
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joanna Michałowska | + 1314 word(s) | 1314 | 2021-02-22 07:17:28 | | | |
| 2 | Camila Xu | Meta information modification | 1314 | 2021-03-18 03:34:35 | | |
Video Upload Options
The term incretin was introduced in 1932 to describe compounds produced by intestinal mucosa in response to nutrient ingestion, which were capable of reducing blood glucose. There are two known incretins: glucose-dependent insulinotropic polypeptide (GIP) produced by the K cells of an upper gut and glucagon-like peptide-1 (GLP-1) produced by the L cells of a lower gut. Incretins play a crucial role in stimulating insulin and glucagon secretion by the pancreas.
1. Introduction
Incretin hormones are released from the intestine after nutrient intake. They play a crucial role in stimulating insulin and glucagon secretion by the pancreas [1][2]. There are two known incretins: glucose-dependent insulinotropic polypeptide (GIP) produced by the K cells of an upper gut and glucagon-like peptide-1 (GLP-1) produced by the L cells of a lower gut. Together, they are responsible for an “incretin effect”, which refers to the observation of two- to three-fold higher insulin secretion after oral glucose intake, in comparison to an equivalent intravenous glucose administration [3][4]. Fasting healthy subjects have low basal plasma concentrations of GIP and GLP-1. They start to rise few minutes after nutrient ingestion, reaching a peak approximately after 1 h, with GIP concentrations being usually higher than GLP-1 concentrations. In patients with T2DM, the incretin effect is diminished or absent. This is due to the fact that the pancreas is no longer responsive to GIP; however, it does remain sensitive to GLP-1 [5]. Thus, incretin-based glucose-lowering medications, in particular GLP-1 receptor agonists (GLP-1RAs), have proven to be effective and are currently used in T2DM treatment [6][7][8][9][10][11]. A similar observation of decreased incretin effect was made in obese individuals with normal glucose tolerance. Several studies reported a reduction in the incretin effect in nondiabetic individuals with obesity, suggesting that incretin hormones may play a role in the pathophysiology of obesity [4][12][13]. Moreover, incretins present pleiotropic actions and effects in various organ systems. GLP-1 is responsible for the reduction in food intake and appetite, increased satiety, and decreased gastric emptying. It affects adipose cells, bone metabolism and the cardiovascular system. GIP addresses fewer organs and functions compared to GLP-1; however, research suggests that it may also influence adipose tissue by promoting fat storage in subcutaneous adipose tissue and bone metabolism by promoting bone formation and limiting bone resorption [3][4]. The summary of the biological effects of GIP and GLP-1 on various organs is presented in Figure 1.
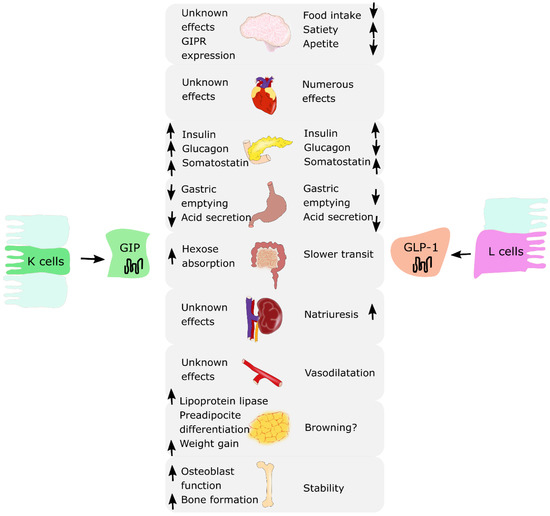
Figure 1. The summary of biological effects of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) on various organs. ↑: increase; ↓: decrease.
2. Biology of Incretins
The term incretin was introduced in 1932 to describe compounds produced by intestinal mucosa in response to nutrient ingestion, which were capable of reducing blood glucose [14]. GIP was discovered in 1970 by John C. Brown in dogs. This hormone was initially named “gastric inhibitory polypeptide”, due to its inhibitory effect on gastric acid secretion [15][16]. The insulinotropic action of GIP in humans was presented three years later, and the acronym GIP was proposed to be changed to its current name—glucose-dependent insulinotropic polypeptide [17][18]. The work in the next several years was focusing on the role of GIP in the pathogenesis of T2DM and its potential for the treatment of this disease, and the evidence that this peptide is not the only incretin emerged [18]. Finally, in 1980s glucagon-like peptides (called GLP-1 and GLP-2) were identified by cloning of the preproglucagon gene, which exhibited insulin-releasing activity. Further investigation demonstrated that truncated GLP-1 (7–36) shows not only insulinotropic effect but also inhibits the secretion of glucagon [14][18].
