| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessio Siciliano | + 1172 word(s) | 1172 | 2020-05-08 19:50:59 | | | |
| 2 | Lily Guo | -18 word(s) | 1154 | 2020-10-28 10:52:40 | | | | |
| 3 | Lily Guo | -18 word(s) | 1154 | 2020-10-28 10:53:11 | | |
Video Upload Options
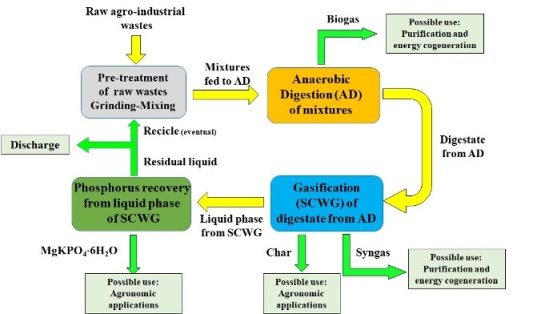
This document reports a synthetic description of a research work in which a new integrated treatment was defined for the production of biofuel and the recovery of phosphorus compounds from agro-industrial residues. As the first step of the proposed process, anaerobic co-digestion was carried out to produce biogas by exploiting raw waste mixtures. Afterwards, the residual digestates were converted to syngas using supercritical wet gasification (SCWG. Finally, the liquid phases from SCWG were treated to recover the phosphorus content as MgKPO4×6H2O crystals. This integrated treatment could be a suitable approach to exploit agro-wastes because it can produce biofuel and valuable chemicals and generates a residual effluent with a very low polluting load
1. Introduction
The identification of novel and suitable approaches for the exploitation of agro-industrial residues is an important environmental and economic issue[1][2][3]. In fact, these wastes are generally not properly disposed of, which causes severe damage to soils and aquatic systems[4][5][6][7]. Therefore, they often constitute a substantial source of pollution. On the other hand, many byproducts of agricultural activities have an enormous potential for energy production and for the recovery of valuable compounds[8][9][10][11]. Anaerobic digestion (AD) is a sustainable, environmentally friendly technology for organic residue exploitation. In anaerobic processes, the organic substrates are metabolized according to a series of sequential steps carried out by different microorganisms species that operate in the absence of dissolved oxygen at two typical temperature ranges, mesophilic (35–40 °C) or thermophilic (55–60 °C)[11]. As a consequence of these biological reactions, a valuable biogas is produced. AD is suitable to efficiently treat seasonal wastes and it can be applied in large facilities and small agricultural companies[12]. Indeed, many batch and continuous digesters with suspended and attached biomass, such as the completely-stirred tank reactor (CSTR), up-flow anaerobic sludge blanket (UASB), up-flow anaerobic filter, etc., have been used[11]. The processes efficiency, in addition to typical operating parameters (OLR: organic load rate, HRT: hydraulic retention time, etc.), is mainly affected by the characteristics of feedstock[11]. The co-digestion of various types of residues is an advantageous method to improve the performance of treatments[11][12][13]. In fact, by properly mixing different matrixes it is possible to obtain a mixture with adequate characteristics in terms of pH, COD/N/P ratio, alkalinity, etc.[13][14][15][16]. Therefore, it is very important to accurately select the type and the amount of waste to be mixed.
