
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rossella Rota | + 4320 word(s) | 4320 | 2020-12-11 06:04:40 |
Video Upload Options
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma of children and adolescents. The fusion-positive (FP)-RMS variant expressing chimeric oncoproteins such as PAX3-FOXO1 and PAX7-FOXO1 is at high risk. The fusion negative subgroup, FN-RMS, has a good prognosis when non-metastatic. Despite a multimodal therapeutic approach, FP-RMS and metastatic FN-RMS often show a dismal prognosis with 5-year survival of less than 30%. Therefore, novel targets need to be discovered to develop therapies that halt tumor progression, reducing long-term side effects in young patients. Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that regulates focal contacts at the cellular edges. It plays a role in cell motility, survival, and proliferation in response to integrin and growth factor receptors’ activation. FAK is often dysregulated in cancer, being upregulated and/or overactivated in several adult and pediatric tumor types. In RMS, both in vitro and preclinical studies point to a role of FAK in tumor cell motility/invasion and proliferation, which is inhibited by FAK inhibitors.
1. Introduction
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma of childhood, accounting for about 50% of all the soft tissue sarcomas and 8% of all pediatric cancers [1]. RMS cells derive from mesenchymal precursors mainly committed to myogenic lineage that are unable to differentiate [2][3]. In agreement, RMS cells express master myogenic factors such as MYOD and myogenin (MYOG), used for diagnostic purposes. Pediatric RMS includes two main histological subtypes, namely embryonal and alveolar RMS. About 70% of alveolar RMS harbors chromosomal translocations among which the most represented are t(2;13)(q36;q14) and t(1:13)(p36;q14), which encode for the chimeric oncoproteins PAX3-FOXO1 and PAX7-FOXO1, respectively [4], and are indicated as fusion-positive (FP)-RMS. PAX3-FOXO1 is the driver of the disease strictly required for the survival of tumor cells [5]. PAX3-FOXO1 being a transcription factor (TF), it is considered undruggable even if new approaches to directly blocks TFs are ongoing objects of deep investigations [6]. Embryonal RMS is devoid of any fusion gene (fusion-negative (FN)-RMS) but frequently harbors RAS mutations ([7] and reviewed in [8]). It has been demonstrated that 20–30% of alveolar RMSs that are fusion negative have the same clinical and molecular features of embryonal RMS and are, thus, considered FN-RMS [9]. FN-RMSs have a favorable prognosis when non-metastatic or in advanced stage (5-year survival rates reaching 70–80%) [10]. Overall, survival of RMS patients with localized non-metastatic disease has greatly improved in the last decades due to the multimodal therapeutic approach including chemotherapy, surgery, and, frequently, radiotherapy [11][12]. Conversely, despite heavy multimodal therapeutic regimens, metastatic FN-RMSs and FP-RMSs have a dismal prognosis, showing a 5-year survival rate <30% [10]. Several clinical trials have been developed to identify therapeutic approaches that could improve the prognosis of RMS at a high risk of relapse, which have been supported by two major cooperative groups such as the Children Oncology Group (COG) in North America and the European Pediatric Soft Tissue Sarcoma Study Group (EpSSG) in Europe.
The first-line standard of care for high-risk RMS includes vincristine and dactinomycin combined with an alkylating agent which is cyclophosphamide for COG (VAC) and ifosfamide for EpSSG (IVA) [13][14]. Recently, the EpSSG evaluated the incorporation of doxorubicin to IVA in the phase 3 trial EpSSG RMS 2005. Unfortunately, RMS patients treated with IVA+Doxo did not show any significant improvement in 3-year event-free survival vs. those treated with IVA alone (65.5% and 63.3% in the IVA-Doxo and IVA, respectively) while adverse effects such as leukopenia, infections, and thrombocytopenia were more common than in the IVA group [15]. In addition, continued maintenance chemotherapy with vinorelbine, a semisynthetic analog of vincristine, and low doses of cyclophosphamide was analyzed in high-risk RMS patients in remission after standard treatment in the phase 3 trial EudraCT (NCT00339118; www.clinicaltrials.gov) (follow-up is still ongoing). Five-year disease-free survival (DFS) and overall survival (OS) resulted both higher than those in the group without maintenance therapy (77.6% vs. 69.8% DFS and 86.5% vs. 73.7% OS, respectively) prompting the EpSSG to incorporate this approach in the new standard of care for these types of patients [16].
Of note, patients with bone or bone marrow metastases or metastases in more than 3 sites show a 5-year survival rate <10% (reviewed in [17]). Therefore, one of the major determinants of tumor progression is the presence of metastases at diagnosis or the development of a metastatic disease during therapy, which are both related to the intrinsic marked ability of mesenchymal muscle precursors to migrate and invade during somitogenesis (reviewed in [18]). Indeed, somites are epithelial-condensed structures of mesodermal cells that undergo secondary epithelial-to-mesenchymal transition (EMT), giving rise to highly migrating mesenchymal progenitors committed to skeletal muscle lineage (reviewed in [18]). As for pediatric cancers in general, the inability to differentiate and the migratory properties of RMS cells are related to abnormalities of developmental pathways that regulate skeletal muscle determination [19]. Among these, FN-RMS receptor tyrosine kinase (RTK) signaling involving the RAS/MEK/ERK and the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) cascade include upstream and downstream mutated components that in the FP-RMS variant are aberrantly regulated by PAX3-FOXO1, suggesting tumorigenic pathways in the two RMS subtypes converge on a common genetic axis [7]. In the last years, several clinical trials have been started using targeted therapy against molecules involved in deregulated pathways in RMS, which were previously validated in the preclinical setting. Results from these clinical trials seem to indicate that therapy with targeted drugs has modest effects on the progression of the disease as single treatment, but should be used in combination with the standard of care as an adjuvant approach [20]. Therefore, novel approaches that should halt tumor metastasis and progression, preventing in the meantime harmful side-effects for young patients affected by RMS, are needed.
