women to paracoccidioidomycosis. Clin. Microbiol. Rev. 2011, 24, 296–313.
- Aristizábal, B.H.H.; Clemons, K.V.V.; Cock, A.M.; Restrepo, A.; Stevens, D.A. Experimental Paracoccidioides brasiliensis infection in mice: Influence of the hormonal status of the host on tissue responses. Med. Mycol. 2002, 40, 169–178.
- dos Santos, R.P.; Maia, A.L.; Goldani, L.Z. Paracoccidioidomycosis in a woman with idiopathic hirsutism. Mycopathologia 2004, 158, 57–59.
- Caixeta, C.A.; de Carli, M.L.; Ribeiro Júnior, N.V.; Sperandio, F.F.; Nonogaki, S.; Nogueira, D.A.; Pereira, A.A.C.; Hanemann, J.A.C. Estrogen Receptor-α Correlates with Higher Fungal Cell Number in Oral Paracoccidioidomycosis in Women. Mycopathologia 2018, 183, 785–791.
- Kurai, H.; Ohmagari, N.; Ito, K.; Kawamura, I.; Suzuki, J.; Hadano, Y.; Endo, M.; Iida, Y.; Okinaka, K.; Kamei, K. A Case of Oral Paracoccidioidomycosis Suspected to be Pharyngeal Cancer. Med. Mycol. J. 2012, 53, 49–52.
- Steinbrück, K.; Fernandes, R. Biliary Paracoccidioidomycosis: An Unusual Infection Simulating Malignant Neoplasm. Ann. Hepatol. 2018, 18, 258–262.
- Garbim, B.B.; D’Ávila, L.; Rigatto, S.Z.P.; da Quadros, K.R.S.; Belangero, V.M.S.; de Oliveira, R.B.; D’Ávila, L.; Rigatto, S.Z.P.; da Quadros, K.R.S.; Belangero, V.M.S.; et al. Hypercalcemia in children: Three cases report with unusual clinical presentations. J. Bras. Nefrol. 2017, 39, 213–216.
- Bernardes Filho, F.; Sgarbi, I.; Flávia da Silva Domingos, S.; Sampaio, R.C.R.; Queiroz, R.M.; Fonseca, S.N.S.; Hay, R.J.; Towersey, L. Acute paracoccidioidomycosis with duodenal and cutaneous involvement and obstructive jaundice. Med. Mycol. Case Rep. 2018, 20, 21–25.
- de Almeida Jr., J.; Peçanha-Pietrobom, P.; Colombo, A. Paracoccidioidomycosis in Immunocompromised Patients: A Literature Review. J. Fungi 2018, 5, 2.
- Barreto, M.M.; Marchiori, E.; Amorim, V.B.; Zanetti, G.; Takayassu, T.C.; Escuissato, D.L.; Souza, A.S.; Rodrigues, R.S. Thoracic Paracoccidioidomycosis: Radiographic and CT findings. Radiographics 2012, 32, 71–84.
- Pinheiro, B.G.; Hahn, R.C.; de Camargo, Z.P.; Rodrigues, A.M. Molecular tools for detection and identification of paracoccidioides species: Current status and future perspectives. J. Fungi 2020, 6, 293.
- do Carmo Silva, L.; de Oliveira, A.A.; de Souza, D.R.; Barbosa, K.L.B.; Freitas e Silva, K.S.; Carvalho Júnior, M.A.B.; Rocha, O.B.; Lima, R.M.; Santos, T.G.; de Almeida Soares, C.M.; et al. Overview of Antifungal Drugs against Paracoccidioidomycosis: How Do We Start, Where Are We, and Where Are We Going? J. Fungi 2020, 6, 300.
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected endemic mycoses. Lancet Infect. Dis. 2017, 17, e367–e377.
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.L.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155.