Besides biogas, anaerobic processes produce a wet residue (digestate) with a remarkable pollutant load. Digestates are often used as organic fertilizers; however, this practice can cause soil deterioration due to their properties such as the high salinity and the potential presence of pathogenic microorganisms [17]. Moreover, spreading wet AD residues leads to the accumulation of nutrients in aquatic systems, and, thus, the eutrophication of water bodies[17]. Therefore, there is a need to develop appropriate technologies for the post-treatment of wet residues generated in anaerobic processes. In this regard, the supercritical wet gasification (SCWG) and the recovery of nutrients as struvite type compounds (MAP: magnesium ammonium phosphate; MPP: magnesium potassium phosphate) can be profitable approaches. In particular, SCWG exploits the organic matter content of biomass for biofuel production[18][19][20]. The process is performed at high temperature (300–600 °C) and pressure (210–400 bar) under supercritical water conditions[21][22][23]. The SCWG process could be schematized in two main stages: an early stage where there is the breakdown of macromolecules to smaller molecules and a second stage, similar to hydrocarbon steam reforming, composed of a water gas shift reaction and methanation reaction[24][25]. In SCWG, water becomes a real reagent for gasification reaction and it is also able to solubilize complex organic compounds. Therefore, this technology is particularly suitable for the treatment of wet residues such as digestates, which are characterized by remarkable levels of organic matter[26][27][28][29]. SCWG has significant advantages over traditional methods for the production of biofuels[30][31], such as higher energy, greater separation efficiency, and the possibility to eliminate the need to pre-dry the matrices[32][33]. Indeed, as demonstrated by previous works, the SCWG process, compared to the conventional technologies, has a great potential because it is a cost-effective process for the treatment of humid wastes and it is highly recommended for energy production from digestates [32][33]. Another advantage is that the liquid resulting from hydrothermal processes has a low amount of organic matter and it is completely sterilized, avoiding, in the case of digestates, the presence of pathogenic organisms such as bacteria and viruses [26,27]. After the SCWG stage, dimensioning ad hoc the CO2 removal section, it is possible to obtain a syngas containing only the molecules necessary for the synthesis of biofuels and/or chemicals such as synthetic natural gas (SNG), pure hydrogen, methanol, dimethyl ether etc.[22]. The gas production could notably change in response to the concentration and type of organic matter, as well as the water content of feedstock[34].
The residual aqueous phase, depending on the waste type subjected to SCWG, could be characterized by a high level of nutrient compounds. In particular, the amount of phosphate could be much more remarkable than that of nitrogen compounds. Indeed, during the gasification reaction, depending on samples’ pH, the ammonium nitrogen could be converted into gaseous ammonia and recovered by means of neutralization in acid solutions. Instead, the phosphorus content remains both in the solid and liquid phase. Some works investigated the possibility of recovering the phosphorus amount from solid residue[35][36]. However, there is a lack of studies focused on the removal and recovery of P dissolved into the liquid phase of SCWG. In this regard, the precipitation of magnesium potassium phosphate hexahydrate (MPP, MgKPO4×6H2O)[37][38] can be considered a suitable and advantageous option. In fact, this process allows the P recovery in the form of one of the struvite-type compounds that are considered potential fertilizers[39][40][41][42][43][44]. MPP precipitation occurs when Mg2+, K+, and PO43- concentration overcomes the solubility product in an alkaline environment[37][38]. Generally, to promote the MPP formation, the pH correction and the addition of potassium and magnesium reactants is required[37][38], which increases the process costs. Therefore, the reduction of chemical consumption is a significant issue for practical applications[45][46][47][48].
In order to define a suitable method to efficiently exploit the agro-industrial residues, an integrated treatment based on AD, SCWG, and MPP precipitation has been developed (Fig.1). This treatment represents a new approach combining all the above techniques. Through an experimental investigation, the factors affecting the production of biogas, from co-digestion of agro-wastes, and of syngas, from gasification of digestate, were identified. Moreover, the application modality of MPP precipitation for the treatment of the liquid phase of SCWG was determined. In particular, this process, in comparison to conventional applications, guarantees a reduction of chemicals consumption.
The developed integrated treatment is advantageous because, in addition to biofuel production and recovery of a valuable phosphorus compound, it can obtain a residual effluent characterized by a very low amount of organic matter and nutrient compounds.
Figure 1. Integrated treatment
The article has been published on 10.3390/su11010052
References
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier-Coussy, H.; Jang, G.-W.; Verniquet, A.; Broeze, J.; et al. A research challenge vision regarding management of agricultural waste in a circular bio-based economy.Crit. Rev. Environ. Sci. Technol.2018, 1–41, doi:10.1080/10643389.2018.1471957.
