| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sylwia Jarmolińska | + 1739 word(s) | 1739 | 2020-10-12 08:36:26 |
Video Upload Options
Mesoporous silicas have enjoyed great interest among scientists practically from the moment of their discovery thanks to their unique attractive properties. Many types of mesoporous silicas have been described in literature, the most thoroughly MCM-41 and SBA-15 ones. The methods of syntheses, characterization and use of mesoporous silicas from SBA (Santa Barbara Amorphous) and HMM (Hybrid Mesoporous Materials) groups are presented. The first group is represented by (i) SBA-1 of three-dimensional cubic structure and (ii) SBA-2 of three-dimensional combined hexagonal and cubic structures. The HMM group is represented by (i) HMM-1 of two-dimensional hexagonal structure and (ii) HMM-2 of three-dimensional structure. The paper provides comprehensive information on the above-mentioned silica materials available so far, also including the data for the silicas modified with metal ions or/and organic functional groups and examples of the materials applications.
1. General Information about Porous Materials
For almost 30 years, much attention has been paid to designing and obtaining new nanomaterials. The interest in such materials stems from the fact that they have at least one component of their structure on the nanoscale (of size from 1 nm to 100 nm) and thus show a number of unique properties and have a wide range of applications [1][2]. A considerable number of such materials belong to nanoporous ones characterized by the presence of channels or pores, classified by IUPAC (International Union of Pure and Applied Chemistry) as micropores (diameter below 2 nm), mesopores (diameter in the range 2–50 nm) and macropores (diameter above 50 nm) [3][4]. The group of porous materials includes for example, zeolites [5], porous carbons [6] and mesoporous silicas [7]. The latter ones are of particular interest as they show highly ordered and stable mesoporous structure, well-developed surface area, ordered system of uniform pores of narrow size distribution and large volume, high thermal, chemical and hydrothermal stability, are nontoxic and their surface can be easily modified [1][8][9].
The history of porous materials started with discovery of natural zeolites, that are microporous aluminosilicates of crystal structure, having a developed system of micropores [10]. Their use in the chemical and petrochemical industry has brought significant benefits both to economy and the natural environment. The success of zeolites has resulted in the syntheses of a number of materials of zeolite structure, however, the size of pores was found to be a substantial limitation as they could not have been used in transformations of larger molecules [11]. The need aroused to obtain mesoporous materials, whose larger pores and large surface area could make them applicable for adsorption, separation, catalysis, as drug delivery carriers, sensors, in photonics for energy storage and conversion and as nanodevices working with large molecules [12].
The first report on the synthesis of mesoporous materials was published in the beginning of the 1990s and it has been a milestone in materials chemistry. In 1992, the first ordered mesoporous silicas were synthesized by the Mobile Research and Development Corporation [13][14]. The materials obtained were called the M41S family and included MCM type materials (Mobile Composition of Matter): MCM-41, MCM-48 and MCM-50, differing in the type of pore ordering. These silicas showed well-developed surface area and a uniform size pore system [15]. The synthesis of M41S materials has opened the way to obtaining new ordered mesoporous silicas [16]: SBA (Santa Barbara Amorphous), MSU (Michigan State University), FSM (Folded Sheet Materials), FDU (Fudan University) and KIT (Korean Advanced Institute of Science and Technology). They were synthesized by modifications of the earlier proposed method by addition of different compounds directing structural development [8][17].
Syntheses of ordered mesoporous silicas of well-defined structure need first of all precise planning of the process, the choice of a suitable compound directing structural development and a suitable precursor of silica. At a proper molar ratio of substrates and proper conditions of synthesis, such as: the time and temperature, pH of solution, time of hydrothermal treatment (ageing), the way and conditions of removal of the structure directing compound from silica pores, it is possible to obtain silicas of desired pore size and structure [18][19].