Focal adhesion kinase (FAK) is a 125 kDa non-receptor tyrosine kinase encoded by the PTK2 gene, mainly localized to cellular focal contacts at the cellular edges, which plays a critical role in adhesion-dependent cell motility, survival, and proliferation in response to integrin and RTK signaling [21]. Overall, FAK coordinates signals between the cytoskeleton of the cells and the extracellular microenvironment. These functions make FAK a crucial factor during tissue development, embryogenesis, and cancer by inhibiting cell death after the disruption of adhesions between cells and the extracellular matrix (ECM), i.e., “anoikis” [22][23]. Anoikis is a form of apoptosis that constitutes one of the key defense mechanisms for preventing cancer metastasis [24]. While in most adult tissues FAK is expressed at low levels, in cancer its expression/activation is frequently upregulated and, in certain tumors, negatively correlates with prognosis [25][26]. FAK has been shown upregulated and overactivated in RMS and its inhibition decreases tumor growth in vivo.
2. FAK Structure and Activity
FAK is not only a sensor of environmental rigidity, but it is also involved in an intricate network of intramolecular interactions existing among the microenvironment, the adhesion receptor complexes, and the nucleus coordinating signals through the focal adhesion multiprotein complex [27]. Functionally, focal adhesion complex works by anchoring the cytoplasmic tails of integrins, which are heterodimeric membrane-spanning proteins, allowing a link with the ECM. This binding provides integrins, lacking for kinase activity, the ability to transduce the signal via FAK in response to changes in cytoskeletal tension.
The structure of FAK consists of multiple domains, including the N-terminal 4.1, ezrin, radixin, moesin homology domain FERM, a central catalytic tyrosine kinase domain important for its activity, and a C-terminal region containing a focal-adhesion targeting (FAT) domain and a proline-rich region [27] (Figure 1). The FERM domain is composed of three lobed structures (lobes F1, F2, F3) arranged in a clover leaf-shaped assembly, and contains a nuclear export sequence (NES) in the lobe F1 and a nuclear localization sequence (NLS) in the lobe F2 [28]. The central kinase domain adopts a typical two-lobed fold. This region contains the activation loop, which extends over 21 residues (564–585) and which is unphosphorylated and highly flexible in the inactive state. It includes two tyrosine residues in the activation loop, Y576 and Y577, which are phosphorylated by the Src family kinases upon activation by cell surface integrins, regulating the kinase activity of FAK. Lastly, the C-terminal domain contains proline-rich regions that consist of two polyproline (PxxP) motifs interacting with the Src homology (SH) 3 domain of several proteins. The extreme C-terminus contains a four-helix bundle that comprises the FAT domain, which interacts with other focal adhesion proteins and is responsible for targeting FAK into the focal adhesion complex [29].
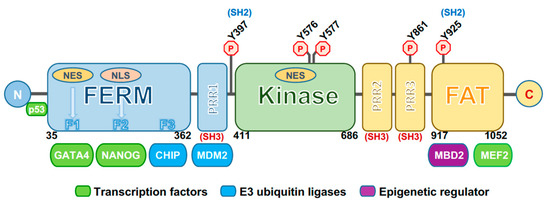
Figure 1. Schematic representation of focal adhesion kinase (FAK). The three main domains of FAK are depicted: The N-terminal 4.1, ezrin, radixin, moesin homology domain (FERM) (in blue), central kinase domain (in green), and focal-adhesion targeting (FAT) domain (in yellow). Domain boundaries are shown. F1, F2, and F3 represent the three lobes of the FERM domain and the regions for binding with transcription factors (such as GATA4 and NANOG) and E3 ubiquitin ligase factors (such as CHIP). Other binding sites are those for MDM2 (E3 ubiquitin ligase), MBD2 (methyl-CpG binding domain protein 2, an epigenetic factor) and MEF2 (transcription factor). Outside the FERM domain the p53-binding site is shown. A nuclear localization sequence (NLS) is located at the F2 FERM lobe, while two nuclear export sequences (NES) are at the F1 FERM lobe and in the kinase domain. PRR1, 2, and 3 represent proline-rich regions (i.e., two polyproline (PxxP) motifs) that interact with the Src homology SH3 domains of several proteins, including Src. Several phosphorylation sites are depicted, among which include Y397, the autophosphorylation site, and Y925, both binding sites for Src. N: N-terminus. C: C-terminus.
The activation of FAK is under the control of different phosphorylation events. An important autoregulatory mechanism occurs through an interaction between the FERM and kinase domains to maintain an autoinhibited state. A main interaction is formed between the FERM F2 lobe and the kinase C-lobe. In addition, an indirect contact is formed in autoinhibited FAK between the FERM-F1 lobe and the kinase N-lobe. This connection includes the Y397 autophosphorylation site, important for the activation of the protein [29]. FERM has a prominent role in controlling FAK activation as demonstrated by the evidence that the deletion of the first 375 residues of the FERM domain results in constitutive activation of FAK [30]. Moreover, in response to different stimuli, such as receptor tyrosine kinases, intracellular pH changes, integrin recruitment to ECM, G-protein-coupled receptors and cytokine receptors, FAK autoinhibition is removed, thus triggering FAK autophosphorylation at the Y397 site. This process is necessary for FAK activation and recruitment at focal adhesions [31].
Once Y397 autophosphorylation occurs, this site is converted into a high affinity-binding site for proteins containing SH2 and SH3 domains, among which is the Src kinase. In this way, Src interacts via its SH2 domain with pY397 and via the SH3 domain with a PxxP motif located between the FERM and the kinase domain. These interactions contribute to Src-dependent activation, triggering phosphorylation of several FAK tyrosines, including Y576 and Y577 in the activation loop of the kinase domain, which induces full catalytic activity of the protein [29]. Furthermore, an intramolecular interaction has been demonstrated between the FERM domain and the C-terminal FAT domain. This FAT/FERM interaction could promote the recruitment of FAK into focal adhesions, triggering protein dimerization [21]. Once activated, FAK is crucial for the regulation of assembly and disassembly of focal adhesion complexes formed by different types of molecules that perceive the mechanical and biochemical signals from the ECM and transmit them inside the cell, by activating different signal transduction pathways. In fact, FAK has long been known as a regulator of cell migration, but also coordinates several cellular processes including polarization, survival, and proliferation via its kinase activity [31]. FAK activation through ADRB2-dependent activation of Src has been involved in the reduction of anoikis levels in ovarian cancer cells under adrenergic modulation [32]. In liver cancer, the Zinc finger protein 32 (ZNF32) enhances the phosphorylation and activation of Src/FAK signaling, contributing to anoikis resistance [33].