- Maurizio Carlini; Enrico Maria Mosconi; Sonia Castellucci; Mauro Villarini; Andrea Colantoni; An Economical Evaluation of Anaerobic Digestion Plants Fed with Organic Agro-Industrial Waste. Energies 2017, 10, 1165, 10.3390/en10081165.
- D. Hernandez; B. Riaño; Mónica Coca; Maria Cruz García González; Treatment of agro-industrial wastewater using microalgae–bacteria consortium combined with anaerobic digestion of the produced biomass. Bioresource Technology 2013, 135, 598-603, 10.1016/j.biortech.2012.09.029.
- Alessio Siciliano; M.A. Stillitano; S. De Rosa; Increase of the anaerobic biodegradability of olive mill wastewaters through a pre-treatment with hydrogen peroxide in alkaline conditions. DESALINATION AND WATER TREATMENT 2014, 55, 1735-1746, 10.1080/19443994.2014.928797.
- Paolo S. Calabrò; Adele Fòlino; Vincenzo Tamburino; Giovanni Zappia; Demetrio Antonio Zema; Increasing the tolerance to polyphenols of the anaerobic digestion of olive wastewater through microbial adaptation. Biosystems Engineering 2018, 172, 19-28, 10.1016/j.biosystemseng.2018.05.010.
- Boubaker Fezzani; Ridha Ben Cheikh; Two-phase anaerobic co-digestion of olive mill wastes in semi-continuous digesters at mesophilic temperature. Bioresource Technology 2010, 101, 1628-1634, 10.1016/j.biortech.2009.09.067.
- Alessio Siciliano; M.A. Stillitano; S. De Rosa; Biogas production from wet olive mill wastes pretreated with hydrogen peroxide in alkaline conditions. Renewable Energy 2016, 85, 903-916, 10.1016/j.renene.2015.07.029.
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review.Energies2013, 6, 4607–4638, doi:10.3390/en6094607.
- Alessio Siciliano; Maria Assuntina Stillitano; Carlo Limonti; Energetic Valorization of Wet Olive Mill Wastes through a Suitable Integrated Treatment: H2O2 with Lime and Anaerobic Digestion. Sustainability 2016, 8, 1150, 10.3390/su8111150.
- Zema, D.A.; Fòlino, A.; Zappia, G.; Calabrò, P.S.; Tamburino, V.; Zimbone, S.M. Anaerobic digestion of orange peel in a semi-continuous pilot plant: An environmentally sound way of citrus waste management in agro-ecosystems.Sci. Total Environ.2018, 630, 401–408, doi:10.1016/j.scitotenv.2018.02.168.
- Khanal, S. Anaerobic Biotechnology for Bioenergy Production: Principles and Applications; Wiley-Blackwell: Ames, IA, USA, 2008.
- Hynek Roubík; Jana Mazancová; Phung Le Dinh; Đinh Văn Dũng; Jan Banout; Biogas Quality across Small-Scale Biogas Plants: A Case of Central Vietnam. Energies 2018, 11, 1794, 10.3390/en11071794.
- Mehariya, S.; Patel, A.K.; Obulisamy, P.K.; Punniyakotti, E.; Wong, J.W.C. Co-digestion of food waste and sewage sludge for methane production: Current status and perspective.Bioresour. Technol.2018,doi:10.1016/j.biortech.2018.04.030.
- Álvarez, J.A.; Otero, L.; Lema, J.M. A methodology for optimising feed composition for anaerobic co-digestion of agro-industrial wastes.Bioresour. Technol.2010, 101, 1153–1158, doi:10.1016/j.biortech.2009.09.061.
- Zahan, Z.; Othman, M.Z.; Muster, T.H. Anaerobic digestion/co-digestion kinetic potentials of different agro-industrial wastes: A comparative batch study for C/N optimisation.Waste Manag.2018, 71, 663–674, doi:10.1016/j.wasman.2017.08.014.