The best known and most thoroughly studied so far are the SBA-1 type silicas. In general SBA type silicas (SBA-11, SBA-12, SBA-15, SBA-16) are obtained using non-ionic surfactants as structural directing agents [20], however, SBA-1 has been for the first time synthesized using a cationic surfactant with a large head component of its molecule [21]. SBA-2 was synthesized using a gemini surfactant. Perhaps because of the necessity of independent synthesis of these two surfactants needed for obtaining SBA-1 and SBA-2, these two silicas have not been so thoroughly described in literature as the other SBA type materials synthesized with the use of commonly available non-ionic surfactants. It should be emphasized that the 3D system of pores present in SBA-1 ensures easier accessibility of these pores to the reagent molecules than the 1D cylindrical pores, so SBA-1 has high application potential, for example, in adsorption and catalysis [22].
HMM materials are organic-inorganic hybrids that have been obtained as a result of condensation of bis-silylated organic compound (R’O)3Si–R–Si(OR’)3 used as a precursor of silica. The type of material obtained, HMM-1 or HMM-2 of different structures, depends on the proportions of the reagents in the reaction mixture [19]. The materials belong to the group of mesoporous materials referred to as PMOs (Periodic Mesoporous Organosilicas).
2. SBA-1
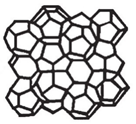
Mesoporous SBA-1 silicas have three-dimensional cubic structure of Pm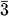
Figure 1. Structure of SBA-1. Reprinted with permission from The Royal Society of Chemistry.
3. SBA-2
SBA-2 type silicas have not been so thoroughly described as SBA-1. The former have a 3D pore network made of spherical cavities ordered in hexagonal close-packed (hcp) system and cubic close-packed system (ccp), joined through cylindrical channels [27][28][29]. It should be emphasized that the structure of SBA-2 silicas is similar to that of SBA-12 showing 3D hexagonal structure and P63/mmc symmetry. The latter have been obtained using triblock copolymer as a mesoporous structure directing compound [30][31]. After the first synthesis of SBA-2 silica, only the hexagonal pore system was identified in its structure, while the presence of the cubic system of pores was found later as a result of detailed investigation [32]. The hitherto literature has provided only a few applications of these silica materials in catalysis and adsorption, as described below.
Figure 2. The network of pores in SBA-2. Reprinted with permission with minor modification.
Copyright (2003) Elsevier B.V.
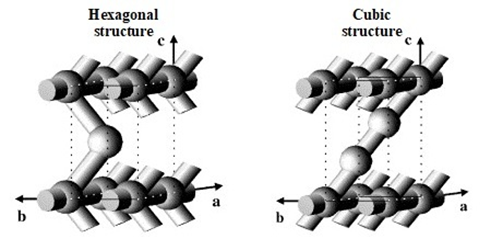
4. HMM-1 and HMM-2
Materials of HMM-1 and HMM-2 types belong to Periodic Mesoporous Organosilicas (PMOs) as they contain in their structure organic and inorganic groups making a hybrid organic-inorganic lattice linked by covalent bonds [33]. The HMM group is divided into HMM-1 and HMM-2 subgroups. Their structure is built of uniform ethyl fragments (–CH2–CH2–) and silica groups (Si2O3), making a network joined through covalent bonds [34][35][36]. The porous structure of these materials is completely different than that in mesoporous materials built of an inorganic lattice with organic modifiers grafted on the surface. HMM-1 and HMM-2 show highly-ordered mesoporous structure and well-defined morphology of hexagonal rods and spherical particles, respectively [34]. These two materials were synthesized using the same reagents: 1,2-Bis(trimethoxysilyl)ethane (BTME) and octadecyltrimethylammonium chloride (ODTMACl), in basic conditions. Their structure was controlled by the temperature of synthesis and molar ratio of the components of the synthetic mixture. The removal of surfactant by extraction with a solvent opened the uniform pores in the materials and it did not deteriorate the ordered structure. The materials were characterized by hydrothermal stability [33][37]. HMM-1 showed a 2D hexagonal structure of p6mm symmetry with 1D pores of diameters smaller than 10 nm and well-developed surface area reaching even 1000 m2/g. Thanks to these properties HMM-1 was applied as a template for the synthesis of nanoparticles and nanowires of metals [38][39]. HMM-2 of a 3D structure of P63/mmc symmetry found similar applications [40].