Otherwise, FAK plays its roles via non-canonical kinase-independent scaffold functions. Lim et al. found that FAK was also functional into the nucleus [34]. Indeed, FAK can shuttle between the cytoplasm and the nucleus via NLS in response to stress signals or detachment of cells from the ECM [35]. Nuclear FAK establishes a direct interaction with p53 and Mdm2, thus enhancing Mdm2-dependent p53 ubiquitination, leading to the degradation of p53 through the ubiquitination pathway and inhibiting apoptosis, thus promoting cell survival [34][36] (Figure 1). Nuclear FAK can also synergize with various E3 ligases to promote the turnover of several transcription factors, thus controlling different networks involved in the inflammatory signaling pathway, in the immune escape and in angiogenesis. In this way, FAK influences multiple cell functions, and FAK signaling activation is a hallmark of tumor cells, forming a nodal interconnection among pathways crucial for cancer progression (Figure 2) (reviewed in [37]).
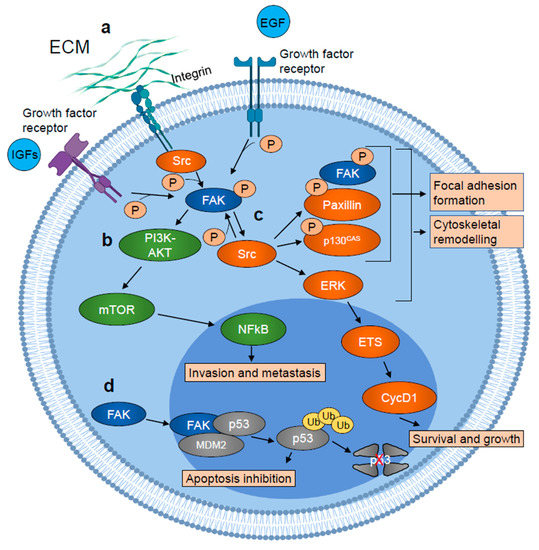
Figure 2. Schematic representation of FAK involvement in tumor growth and metastasis. (a) FAK is autophosphorylated in response to growth factor receptors and integrins activation and activated by Src. (b) Active FAK promotes tumor cell invasion and metastasis, activating PI3K-AKT-mTOR signaling cascade, which results in increased NFkB transcriptional activity. (c) Active FAK also stimulates cytoskeletal remodeling and focal adhesion formation/turnover, inducing SRC-dependent phosphorylation of paxillin and p130cas, leading to the formation of a focal adhesion complex which includes phosphorylated/active FAK, paxillin, and p130cas. SRC also stimulates ERK signaling cascade which results in the ETS transcription factor-dependent induction of cyclin D1 (CycD1) expression which in turn promotes tumor cell survival and growth. (d) Nuclear FAK acts as a scaffold protein for the p53–MDM2 interaction, inducing p53 ubiquitination and its proteasomal degradation which results in apoptosis inhibition. Figure realized with BioRender.com.
However, the molecular mechanisms are still unclear and further studies on the roles of nuclear FAK are needed [36].
3. FAK in Skeletal Muscle
During muscle development, FAK is involved in several processes including differentiation and migration. Crosstalk between myoblasts and ECM, in particular laminin, regulates myogenic processes by stabilizing multinucleated structures that will form muscle fibers (reviewed in [38]. Forced overexpression of a constitutively active membrane-bound FAK in quail myoblasts prompted them to proliferate faster, while overexpression of an inactive FAK mutated in the activation site resulted in differentiation and myotube fusion [39].
This result has been one of the first experimental evidences of an involvement of FAK in myogenesis. A schematic representation of FAK involvement in myogenesis is reported in Figure 3. Interestingly, the activation of FAK under muscle differentiation cues in vitro appears to be biphasic, with a decrease in phosphorylation during the early phases, after the switch of myoblasts from the proliferating to differentiation medium, followed by a gradual increase in cells maintained in differentiation medium for 6 days [40]. This phenomenon is mirrored by treatment with insulin, which stimulates FAK phosphorylation in proliferating myoblasts, followed by a rapid decrease in differentiated cells and again an increase to return to the baseline FAK phosphorylation levels after a half-hour [41]. Moreover, when differentiating myoblasts are transfected with a wild-type FAK, the reduction of the pro-proliferative cyclin D1 is inhibited together with increase of MYOG and muscle creatin kinase levels [40].
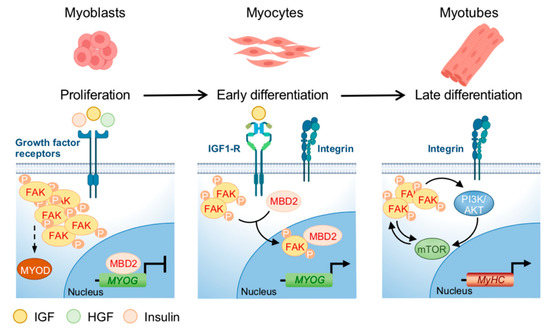
Figure 3. FAK regulation during myoblasts differentiation. The expression of FAK and its phosphorylation are related to specific points in the differentiation of myoblasts into myotubes. FAK is activated by growth factor receptors and regulates the development of myoblasts and the formation of muscle fibers. During the proliferation phase, the activation of growth factor receptors leads to FAK phosphorylation/activation, needed for MYOD expression, while the expression of MYOG is blocked by the binding of MBD2 to its promoter. In the early differentiation phase, phosphorylated-FAK fraction decreases and FAK cytoplasmic interaction with MBD2 promotes the translocation of the FAK/MBD2 complex into the nucleus, where MBD2 interaction with the MYOG promoter is prevented, leading to MYOG expression. During myotubes formation, FAK levels increase, even if not at the same level as in the proliferative phase. In this terminal phase, characterized by MyHC expression, integrins activation induces multiple pathways such as PI3K/AKT/mTOR which crosstalk with FAK to control protein synthesis and the size of the muscle fibers. Figure realized with BioRender.com.