- Keucken, A.; Habagil, M.; Batstone, D.; Jeppsson, U.; Arnell, M. Anaerobic Co-Digestion of Sludge and Organic Food Waste—Performance, Inhibition, and Impact on the Microbial Community.Energies2018,11,2325, doi:10.3390/en11092325.
- Marta Goberna; Sabine Marie Podmirseg; S. Waldhuber; B. A. Knapp; C. Garcia; Heribert Insam; Pathogenic bacteria and mineral N in soils following the land spreading of biogas digestates and fresh manure. Applied Soil Ecology 2011, 49, 18-25, 10.1016/j.apsoil.2011.07.007.
- Codignole Luz, F.; Volpe, M.; Fiori, L.; Manni, A.;Cordiner, S.;Mulone, V.; Rocco, V. Spent coffee enhanced biomethane potential via an integrated hydrothermal carbonization-anaerobic digestion process.Bioresour. Technol.2018, 256, 102–109, doi:10.1016/j.biortech.2018.02.021.
- P. Basu; Vichuda Mettanant; Biomass Gasification in Supercritical Water -- A Review. International Journal of Chemical Reactor Engineering 2009, 7, , 10.2202/1542-6580.1919.
- Nanda, S.; Isen, J.; Dalai, A.K.; Kozinski, J.A. Gasification of fruit wastes and agro-food residues in supercritical water.Energy Convers. Manag.2016, 110, 296–306, doi:10.1016/j.enconman.2015.11.060.
- Amrullah, A.; Matsumura, Y. Supercritical water gasification of sewage sludge in continuous reactor.Bioresour. Technol.2018, 249, 276–283, doi:10.1016/j.biortech.2017.10.002.
- Berta Matas Güell; Judit Sandquist; Lars So̸rum; Gasification of Biomass to Second Generation Biofuels: A Review. ASME 2011 5th International Conference on Energy Sustainability, Parts A, B, and C 2011, null, 1119-1129, 10.1115/es2011-54140.
- Kruse, A. Supercritical water gasification.Biofuels Bioprod. Biorefining2008, 2, 415–437, doi:10.1002/bbb.93.
- Yakaboylu, O.; Harinck, J.; Smit, K.G.; de Jong, W. Supercritical Water Gasification of Biomass: A Literature and Technology Overview.Energies2015, 8, 859–894, doi:10.3390/en8020859.
- Matsumura, Y.; Minowa, T. Fundamental design of a continuous biomass gasification process using a supercritical water fluidized bed.Int. J. Hydrogen Energy2004, 29, 701–707, doi:10.1016/j.ijhydene.2003.09.005.
- Molino, A.; Nanna, F.; Villone, A.; Iovane, P.; Tarquini, P.; Migliori, M.; Giordano, G.; Braccio, G. Pressure and time effect over semi-continuous gasification of zootechnical sludge near critical condition of water for green chemicals production.Fuel2014, 136, 172–176, doi:10.1016/j.fuel.2014.07.024.
- Molino, A.; Migliori, M.; Blasi, A.; Davoli, M.; Marino, T.; Chianese, S.; Catizone, E.; Giordano, G. Municipal waste leachate conversion via catalytic supercritical water gasification process.Fuel2017, 206, 155–161, doi:10.1016/j.fuel.2017.05.091.
- Chianese, S.; Loipersböck, J.; Malits, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Musmarra, D. Hydrogen from the high temperature water gas shift reaction with an industrial Fe/Cr catalyst using biomass gasification tar rich synthesis gas.Fuel Process. Technol.2015, 132, 39–48, doi:10.1016/j.fuproc.2014.12.034.
- Molino, A.; Larocca, V.; Valerio, V.; Martino, M.; Marino, T.;Rimauro, J.; Casella, P. Biofuels and Bio-based Production via Supercritical Water Gasification of Peach Scraps.Energy Fuels2016, 30, 10443–10447, doi:10.1021/acs.energyfuels.6b01743.