5. Application of Mesoporous Silica Materials - Summary
Nanoporous silica materials, due to their physicochemical properties, show a number of applications [41][42][43]. Table 1 summarizes potential applications of SBA-1, SBA-2, HMM-1 and HMM-2 materials.
Table 1. Potential use of the SBA and HMM-type materials.
| Type of material | Metal/Organic Groups | Application | Ref. |
|---|---|---|---|
| Synthesis in Acidic Conditions | |||
| SBA-1 | Ti | Oxidation of styrene with hydrogen peroxide | [44] |
| SBA-1 | Fe | ||
| SBA-1 | Mo | Partial oxidation of methane with oxygen | [45] |
| SBA-1 | Al | Isomerization of n-decane | [46] |
| SBA-1 | Al | Synthesis of 7-methoxy-4-methylcoumarin | [47] |
| SBA-1 | Al Al and Mg |
Acetalization of heptanal | [48] |
| SBA-1 | Ti | Epoxidation of styrene to styrene oxide | [49] |
| SBA-1 | Cr | Dehydrogenation of ethane with the use of CO2 | [50] |
| Dehydrogenation of propane with the use of CO2 | [51] | ||
| SBA-1 | Ga | tert-butylation of phenol | [25] |
| Alkylation of naphthalene with propylene | [52] | ||
| SBA-1 | Mn | Oxidation of ethylbenzene with the use of TBHP | [53] |
| SBA-1 | alkali metals Li, Na, K, Rb, Cs |
Knoevenagel condensation between benzaldehyde or benzylacetone and ethyl cyanoacetate | [54] |
| SBA-1 | amino groups | Adsorption of oxyanions (chromates and arsenates) | [55] |
| SBA-1 | Hoveyd-Grubbs catalyst | Olefin metathesis catalyst | [56] |
| SBA-1 | unmodified | For obtaining highly-ordered carbon materials | [57] |
| Synthesis in Basic Conditions | |||
| SBA-1 | Al | Alkylation of 2, 4-Di-tert-butylphenol with cinnamyl alcohol | [58][59] |
| Alkylation of toluene with benzyl alcohol | [60] | ||
| SBA-1 | Ti | Oxidation of 2, 3, 6-trimethylphenol | [61] |
| SBA-1 | unmodified | Immobilization of lysozyme | [62] |
| SBA-1 | carboxyl groups | Immobilization of papain | [63] |
| SBA-1 | carboxyl and amino groups | Adsorption of toxic anionic or cationic dyes | [27] |
| SBA-2 | thiol groups | Esterification of glycerol with oleic acid (very low activity) | [64] |
| SBA-2 | sulfonic groups | Esterification of glycerol with oleic or lauric acid (very low activity) | [65] |
| SBA-2 | Ti | Oxidizing desulfurization of Diesel oil | [28] |
| SBA-2 | unmodified | Adsorbent of volatile organic compounds (adsorbent for separation of a mixture of benzene/cyclohexene) | [66] |
| HMM-1 | Rh or Pt and Rh | Hydrogenation of n-butane | [36] |
| HMM-1 | sulfonic groups | Hydrolysis of saccharose of starch | [67] |
| HMM-1 | unmodified | Matrices for syntheses of nanowires and metal nanoparticles (bimetallic Pt-Rh, Pt-Pd as well as monometallic Pt or Rh) | [68] |
| HMM-1 | Pd nanowires or nanoparticles | CO oxidation | [38] |
| HMM-2 | unmodified | Matrices for syntheses of nanowires and metal nanoparticles (Au, Pt) | [69] |
| HMM-2 | Au nanoparticles | CO oxidation | [70][71] |
References
- Sharma, N.; Ojha, H.; Bharadwaj, A.; Pathak, D.P.; Sharma, R.K. Preparation and catalytic applications of nanomaterials: A review. RSC Adv. 2015, 5, 53381–53403.