Notably, FAK activation is needed for MyoD expression in murine myoblasts and, in addition, FAK cytoplasmic interaction with the methylating protein MBD2 promotes its shuttling to the nucleus and decreases the interactions of MBD2 with the myogenin promoter, thus inducing the expression of the gene (reviewed in [38]). Therefore, FAK activity seems to be strictly related to myogenesis, being downregulated in the early phases to impede proliferation and then present during differentiation, when presumably the function of integrins in regulating myoblasts fusion is mandatory. In agreement with a role in the homeostasis of muscle fibers, FAK is more expressed in myotubes than in myoblasts and involved in muscle regeneration (reviewed in [38]). A crosstalk between FAK and PI3K/AKT/mTOR in muscle cells has been suggested by data showing that IGF1 induces phosphorylation of both FAK and tuberous sclerosis complex 2 (TSC2), which is a negative regulator of mTOR and a target of FAK [42]. This phenomenon leads to the activation of mTOR, which controls protein synthesis and the cell size of the muscle fibers. In agreement, silencing of FAK reduces TSC2 phosphorylation, which is associated with decreased phosphorylation of the mTOR target genes S6K1 kinase and 4E-BP1 [43]. A direct interaction between FAK and PI3K, after binding of FAK to the SH2 domain of the 85 kDa PI3K, increases PI3K activity [44]. Finally, in murine myoblasts, silencing of PTEN or SHP2, two negative regulators of PI3K, results in FAK activity enhancement [45].
Altogether, these data suggest that FAK plays fundamental roles in skeletal muscle participating, in addition to myogenesis/regeneration, in the control of energy metabolism also via PI3K signaling.
4. FAK in Rhabdomyosarcoma
The levels of FAK expression cannot be used as a prognostic indicator in RMS since FAK and Y379 phosphorylated FAK are both also expressed in normal skeletal muscle. The expression of FAK in RMS has been evaluated in a recent study showing that, in a small cohort of pediatric RMSs including both alveolar and embryonal histotypes, FAK was expressed and phosphorylated with no difference between the two subtypes [46]. Functionally, a first report has involved FAK in RMS cell motility using a model of inhibition of mTOR signaling (Figure 4) [47]. Of the two mTOR kinase complexes, mTORC1 and mTORC2, both including mTOR but associated with different interactors, only the former phosphorylates the S6K1 kinase and 4E-BP1 to regulate protein biosynthesis, controlling cell growth and size and is sensitive to the macrolide antibiotic rapamycin [48][49]. However, the two complexes are closely related, and they can regulate each other. Then, rapamycin can inhibit mTORC2 too in certain conditions (reviewed in [50]). mTORC1 is downstream to the PI3K pathway and is activated by growth factors (reviewed in [50]). Dysregulated mTOR signaling is a hallmark of many tumors and rapamycin analogs are widely used in clinics and have been investigated in clinical trials (www.clinicaltrials.org) on several adult and pediatric tumor types including RMS ([51] and reviewed in [50]). Activation of mTOR has been reported in RMS [52] and mTORC1 inhibitors have entered clinical trials even if they did not show any significant effect on RMS patients’ prognosis [53]. Liu et al. [47] studied the mechanism of cell motility inhibition of rapamycin on the PAX3-FOXO1 RMS cell line RH30 upon treatment with IGF1. Treatment with the growth factor quickly induced the reorganization of cytoskeleton with F-actin fibers condensed at the leading edge of cells and lamellipodia formation [47]. This phenomenon was inhibited by rapamycin [47]. mTOR was directly involved in the effects of IGF1 as demonstrated by the inability of rapamycin to inhibit cytoskeletal reorganization in the presence of a rapamycin-resistant mutant mTOR (mTORrr). In agreement, genetic silencing of mTOR or the mTOR-associated protein raptor mirrored the rapamycin-dependent effects in IGF1-treated cells. While the levels of focal adhesion components FAK, paxillin, and p130Cas were unmodified by IGF1, the growth factor caused robust phosphorylation of the three proteins, which was prevented by pretreatment with rapamycin which was ineffective in cells expressing mTORrr [47]. IGF1-dependent phosphorylation of FAK and the other focal adhesion proteins was inhibited by the silencing of both raptor and rictor, the latter an mTORC2 component, while rapamycin affected only mTORC1 signaling, suggesting that both complexes participate in this process. Moreover, the effects of rapamycin were abrogated by overexpression of S6K1, the constitutively active effector of mTORC1, and mimicked by S6K1 depletion [47]. Considering that RMS is characterized by high invasiveness and RMS cells exhibit elevated migration properties, these results unveiled the potential of an FAK blockade via pharmacologic mTORC1 inhibition in RMS.
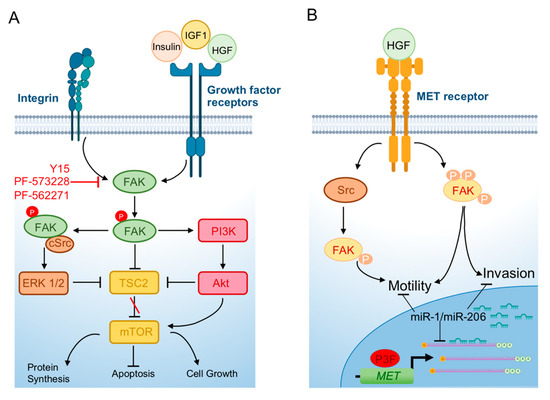
Figure 4. FAK involvement in rhabdomyosarcoma. (A) FAK is phosphorylated and activated upon binding of growth factors to the RTK receptors and integrins activation. Phosphorylated FAK promotes cell growth and protein synthesis and blocks apoptosis through activation of PI3K-AKT-mTOR signaling. The FAK–Src complex (i) induces the ERK1/2 pathway, leading to the reduction of phosphorylated TSC2 which results in increased mTOR activity; and (ii) blocks TSC2 directly. (B) In rhabdomyosarcoma, MET, which is a transcriptional target of PAX3-FOXO1 (P3F), is overexpressed and induces signal cascades, promoting motility and invasion also through FAK activation. In these tumor cells, the “MyomiRs” miR-1/miR-206 are downregulated and when forcedly expressed, they target MET mRNA and block its translation, resulting in a block of invasion and motility. Figure realized with BioRender.com.