- Theodoro, R.C.; Teixeira, M.D.M.; Felipe, M.S.S.; Paduan, K.D.S.; Ribolla, P.M.; San-Blas, G.; Bagagli, E. Genus paracoccidioides: Species recognition and biogeographic aspects. PLoS ONE 2012, 7, e37694.
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25.
- de Macedo, P.M.; Almeida-Paes, R.; Freitas, D.F.S.; Brito-Santos, F.; Figueiredo-Carvalho, M.H.G.; de Almeida Soares, J.C.; Freitas, A.D.; Zancopé-Oliveira, R.M.; do Valle, A.C.F. Hepatic Disease with Portal Hypertension and Acute Juvenile Paracoccidioidomycosis: A Report of Two Cases and Literature Review. Mycopathologia 2017, 68, 352–359.
- Hahn, R.C.; Rodrigues, A.M.; Della Terra, P.P.; Nery, A.F.; Hoffmann-Santos, H.D.; Góis, H.M.; Fontes, C.J.F.; de Camargo, Z.P.; Terra, P.P.D.; Nery, A.F.; et al. Clinical and epidemiological features of paracoccidioidomycosis due to paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2019, 13, 1–13.
- Vaz, C.A.; Singer-Vermes, L.M.; Calich, V.L. Comparative studies on the antibody repertoire produced by susceptible and resistant mice to virulent and nonvirulent Paracoccidioides brasiliensis isolates. Am. J. Trop. Med. Hyg. 1998, 59, 971–977.
- Kurokawa, C.S.; Lopes, C.R.; Sugizaki, M.F.; Kuramae, E.E.; Franco, M.F.; Peraçoli, M.T.S. Virulence profile of ten Paracoccidioides brasiliensis isolates: Association with morphologic and genetic patterns. Rev. Inst. Med. Trop. Sao Paulo 2005, 47, 257–262.
- Castilho, D.G.; Chaves, A.F.A.; Xander, P.; Zelanis, A.; Kitano, E.S.; Serrano, S.M.T.; Tashima, A.K.; Batista, W.L. Exploring potential virulence regulators in Paracoccidioides brasiliensis isolates of varying virulence through quantitative proteomics. J. Proteome Res. 2014, 13, 4259–4271.
- Headley, S.A.; Pretto-Giordano, L.G.; Di Santis, G.W.; Gomes, L.A.; Macagnan, R.; da Nóbrega, D.F.; Leite, K.M.; de Alcântara, B.K.; Itano, E.N.; Alfieri, A.A.; et al. Paracoccidioides brasiliensis-associated dermatitis and lymphadenitis in a dog. Mycopathologia 2017, 182, 425–434.
- Bagagli, E.; Bosco, S.M.G.; Theodoro, R.C.; Franco, M. Phylogenetic and evolutionary aspects of Paracoccidioides brasiliensis reveal a long coexistence with animal hosts that explain several biological features of the pathogen. Infect. Genet. Evol. 2006, 6, 344–351.
- Vidal, M.S.; de Melo, N.T.; Garcia, N.M.; Del Negro, G.M.; de Assis, C.M.; Heins-Vaccari, E.M.; Naiff, R.D.; Mendes, R.P.; da Silva Lacaz, C. Paracoccidioides brasiliensis. A mycologic and immunochemical study of a sample isolated from an armadillo (Dasipus novencinctus). Rev. Inst. Med. Trop. Sao Paulo 1995, 37, 43–49.
- Hrycyk, M.F.; Garcia Garces, H.; de Bosco, S.M.G.; de Oliveira, S.L.; Marques, S.A.; Bagagli, E. Ecology of Paracoccidioides brasiliensis, P. lutzii and related species: Infection in armadillos, soil occurrence and mycological aspects. Med. Mycol. 2018, 56, 950–962.
- Storrs, E.E.; Walsh, G.P.; Burchfield, H.P.; Binford, C.H. Leprosy in the armadillo: New model for biomedical research. Science 1974, 183, 851–852.