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074.
- Welborn, S.S.; Detsi, E. Small-angle X-ray scattering of nanoporous materials. Nanoscale Horiz. 2020, 5, 12–24.
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069.
- Feliczak-Guzik, A. Hierarchical zeolites: Synthesis and catalytic properties. Microporous Mesoporous Mater. 2018, 259, 33–45.
- Malgras, V.; Tang, J.; Wang, J.; Kim, J.; Torad, N.L.; Dutta, S.; Ariga, K.; Hossain, M.S.A.; Yamauchi, Y.; Wu, K.C.W. Fabrication of nanoporous carbon materials with hard- and soft-templating approaches: A review. J. Nanosci. Nanotechnol. 2019, 19, 3673–3685.
- Naik, B.; Ghosh, N. A review on chemical methodologies for preparation of mesoporous silica and alumina based materials. Recent Pat. Nanotechnol. 2009, 3, 213–224.
- Kumar, S.; Malik, M.M.; Purohit, R. Synthesis methods of mesoporous silica materials. Mater. Today Proc. 2017, 4, 350–357.
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902.
- Pal, N.; Bhaumik, A. Soft templating strategies for the synthesis of mesoporous materials: Inorganic, organic-inorganic hybrid and purely organic solids. Adv. Colloid Interface Sci. 2013, 189–190, 21–41.
- Perego, C.; Millini, R. Porous materials in catalysis: Challenges for mesoporous materials. Chem. Soc. Rev. 2013, 42, 3956–3976.
- Li, W.; Zhao, D. An overview of the synthesis of ordered mesoporous materials. Chem. Commun. 2013, 49, 943–946.
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843.
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712.
- Chen, H.; Yang, H.; Xi, Y. Highly ordered and hexagonal mesoporous silica materials with large specific surface from natural rectorite mineral. Microporous Mesoporous Mater. 2019, 279, 53–60.
- Asefa, T.; Tao, Z. Mesoporous silica and organosilica materials—Review of their synthesis and organic functionalization. Can. J. Chem. 2012, 90, 1015–1031.
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.V.N. Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A Gen. 2018, 559, 57–74.
- Meynen, V.; Cool, P.; Vansant, E.F. Verified syntheses of mesoporous materials. Microporous Mesoporous Mater. 2009, 125, 170–223.
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chemie Int. Ed. 2006, 45, 3216–3251.
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric sufactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036.
- Huo, Q.; Margolese, D.I.; Ciesla, U.; Feng, P.; Gier, T.E.; Sieger, P.; Leon, R.; Petroff, P.M.; Schüth, F.; Stucky, G.D. Generalized synthesis of periodic surfactant/inorganic composite materials. Nature 1994, 368, 317–321.
- Liu, M.C.; Sheu, H.S.; Cheng, S. Drying induced phase transformation of mesoporous silica. Chem. Commun. 2002, 23, 2854–2855.
- Che, S.; Sakamoto, Y.; Yoshitake, H.; Terasaki, O.; Tatsumi, T. Synthesis and characterization of Mo–SBA-1 cubic mesoporous molecular sieves. J. Phys. Chem. B 2001, 105, 10565–10572.
- Sakamoto, Y.; Kaneda, M.; Terasaki, O.; Zhao, D.Y.; Kim, J.M.; Stucky, G.; Shin, H.J.; Ryoo, R. Direct imaging of the pores and cages of three-dimensional mesoporous materials. Nature 2000, 408, 449–453.
- Srinivasu, P.; Vinu, A. Three-dimensional mesoporous gallosilicate with Pm3n symmetry and its unusual catalytic performances. Chem. Eur. J. 2008, 14, 3553–3561.
- Michorczyk, P.; Ogonowski, J.; Niemczyk, M. Investigation of catalytic activity of CrSBA-1 materials obtained by direct method in the dehydrogenation of propane with CO2. Appl. Catal. A Gen. 2010, 374, 142–149.