Cell motility can be regulated by FAK as a downstream effector of the MET oncogene [54], which is a regulator of the invasive abilities of cancer cells and overexpressed in both RMS subtypes, in addition to be induced by PAX3-FOXO1 [55][56]. MET is a target of the muscle-specific microRNAs miR-1/miR-206 that drive skeletal muscle differentiation in muscle thus called “MyomiRs” (reviewed in [57]) (Figure 4). In RMS, both these microRNAs are highly downregulated and when forcedly reintroduced in tumor cells are able to halt cell proliferation and migration causing myogenic tumor cell differentiation in vitro and in vivo partly by decreasing MET [2][58]. Yan et al. showed that FAK activation/phosphorylation was impaired after miR-1 and miR-206 overexpression in the FN-RMS cell line, RD, in parallel with MET downregulation and the blockade of cell migration and proliferation [58]. This report suggested an interconnection between the functions of a known oncogene in RMS and FAK activation.
A subsequent study directly involved FAK in cell survival, invasion, and migration of RMS cells, regardless their fusion gene status [46]. As a matter of fact, FAK depletion via siRNAs in FN-RMS RD cells and in PAX3-FOXO1 RH30 cells significantly reduced both cell viability and proliferation. Moreover, the FAK inhibitor PF-573228 [59], which competitively targets the ATP-binding pocket of FAK, blocking autophosphorylation at the Y397 site, also decreased cell survival and cell proliferation, promoting apoptosis via the PARP cleavage and Caspase-3 activation in both cell lines. Further, significant reduction of cell invasion through a Matrigel® layer and cell migration was detected after PF-573228 treatment at different concentrations. The effects of FAK inhibition in vivo were evaluated using Y15, a small molecule that inhibits FAK by decreasing its protein levels [60]. Y15 treatment of RD and RH30 cell lines mirrored in vitro the results obtained with PF-573228, inhibiting cell proliferation, viability, migration, and invasion [46]. When treated with Y15, tumor xenografts, obtained by injecting RD and RH30 cells into immunocompromised mice, grew more slowly and exhibited a smaller mass and lower percentage of the proliferative marker Ki67 compared to those treated with the vehicle [46]. This work suggested for the first time that FAK inhibition could have an impact on RMS tumorigenic features in vivo, paving the way to preclinical investigation on inhibition of this kinase in this soft tissue tumor.
The Y397 phosphorylated FAK was detected in the FN-RMS cell line RD18, a subclone of RD cells [61], by mass spectrometry (MS)-based phosphoproteomics [62]. Recently, Narendran et al. showed that in FN-RMS RD cells, FAK autophosphorylation at Y379 appeared higher, while FAK Y576/577 phosphorylation levels at the activation loop were lower than those in the hTERT-immortalized normal primary fibroblasts [63]. Conversely, no difference in the levels of expression was found in the phosphorylation of Y925 FAK between the two cell types [63]. Treatment with the ATP-competitive, reversible inhibitor of FAK, PF-562271, markedly reduced Y397 phosphorylation of FAK in RD cells and suppressed the co-localization of FAK Y397 phosphorylation with F-actin stress fibers and the cell edges, indicative of loss of the motile cell phenotype [63]. FAK inhibition also determined a significant cell cycle arrest in the G1 phase of tumor cells compared to the vehicle. Then, the authors explored the migratory phenotype of RD cells, showing that PF-562271 treatment prevented cell migration, as reported by Waters et al. [46] for PF-573228. These findings directly linked FAK activation with cell motility and F-actin fibers’ organization in FN-RMS cells [63].
Interestingly, the alkylating agent Temozolomide has been shown to impair RH30 FP-RMS cells’ survival and, at the same time, to activate autophagy, the inhibition of which synergizes with the drug to induce tumor cell death [64]. Through autophagy, tumor cells can enzymatically degrade and recycle cytosolic components under stressful conditions such as the extracellular matrix (ECM) detachment and starvation [65][66]. Autophagy is a mechanism through which anoikis can be inhibited and, thus, involved in anoikis resistance in solid tumors [67]. FAK being involved in the inhibition of autophagy by phosphorylating Beclin-1, a component of the complex promoting autophagosome biogenesis [68], and by activating mTOR [31], the involvement of FAK in the response to Temozolomide in FP-RMS cells deserves further investigations.
Recently, Fanzani et al. demonstrated that caveolin-1, when overexpressed in FN-RMS cells in vitro, promotes migration and invasion of tumor cells, a phenomenon blocked by ERK inhibition [69]. Caveolin-1 is one of the major Src kinase substrates that localizes to the caveolae and to focal adhesions sites to drive directional motility and, when phosphorylated/activated by Src, stabilizes FAK, stimulating cell motility in prostate cancer cells [70]. Moreover, caveolin-1 is a target of mTORC2 that, through caveolin-1 phosphorylation, regulates the formation of caveolae [71]. Since mTORC2 cooperates with FAK in mesenchymal stem cells [72] and caveolin-1 stabilizes FAK [70], these pathways could also be interconnected in RMS, where all these signalings are deregulated and warrant future investigations.
References
- Sultan, I.; Qaddoumi, I.; Yaser, S.; Rodriguez-Galindo, C.; Ferrari, A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: An analysis of 2,600 patients. J. Clin. Oncol. 2009, 20, 3391–3397.
- Taulli, R.; Bersani, F.; Foglizzo, V.; Linari, A.; Vigna, E.; Ladanyi, M.; Tuschl, T.; Ponzetto, C. The muscle-specific microRNA miR-206 blocks human rhabdomyosarcoma growth in xenotransplanted mice by promoting myogenic differentiation. J. Clin. Investig. 2009, 119, 2366–2378.
- Raimondi, L.; Ciarapica, R.; De Salvo, M.; Verginelli, F.; Gueguen, M.; Martini, C.; De Sio, L.; Cortese, G.; Locatelli, M.; Dang, T.P.; et al. Inhibition of Notch3 signalling induces rhabdomyosarcoma cell differentiation promoting p38 phosphorylation and p21 Cip1 expression and hampers tumour cell growth in vitro and in vivo. Cell Death Differ. 2012, 19, 871–881.
- Barr, F.G.; Nauta, L.E.; Davis, R.J.; Schäfer, B.W.; Nycum, L.M.; Biegel, J.A. In vivo amplification of the PAX3-FKHR and PAX7-FKHR fusion genes in alveolar rhabdomyosarcoma. Hum. Mol. Genet. 1996, 5, 15–21.