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis —A Technological Review.Energies2012, 5, 4952–5001, doi:10.3390/en5124952.
- Panepinto, D.; Fiore, S.; Genon, G.; Acri, M. Thermal valorization of sewer sludge: Perspectives for large wastewater treatment plants, J. Clean. Prod. 2016, 137, 1323–1329, doi:10.1016/j.jclepro.2016.08.014.
- Sabino De Gisi; Girolamo Giordano; M. Migliori; V. Lauro; G. Santarcangelo; T. Marino; V. LaRocca; P. Tarquini; Process Innovation Via Supercritical Water Gasification to Improve the Conventional Plants Performance in Treating Highly Humid Biomass. Waste and Biomass Valorization 2016, 7, 1289-1295, 10.1007/s12649-016-9528-y.
- Edgar Gasafi; Marion-Yvonne Reinecke; Andrea Kruse; Liselotte Schebek; Economic analysis of sewage sludge gasification in supercritical water for hydrogen production. Biomass and Bioenergy 2008, 32, 1085-1096, 10.1016/j.biombioe.2008.02.021.
- Gong, M.; Zhu, W.; Xu, Z.R.; Zhang, H.W.; Yang, H.P. Influence of sludge properties on the direct gasification of dewatered sewage sludge in supercritical water.Renew. Energy2014, 66, 605–611, doi:10.1016/j.renene.2014.01.006.
- Katarzyna Gorazda; Barbara Tarko; Sebastian Werle; Zbigniew Wzorek; Sewage sludge as a fuel and raw material for phosphorus recovery: Combined process of gasification and P extraction.. Waste Management 2017, 73, 404-415, 10.1016/j.wasman.2017.10.032.
- Acelas, N.Y.; López, D.P.; Brilman, D.W.F.; Kersten, S.R.A.; Kootstra, A.M.J. Supercritical water gasification of sewage sludge: Gas production and phosphorus recovery.Bioresour. Technol.2014, 174, 167–175, doi:10.1016/j.biortech.2014.10.003.
- Kangning Xu; Chengwen Wang; Haiyan Liu; Yi Qian; Simultaneous removal of phosphorus and potassium from synthetic urine through the precipitation of magnesium potassium phosphate hexahydrate. Chemosphere 2011, 84, 207-212, 10.1016/j.chemosphere.2011.04.057.
- J.A. Wilsenach; C.A.H. Schuurbiers; Mark Van Loosdrecht; Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Research 2007, 41, 458-466, 10.1016/j.watres.2006.10.014.
- Alessio Siciliano; S. De Rosa; Recovery of ammonia in digestates of calf manure through a struvite precipitation process using unconventional reagents. Environmental Technology 2013, 35, 841-850, 10.1080/09593330.2013.853088.
- L. Karabegovic; M. Uldal; A. Werker; F. Morgan-Sagastume; Phosphorus recovery potential from a waste stream with high organic and nutrient contents via struvite precipitation. Environmental Technology 2013, 34, 871-883, 10.1080/09593330.2012.720718.
- Işık Kabdaşlı; O. Tunay; P. Ozcan; Application of struvite precipitation coupled with biological treatment to slaughterhouse wastewaters. Environmental Technology 2009, 30, 1095-1101, 10.1080/09593330903136856.
- Battistoni, P.; Boccadoro, R.; Fatone, F.; Pavan, P. Auto-Nucleation and Crystal Growth of Struvite in a Demonstrative Fluidized Bed Reactor (FBR). Environ. Technol.2010, 26, 975–982,doi:10.1080/09593332608618486.
- Alessio Siciliano; Assessment of fertilizer potential of the struvite produced from the treatment of methanogenic landfill leachate using low-cost reagents. Environmental Science and Pollution Research 2015, 23, 5949-5959, 10.1007/s11356-015-5846-z.