- Pérez-Mendoza, M.; Gonzalez, J.; Wright, P.A.; Seaton, N.A. Structure of the mesoporous silica SBA-2, determined by a percolation analysis of adsorption. Langmuir 2004, 20, 9856–9860.
- Shi, C.; Wang, W.; Liu, N.; Xu, X.; Wang, D.; Zhang, M.; Sun, P.; Chen, T. Low temperature oxidative desulfurization with hierarchically mesoporous titaniumsilicate Ti-SBA-2 single crystals. Chem. Commun. 2015, 51, 11500–11503.
- Zhao, D.; Wan, Y.; Zhou, W. Ordered Mesoporous Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 171–181.
- Sakamoto, Y.; Díaz, I.; Terasaki, O.; Zhao, D.; Pérez-Pariente, J.; Kim, J.M.; Stucky, G.D. Three-dimensional cubic mesoporous structures of SBA-12 and related materials by electron crystallography. J. Phys. Chem. B 2002, 106, 3118–3123.
- Garcia-Bennett, A.E.; Williamson, S.; Wright, P.A.; Shannon, I.J. Control of structure, pore size and morphology of three-dimensionally ordered mesoporous silicas prepared using the dicationic surfactant [CH3(CH2)15N(CH3)2(CH2)3N(CH3)3]Br2. J. Mater. Chem. 2002, 12, 3533–3540.
- Zhou, W.; Hunter, H.M.A.; Wright, P.A.; Ge, Q.; Thomas, J.M. Imaging the pore structure and polytypic intergrowths in mesoporous silica. J. Phys. Chem. B 1998, 102, 2–5.
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614.
- Guan, S.; Inagaki, S.; Ohsuna, T.; Terasaki, O. Cubic hybrid organic-inorganic mesoporous crystal with a decaoctahedral shape. J. Am. Chem. Soc. 2000, 122, 5660–5661.
- Guan, S.; Inagaki, S.; Ohsuna, T.; Terasaki, O. Hybrid ethane-siloxane mesoporous materials with cubic symmetry. Microporous Mesoporous Mater. 2001, 44–45, 165–172.
- Dhepe, P.L.; Fukuoka, A.; Ichikawa, M. Novel fabrication and catalysis of nano-structured Rh and RhPt alloy particles occluded in ordered mesoporous silica templates using supercritical carbon dioxide. Phys. Chem. Chem. Phys. 2003, 5, 5565–5573.
- Kruk, M.; Jaroniec, M.; Guan, S.; Inagaki, S. Adsorption and thermogravimetric characterization of mesoporous materials with uniform organic-inorganic frameworks. J. Phys. Chem. B 2001, 105, 681–689.
- Fukuoka, A.; Araki, H.; Sakamoto, Y.; Inagaki, S.; Fukushima, Y.; Ichikawa, M. Palladium nanowires and nanoparticles in mesoporous silica templates. Inorg. Chim. Acta 2003, 350, 371–378.
- Fukuoka, A.; Higuchi, T.; Ohtake, T.; Oshio, T.; Kimura, J.-I.; Sakamoto, Y.; Shimomura, N.; Inagaki, S.; Ichikawa, M. Nanonecklaces of platinum and gold with high aspect ratios synthesized in mesoporous organosilica templates by wet hydrogen reduction. Chem. Mater. 2006, 18, 337–343.
- Ravikovitch, P.I.; Neimark, A.V. Density functional theory of adsorption in spherical cavities and pore size characterization of templated nanoporous silicas with cubic and three-dimensional hexagonal structures. Langmuir 2002, 18, 1550–1560.
- Yang, J.; Su, H.; Lian, C.; Shang, Y.; Liu, H.; Wu, J. Understanding surface charge regulation in silica nanopores. Phys. Chem. Chem. Phys. 2020, 22, 15373–15380.