- Gryder, B.E.; Yohe, M.E.; Chou, H.C.; Zhang, X.; Marques, J.; Wachtel, M.; Schaefer, B.; Sen, N.; Song, Y.; Gualtieri, A.; et al. PAX3-FOXO1 establishes myogenic super enhancers and confers BET bromodomain vulnerability. Cancer Discov. 2017, 7, 884–899.
- Bushweller, J.H. Targeting transcription factors in cancer—From undruggable to reality. Nat. Rev. Cancer 2019, 19, 611–624.
- Shern, J.F.; Chen, L.; Chmielecki, J.; Wei, J.S.; Patidar, R.; Rosenberg, M.; Ambrogio, L.; Auclair, D.; Wang, J.; Song, Y.K.; et al. Comprehensive Genomic Analysis of Rhabdomyosarcoma Reveals a Landscape of Alterations Affecting a Common Genetic Axis in Fusion-Positive and Fusion-Negative Tumors. Cancer Discov. 2014, 4, 216–231.
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Prim. 2019, 5, 1–19.
- Williamson, D.; Missiaglia, E.; De Reyniès, A.; Pierron, G.; Thuille, B.; Palenzuela, G.; Thway, K.; Orbach, D.; Laé, M.; Fréneaux, P.; et al. Fusion gene-negative alveolar rhabdomyosarcoma is clinically and molecularly indistinguishable from embryonal rhabdomyosarcoma. J. Clin. Oncol. 2010, 28, 2151–2158.
- Missiaglia, E.; Williamson, D.; Chisholm, J.; Wirapati, P.; Pierron, G.; Petel, F.; Concordet, J.-P.; Thway, K.; Oberlin, O.; Pritchard-Jones, K.; et al. PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. J. Clin. Oncol. 2012, 30, 1670–1677.
- Stevens, M.C.G.; Rey, A.; Bouvet, N.; Ellershaw, C.; Flamant, F.; Habrand, J.L.; Marsden, H.B.; Martelli, H.; De Toledo, J.S.; Spicer, R.D.; et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: Third study of the International Society of Paediatric Oncology-SIOP malignant mesenchymal tumor 89. J. Clin. Oncol. 2005, 23, 2618–2628.
- Oberlin, O.; Rey, A.; Sanchez De Toledo, J.; Martelli, H.; Jenney, M.E.M.; Scopinaro, M.; Bergeron, C.; Merks, J.H.M.; Bouvet, N.; Ellershaw, C.; et al. Randomized comparison of intensified six-drug versus standard three-drug chemotherapy for high-risk nonmetastatic rhabdomyosarcoma and other chemotherapy-sensitive childhood soft tissue sarcomas: Long-term results from the International Society of Pediatric Oncology MMT95 study. J. Clin. Oncol. 2012, 30, 2457–2465.
- Sultan, I.; Ferrari, A. Selecting multimodal therapy for rhabdomyosarcoma. Expert Rev. Anticancer Ther. 2010, 10, 1285–1301.
- Hawkins, D.S.; Gupta, A.A.; Rudzinski, E.R. What is new in the biology and treatment of pediatric rhabdomyosarcoma? Curr. Opin. Pediatr. 2014, 26, 50–56.
- Bisogno, G.; Jenney, M.; Bergeron, C.; Gallego Melcón, S.; Ferrari, A.; Oberlin, O.; Carli, M.; Stevens, M.; Kelsey, A.; De Paoli, A.; et al. Addition of dose-intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): A multicentre, open-label, randomised controlled, phase 3 trial. Lancet Oncol. 2018, 19, 1061–1071.
- Bisogno, G.; De Salvo, G.L.; Bergeron, C.; Gallego Melcón, S.; Merks, J.H.; Kelsey, A.; Martelli, H.; Minard-Colin, V.; Orbach, D.; Glosli, H.; et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 1566–1575.
- Ramadan, F.; Fahs, A.; Ghayad, S.E.; Saab, R. Signaling pathways in Rhabdomyosarcoma invasion and metastasis. Cancer Metastasis Rev. 2020, 39, 287–301.
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890.
- Monje, M. Open questions: Why are babies rarely born with cancer? BMC Biol. 2018, 16, 129.
- Schöffski, P.; Wozniak, A.; Leahy, M.G.; Aamdal, S.; Rutkowski, P.; Bauer, S.; Richter, S.; Grünwald, V.; Debiec-Rychter, M.; Sciot, R.; et al. The tyrosine kinase inhibitor crizotinib does not have clinically meaningful activity in heavily pre-treated patients with advanced alveolar rhabdomyosarcoma with FOXO rearrangement: European Organisation for Research and Treatment of Cancer phase 2 trial 90101 ‘CREATE’. Eur. J. Cancer 2018, 94, 156–167.
- Brami-Cherrier, K.; Gervasi, N.; Arsenieva, D.; Walkiewicz, K.; Boutterin, M.C.; Ortega, A.; Leonard, P.G.; Seantier, B.; Gasmi, L.; Bouceba, T.; et al. FAK dimerization controls its kinase-dependent functions at focal adhesions. EMBO J. 2014, 33, 356–370.
- Bouchard, V.; Demers, M.J.; Thibodeau, S.; Laquerre, V.; Fujita, N.; Tsuruo, T.; Beaulieu, J.F.; Gauthier, R.; Vézina, A.; Villeneuve, L.; et al. Fak/Src signaling in human intestinal epithelial cell survival and anoikis: Differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways. J. Cell. Physiol. 2007, 212, 717–728.
- Horowitz, J.C.; Rogers, D.S.; Sharma, V.; Vittal, R.; White, E.S.; Cui, Z.; Thannickal, V.J. Combinatorial activation of FAK and AKT by transforming growth factor-β1 confers an anoikis-resistant phenotype to myofibroblasts. Cell. Signal. 2007, 19, 761–771.
- Grassian, A.R.; Coloff, J.L.; Brugge, J.S. Extracellular matrix regulation of metabolism and implications for tumorigenesis. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 313–324.
- Nana, F.A.; Vanderputten, M.; Ocak, S. Role of focal adhesion kinase in small-cell lung cancer and its potential as a therapeutic target. Cancers 2019, 11, 1683.
- Zhang, Q.; Wang, H.; Wei, H.; Zhang, D. Focal adhesion kinase (FAK) is associated with poor prognosis in urinary bladder carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 831–838.