- Atef Korchef; Hassidou Saidou; Mohamed Ben Amor; Phosphate recovery through struvite precipitation by CO2 removal: Effect of magnesium, phosphate and ammonium concentrations. Journal of Hazardous Materials 2011, 186, 602-613, 10.1016/j.jhazmat.2010.11.045.
- Alessio Siciliano; Maria Assuntina Stillitano; Carlo Limonti; Francesco Marchio; Ammonium Removal from Landfill Leachate by Means of Multiple Recycling of Struvite Residues Obtained through Acid Decomposition. Applied Sciences 2016, 6, 375, 10.3390/app6110375.
- J. Sánchez; M. Acedo; A. Alonso; J. A. Alonso; P. Alvarez; Enrique Ascasibar; Alfonso Baciero; R. Balbin; L. Barrera; Ivan Vargas Blanco; J. Botija; A. De Bustos; E. De La Cal; Ivan Calvo; Alvaro Cappa; J.M. Carmona; D. Carralero; R. Carrasco; B.A. Carreras; Francisco Castejon; Rodrigo Castro; G. Catalan; A.A. Chmyga; M. Chamorro; Leonid Eliseev; Luis Esteban; Teresa Estrada; A. Fernández; R. Fernández-Gavilán; J.A. Ferreira; J.M. Fontdecaba; C. Fuentes; Luis Garcia; Isabel Garcia-Cortes; R. Garcia-Gomez; J.M. García-Regaña; J. Guasp; Luis Guimarais; T. Happel; J. Hernanz; J. Herranz; C. Hidalgo; J.A. Jiménez; A. Jiménez-Denche; R. Jiménez-Gomez; David Jimenez-Rey; I. Kirpitchev; A.D. Komarov; A.S. Kozachok; L. Krupnik; F. Lapayese; M. Liniers; Daniel Lopez-Bruna; A. López-Fraguas; J. López-Rázola; A. López-Sánchez; S. Lysenko; G. Marcon; F. Martin; V. Maurin; K.J. McCarthy; F. Medina; M. Medrano; A.V. Melnikov; P. Mendez; Boudewijn Ph Van Milligen; E. Mirones; Igor Nedzelskiy; M.A. Ochando; J. Olivares; Jose Luis De Pablos; L. Pacios; Ignacio Pastor; M.A. Pedrosa; A. De La Peña; A. Pereira; G. Perez; D. Pérez-Risco; A. Petrov; S. Petrov; A. Portas; D. Pretty; D. Rapisarda; Giuseppe A. Rattá; Jose M. Reynolds-Barredo; E. Rincon; L. Ríos; C. Rodriguez; J.A. Romero; A. Ros; A. Salas; M. Sánchez; Edilberto Sanchez; E. Sánchez-Sarabia; K. Sarksian; J.A. Sebastián; C. Silva; S. Schchepetov; Nina Skvortsova; Emilia R Solano; A. Soleto; Francisco Tabares; David Tafalla; A. Tarancón; Yu. Taschev; J. Tera; A. Tolkachev; Victor Tribaldos; V.I. Vargas; J. Vega; G. Velasco; Jose Luis Velasco; M. Weber; G. Wolfers; B. Zurro; Confinement transitions in TJ-II under Li-coated wall conditions. Nuclear Fusion 2009, 49, 104018, 10.1088/0029-5515/49/10/104018.
- Alessio Siciliano; C. Ruggiero; S. De Rosa; A new integrated treatment for the reduction of organic and nitrogen loads in methanogenic landfill leachates. Process Safety and Environmental Protection 2013, 91, 311-320, 10.1016/j.psep.2012.06.008.
- Ahmet Günay; Dogan Karadag; Ismail Tosun; Mustafa Ozturk; Use of magnesit as a magnesium source for ammonium removal from leachate. Journal of Hazardous Materials 2008, 156, 619-623, 10.1016/j.jhazmat.2007.12.067.