- Lian, C.; Xian, K.; Liu, H.; Wu, J. Flow effects on silicate dissolution and ion transport at an aqueous interface. Phys. Chem. Chem. Phys. 2019, 21, 6970–6975.
- Lis, D.; Backus, E.H.G.; Hunger, J.; Parekh, S.H.; Bonn, M. Liquid flow along a solid surface reversibly alters interfacial chemistry. Science 2014, 644, 1138–1142.
- Gabaldon, J.P.; Bore, M.; Datye, A.K. Mesoporous silica supports for improved thermal stability in supported Au catalysts. Top. Catal. 2007, 44, 253–262.
- Dai, L.-X.; Tabata, K.; Suzuki, E.; Tatsumi, T. Synthesis and characterization of V-SBA-1 cubic mesoporous molecular sieves. Chem. Mater. 2001, 13, 208–212.
- Peng, M.M.; Hemalatha, P.; Ganesh, M.; Palanichamy, M.; Jang, H.T. Solvent free synthesis of coumarin derivative by the use of AlSBA-1 molecular sieves. J. Ind. Eng. Chem. 2014, 20, 953–960.
- Venkatachalam, K.; Palanichamy, M.; Murugesan, V. Acetalization of heptanal over Al-SBA-1 molecular sieve. Catal. Commun. 2010, 12, 299–303.
- Ji, D.; Ren, T.; Yan, L.; Suo, J. Synthesis of Ti-incorporated SBA-1 cubic mesoporous molecular sieves. Mater. Lett. 2003, 57, 4474–4477.
- Ji, D.; Zhao, R.; Lv, G.; Qian, G.; Yan, L.; Suo, J. Direct synthesis, characterization and catalytic performance of novel Ti-SBA-1 cubic mesoporous molecular sieves. Appl. Catal. A Gen. 2005, 281, 39–45.
- Michorczyk, P.; Pietrzyk, P.; Ogonowski, J. Preparation and characterization of SBA-1-supported chromium oxide catalysts for CO2 assisted dehydrogenation of propane. Microporous Mesoporous Mater. 2012, 161, 56–66.
- Brzozowski, R.; Vinu, A. Alkylation of naphthalene over mesoporous Ga-SBA-1 catalysts. Top. Catal. 2009, 52, 1001–1004.
- Imran, G.; Maheswari, R. Mn-incorporated SBA-1 cubic mesoporous silicates: Synthesis and characterization. Mater. Chem. Phys. 2015, 161, 237–242.
- Gracia, M.D.; Jurado, M.J.; Luque, R.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Modified SBA-1 materials for the Knoevenagel condensation under microwave irradiation. Microporous Mesoporous Mater. 2009, 118, 87–92.
- Corma, A.; Iborra, S. Optimization of alkaline earth metal oxide and hydroxide catalysts for base-catalyzed reactions. Adv. Catal. 2006, 49, 239–302.
- Kao, H.-M.; Liao, C.-H.; Palani, A.; Liao, Y.-C. One-pot synthesis of ordered and stable cubic mesoporous silica SBA-1 functionalized with amino functional groups. Microporous Mesoporous Mater. 2008, 113, 212–223.
- Li, H.; Sakamoto, Y.; Li, Y.; Terasaki, O.; Thommes, M.; Che, S. Synthesis of carbon replicas of SBA-1 and SBA-7 mesoporous silicas. Microporous Mesoporous Mater. 2006, 95, 193–199.
- Liu, M.-C.; Chang, C.-S.; Chan, J.C.C.; Sheu, H.-S.; Cheng, S. An alkaline route to prepare hydrothermally stable cubic Pm3n mesoporous silica using CTEA template. Microporous Mesoporous Mater. 2009, 121, 41–51.
- Lin, T.-H.; Chen, C.-H.; Chang, C.-S.; Liu, M.-C.; Huang, S.-J.; Cheng, S. Cubic Pm3n mesoporous aluminosilicate assembled from zeolite seeds as strong acidic catalysts. Catal. Sci. Technol. 2015, 5, 3182–3193.