- Schaller, M.D. Cellular functions of FAK kinases: Insight into molecular mechanisms and novel functions. J. Cell Sci. 2010, 123, 1007–1013.
- Ceccarelli, D.F.J.; Hyun, K.S.; Poy, F.; Schaller, M.D.; Eck, M.J. Crystal structure of the FERM domain of focal adhesion kinase. J. Biol. Chem. 2006, 281, 252–259.
- Martínez, P.T.; Navajas, P.L.; Lietha, D. FAK structure and regulation by membrane interactions and force in focal adhesions. Biomolecules 2020, 10, 179.
- Cooper, L.A.; Shen, T.-L.; Guan, J.-L. Regulation of Focal Adhesion Kinase by Its Amino-Terminal Domain through an Autoinhibitory Interaction. Mol. Cell. Biol. 2003.
- Liu, G.; Guibao, C.D.; Zheng, J. Structural Insight into the Mechanisms of Targeting and Signaling of Focal Adhesion Kinase. Mol. Cell. Biol. 2002, 22, 2751–2760.
- Sood, A.K.; Armaiz-Pena, G.N.; Halder, J.; Nick, A.M.; Stone, R.L.; Hu, W.; Carroll, A.R.; Spannuth, W.A.; Deavers, M.T.; Allen, J.K.; et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J. Clin. Investig. 2010, 120, 1515–1523.
- Li, K.; Zhao, G.; Ao, J.; Gong, D.; Zhang, J.; Chen, Y.; Li, J.; Huang, L.; Xiang, R.; Hu, J.; et al. ZNF32 induces anoikis resistance through maintaining redox homeostasis and activating Src/FAK signaling in hepatocellular carcinoma. Cancer Lett. 2019, 442, 271–278.
- Lim, S.T.; Chen, X.L.; Lim, Y.; Hanson, D.A.; Vo, T.T.; Howerton, K.; Larocque, N.; Fisher, S.J.; Schlaepfer, D.D.; Ilic, D. Nuclear FAK Promotes Cell Proliferation and Survival through FERM-Enhanced p53 Degradation. Mol. Cell 2008, 29, 9–22.
- Golubovskaya, V.M.; Finch, R.; Cance, W.G. Direct interaction of the N-terminal domain of focal adhesion kinase with the N-terminal transactivation domain of p53. J. Biol. Chem. 2005.
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on Cancer: A focused review. J. Exp. Clin. Cancer Res. 2019, 38, 1–11.
- Mousson, A.; Sick, E.; Carl, P.; Dujardin, D.; De Mey, J.; Rondé, P. Targeting focal adhesion kinase using inhibitors of protein-protein interactions. Cancers 2018, 10, 278.
- Graham, Z.A.; Gallagher, P.M.; Cardozo, C.P. Focal adhesion kinase and its role in skeletal muscle. J. Muscle Res. Cell Motil. 2015, 36, 305–315.
- Sastry, S.K.; Lakonishok, M.; Wu, S.; Truong, T.Q.; Huttenlocher, A.; Turner, C.E.; Horwitz, A.F. Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J. Cell Biol. 1999, 144, 1295–1309.
- Clemente, C.F.M.Z.; Corat, M.A.F.; Saad, S.T.O.; Franchini, K.G. Differentiation of C2C12 myoblasts is critically regulated by FAK signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005.
- Goel, H.L.; Dey, C.S. Focal adhesion kinase tyrosine phosphorylation is associated with myogenesis and modulated by insulin. Cell Prolif. 2002, 35, 131–142.
- Gan, B.; Yoo, Y.; Guan, J.L. Association of focal adhesion kinase with tuberous sclerosis complex 2 in the regulation of S6 kinase activation and cell growth. J. Biol. Chem. 2006, 281, 37321–37329.
- Crossland, H.; Kazi, A.A.; Lang, C.H.; Timmons, J.A.; Pierre, P.; Wilkinson, D.J.; Smith, K.; Szewczyk, N.J.; Atherton, P.J. Focal adhesion kinase is required for IGF-I-mediated growth of skeletal muscle cells via a TSC2/mTOR/S6K1-associated pathway. Am. J. Physiol. Endocrinol. Metab. 2013, 305.
- Chen, H.C.; Appeddu, P.A.; Isoda, H.; Guan, J.L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 1996, 271, 26329–26334.
- Gupta, A.; Dey, C.S. PTEN and SHIP2 regulates PI3K/Akt pathway through focal adhesion kinase. Mol. Cell. Endocrinol. 2009, 309, 55–62.
- Waters, A.M.; Stafman, L.L.; Garner, E.F.; Mruthyunjayappa, S.; Stewart, J.E.; Mroczek-Musulman, E.; Beierle, E.A. Targeting focal adhesion kinase suppresses the malignant phenotype in rhabdomyosarcoma cells. Transl. Oncol. 2016, 9, 263–273.
- Liu, L.; Chen, L.; Chung, J.; Huang, S. Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene 2008, 27, 4998–5010.
- Guertin, D.A.; Sabatini, D.M. Defining the Role of mTOR in Cancer. Cancer Cell 2007, 12, 9–22.
- Loewith, R.; Jacinto, E.; Wullschleger, S.; Lorberg, A.; Crespo, J.L.; Bonenfant, D.; Oppliger, W.; Jenoe, P.; Hall, M.N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 2002, 10, 457–468.
- Chen, Y.; Zhou, X. Research progress of mTOR inhibitors. Eur. J. Med. Chem. 2020, 208, 112820.
- Amin, H.M.; Morani, A.C.; Daw, N.C.; Lamhamedi-Cherradi, S.E.; Subbiah, V.; Menegaz, B.A.; Vishwamitra, D.; Eskandari, G.; George, B.; Benjamin, R.S.; et al. IGF-1R/mTOR targeted therapy for ewing sarcoma: A meta-analysis of five IGF-1R-related trials matched to proteomic and radiologic predictive biomarkers. Cancers 2020, 12, 1768.
- McKinnon, T.; Venier, R.; Yohe, M.; Sindiri, S.; Gryder, B.E.; Shern, J.F.; Kabaroff, L.; Dickson, B.; Schleicher, K.; Chouinard-Pelletier, G.; et al. Functional screening of FGFR4-driven tumorigenesis identifies PI3K/mTOR inhibition as a therapeutic strategy in rhabdomyosarcoma. Oncogene 2018, 37, 2630–2644.
- Geoerger, B.; Kieran, M.W.; Grupp, S.; Perek, D.; Clancy, J.; Krygowski, M.; Ananthakrishnan, R.; Boni, J.P.; Berkenblit, A.; Spunt, S.L. Phase II trial of temsirolimus in children with high-grade glioma, neuroblastoma and rhabdomyosarcoma. Eur. J. Cancer 2012, 48, 253–262.
- Chen, S.-Y.; Chen, H.-C. Direct Interaction of Focal Adhesion Kinase (FAK) with Met Is Required for FAK To Promote Hepatocyte Growth Factor-Induced Cell Invasion. Mol. Cell. Biol. 2006, 11, e1004593.
- Ferracini, R.; Olivero, M.; Di Renzo, M.F.; Martano, M.; De Giovanni, C.; Nanni, P.; Basso, G.; Scotlandi, K.; Lollini, P.L.; Comoglio, P.M. Retrogenic expression of the MET proto-oncogene correlates with the invasive phenotype of human rhabdomyosarcomas. Oncogene 1996, 12, 1697–1705.
- Ginsberg, J.P.; Davis, R.J.; Bennicelli, J.L.; Nauta, L.E.; Barr, F.G. Up-regulation of MET but not neural cell adhesion molecule expression by the PAX3-FKHR fusion protein in alveolar rhabdomyosarcoma. Cancer Res. 1998, 58, 3542–3546.
- Rota, R.; Ciarapica, R.; Giordano, A.; Miele, L.; Locatelli, F. MicroRNAs in rhabdomyosarcoma: Pathogenetic implications and translational potentiality. Mol. Cancer 2011, 10, 1–14.
- Yan, D.; Dong, X.D.; Chen, X.; Wang, L.; Lu, C.; Wang, J.; Qu, J.; Tu, L. MicroRNA-1/206 targets c-met and inhibits rhabdomyosarcoma development. J. Biol. Chem. 2009, 284, 29596–29604.
- Slack-Davis, J.K.; Martin, K.H.; Tilghman, R.W.; Iwanicki, M.; Ung, E.J.; Autry, C.; Luzzio, M.J.; Cooper, B.; Kath, J.C.; Roberts, W.G.; et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 2007, 282, 148145–148152.
- Hochwald, S.N.; Nyberg, C.; Zheng, M.; Zheng, D.; Wood, C.; Massoll, N.A.; Magis, A.; Ostrov, D.; Cance, W.G.; Golubovskaya, V.M. A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle 2009, 8, 2435–2443.
- Lollini, P.L.; De Giovanni, C.; Landuzzi, L.; Nicoletti, G.; Scotlandi, K.; Nanni, P. Reduced metastatic ability of in vitro differentiated human rhabdomyosarcoma cells. Invasion Metastasis 1991, 11, 116–124.
- Bai, Y.; Li, J.; Fang, B.; Edwards, A.; Zhang, G.; Bui, M.; Eschrich, S.; Altiok, S.; Koomen, J.; Haura, E.B. Phosphoproteomics identifies driver tyrosine kinases in sarcoma cell lines and tumors. Cancer Res. 2012, 70, 2501–2511.
- Al-Ghabkari, A.; Qasrawi, D.O.; Alshehri, M.; Narendran, A. Focal adhesion kinase (FAK) phosphorylation is a key regulator of embryonal rhabdomyosarcoma (ERMS) cell viability and migration. J. Cancer Res. Clin. Oncol. 2019, 145, 1461–1469.
- Moghadam, A.R.; da Silva Rosa, S.C.; Samiei, E.; Alizadeh, J.; Field, J.; Kawalec, P.; Thliveris, J.; Akbari, M.; Ghavami, S.; Gordon, J.W. Autophagy modulates temozolomide-induced cell death in alveolar Rhabdomyosarcoma cells. Cell Death Discov. 2018, 4.
- Fung, C.; Lock, R.; Gao, S.; Salas, E.; Debnath, J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol. Biol. Cell 2008, 19, 797–806.
- Shang, L.; Chen, S.; Du, F.; Li, S.; Zhao, L.; Wang, X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc. Natl. Acad. Sci. USA 2011, 108, 4788–4793.
- Debnath, J. Detachment-induced autophagy during anoikis and lumen formation in epithelial acini. Autophagy 2008, 4, 351–353.
- Cheng, Z.; Zhu, Q.; Dee, R.; Opheim, Z.; Mack, C.P.; Cyr, D.M.; Taylor, J.M. Focal adhesion kinase-mediated phosphorylation of Beclin1 protein suppresses cardiomyocyte autophagy and initiates hypertrophic growth. J. Biol. Chem. 2017, 292, 2065–2079.
- Codenotti, S.; Faggi, F.; Ronca, R.; Chiodelli, P.; Grillo, E.; Guescini, M.; Megiorni, F.; Marampon, F.; Fanzani, A. Caveolin-1 enhances metastasis formation in a human model of embryonal rhabdomyosarcoma through Erk signaling cooperation. Cancer Lett. 2019, 449, 135–144.
- Meng, F.; Saxena, S.; Liu, Y.; Joshi, B.; Wong, T.H.; Shankar, J.; Foster, L.J.; Bernatchez, P.; Nabi, I.R. The phospho-caveolin-1 scaffolding domain dampens force fluctuations in focal adhesions and promotes cancer cell migration. Mol. Biol. Cell 2017, 28, 2190–2201.
- Hau, A.M.; Gupta, S.; Leivo, M.Z.; Nakashima, K.; Macias, J.; Zhou, W.; Hodge, A.; Wulfkuhle, J.; Conkright, B.; Bhuvaneshwar, K.; et al. Dynamic Regulation of Caveolin-1 Phosphorylation and Caveolae Formation by Mammalian Target of Rapamycin Complex 2 in Bladder Cancer Cells. Am. J. Pathol. 2019, 189, 1846–1862.
- Thompson, W.R.; Guilluy, C.; Xie, Z.; Sen, B.; Brobst, K.E.; Yen, S.S.; Uzer, G.; Styner, M.; Case, N.; Burridge, K.; et al. Mechanically activated fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem Cells 2013, 31, 2528–2537.