- Wang, J.-G.; Zhou, H.-J.; Sun, P.-C.; Ding, D.-T.; Chen, T.-H. Hollow carved single-crystal mesoporous silica templated by mesomorphous polyelectrolyte-surfactant complexes. Chem. Mater. 2010, 22, 3829–3831.
- Lin, T.-H.; Chen, C.-C.; Jang, L.-Y.; Lee, J.-F.; Cheng, S. Preparation and catalytic properties of mesoporous titanosilicate of cubic Pm3n structure. Microporous Mesoporous Mater. 2014, 198, 194–202.
- Li, N.; Wang, J.-G.; Zhou, H.-J.; Sun, P.-C.; Chen, T.-H. Facile fabrication of hierarchically nanoporous SBA-1 nanoparticles. RSC Adv. 2012, 2, 2229–2231.
- Xu, J.; Liu, W.; Yu, Y.; Du, J.; Li, N.; Xu, L. Synthesis of mono-dispersed mesoporous SBA-1 nanoparticles with tunable pore size and their application in lysozyme immobilization. RSC Adv. 2014, 4, 37470–37478.
- Lin, C.-H.; Deka, J.R.; Wu, C.-E.; Tsai, C.-H.; Saikia, D.; Yang, Y.-C.; Kao, H.-M. Bifunctional cage-type cubic mesoporous silica SBA-1 nanoparticles for selective adsorption of dyes. Chem. An. Asian J. 2017, 12, 1314–1325.
- Díaz, I.; Mohino, F.; Pérez-Pariente, J.; Sastre, E.; Wright, P.A.; Zhou, W. 07-P-17-A direct synthesis route to the mesoporous silicate SBA-2 bearing thiol groups. Stud. Surf. Sci. Catal. 2001, 135, 1253–1284.
- Pérez-Pariente, J.; Díaz, I.; Mohino, F.; Sastre, E. Selective synthesis of fatty monoglycerides by using functionalised mesoporous catalysts. Appl. Catal. A Gen. 2003, 254, 173–188.
- Emparan-Legaspi, M.J.; Gonzalez, J.; Gonzalez-Carrillo, G.; Ceballos-Magaña, S.G.; Canales-Vazquez, J.; Aguayo-Villarreal, I.A.; Muñiz-Valencia, R. Dynamic adsorption separation of benzene/cyclohexane mixtures on micro-mesoporous silica SBA-2. Microporous Mesoporous Mater. 2020, 294, 109942.
- Dhepe, P.L.; Ohashi, M.; Inagaki, S.; Ichikawa, M.; Fukuoka, A. Hydrolysis of sugars catalyzed by water-tolerant sulfonated mesoporous silicas. Catal. Lett. 2005, 102, 163–169.
- Fukuoka, A.; Sakamoto, Y.; Guan, S.; Inagaki, S.; Sugimoto, N.; Fukushima, Y.; Hirahara, K.; Iijima, S.; Ichikawa, M. Novel templating synthesis of necklace-shaped mono- and bimetallic nanowires in hybrid organic-inorganic mesoporous material. J. Am. Chem. Soc. 2001, 123, 3373–3374.
- Fukuoka, A.; Araki, H.; Kimura, J.; Sakamoto, Y.; Higuchi, T.; Sugimoto, N.; Inagaki, S.; Ichikawa, M. Template synthesis of nanoparticle arrays of gold, platinum and palladium in mesoporous silica films and powders. J. Mater. Chem. 2004, 14, 752–756.
- Bore, M.T.; Pham, H.N.; Switzer, E.E.; Ward, T.L.; Fukuoka, A.; Datye, A.K. The role of pore size and structure on the thermal stability of gold nanoparticles within mesoporous silica. J. Phys. Chem. B 2005, 109, 2873–2880.
- Gabaldon, J.P.; Bore, M.; Datye, A.K. Mesoporous silica supports for improved thermal stability in supported Au catalysts. Top. Catal. 2007, 44, 253–262.