GIP is a peptide, which is synthesized and secreted mainly from K cells located in the duodenum and proximal jejunum; however, expression of this incretin by the central nervous system (CNS) has also been observed [19]. GIP expression has been detected in the brain, including hippocampus, thalamus, cerebellum, brainstem, and cortex in rats [20], and hypothalamus in humans [21]. This incretin derives from a precursor pro-peptide (pro-GIP), which is posttranslationally processed at residue 65 by proprotein convertase subtilisin/kexin type 1 to 42 amino acid (aa) form of GIP(1–42). Another amidated isoform of GIP(1–30) has also been detected as the pro-GIP peptide sequence contains a consensus cleavage site for prohormone convertase 2 (PC2) at residues 52–55 [22]. Both isoforms bind to GIP receptor (GIPR) and present intrinsic activity; however, it was speculated that they might have different pharmacokinetic profiles [23].
GLP-1 is synthesized and secreted mainly from L cells located in the small and large intestine, but it is also expressed in the CNS, primarily in the brainstem [19]. GCG gene encodes preproglucagon, which is cleaved to form proglucagon—160-aa long protein that undergoes differential posttranslational processing in particular types of cells, based on the relative activities of the prohormone convertases 1/3 (PC1,3) and 2. In L-cells of the intestine and specific CNS neurons, PC1 predominates, and proglucagon is cleaved to five functional regions: two carboxy-terminal glucagon-like peptides (GLP-1 and GLP-2), oxyntomodulin, glicentin, and intervening peptide 2. In α cells of the pancreas, the action of PC2 is greater, and proglucagon is processed to glucagon, glicentin-related pancreatic polypeptide (GRPP), intervening peptide 1, and major proglucagon fragment [24] (Figure 2). There are two biologically active forms of GLP-1 classified as amidated GLP-1 (GLP-1(7–36) amide) and glycine-extended GLP-1 (GLP-1(7–37)) [3].
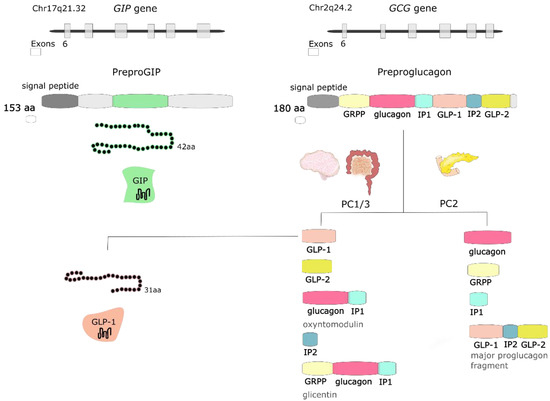
Figure 2. The GIP gene is localized on human chromosome Chr17q21.32 and encodes 153-amino acid (aa) proprotein, which is processed to 42-aa protein glucose-dependent insulinotropic peptide (GIP). GCG gene is localized on human chromosome Chr2q24.2 and encodes preproglucagon—180-aa protein, which undergoes differential posttranslational processing in a particular type of cell. Proteolytic processing of proglucagon by prohormone convertase 1/3 (PC1/3) in the intestine generates glucagon-like peptide 1 (GLP-1), glucagon-like peptide 2 (GLP-2), oxyntomodulin, intervening peptide 2 (IP2) and glicentin. Prohormone convertase 2 (PC2) activity in the pancreas forms glucagon, glicentin-related pancreatic polypeptide (GRPP), intervening peptide 1 (IP1), and major proglucagon fragment.
Glucose is one of the nutrients stimulating the secretion of GIP and GLP-1; however other nutrients, including carbohydrates (sucrose, starch), triglycerides, some amino acids and proteins, are also responsible for increasing incretin levels [25]. About two-thirds of the insulin response to an oral glucose load is a result of incretin hormone release, where both GIP and GLP-1 stimulate insulin secretion in a glucose-dependent manner [26]. They exert their effect by binding to their specific receptors—GIPR and GLP-1R, which belong to the G-protein coupled receptor family [27]. GIPR and GLP-1R stimulation activates adenylate cyclase and results in the increase of intracellular cyclic adenosine monophosphate (cAMP), leading to the activation of protein kinase A (PKA) and cAMP-regulated guanine nucleotide exchange factor II (cAMP-GEFII). These two proteins regulate insulin release via the formation of ATP, closure of ATP-sensitive K+ (KATP) channels, β-cell depolarization, the opening of voltage-dependent Ca2+ channels, the influx of the ions and elevation of the intracellular Ca2+ concentration, which triggers exocytosis of insulin granules. This mechanism accounts for approx. 70% of total insulinotropic activity exerted by both incretins [26]. Except for insulinotropic activity, incretin hormones affect pancreatic α cells and glucagon secretion. The evidence indicates that GIP stimulates glucagon release, whereas GLP-1 inhibits glucagon secretion (especially at high glucose concentrations). The glucagonostatic effect of GLP-1 leads to reduced hepatic glucose production [3]. Both GIP and GLP-1 are inactivated by dipeptidyl peptidase-4 (DPP-4), which converts active forms to inactive GIP(3–42), GLP-1(9–36) amide and/or GLP-1(9–37), which are subsequently excreted from the kidneys (Figure 3). DPP-4 processed incretins do not exhibit insulinotropic effect; however, recent studies showed that those shorter forms might have some additional biologic activities, as truncated forms of GLP-1—GLP-1(9–36) amide and GLP-1(9–37) have been demonstrated to be associated with GLP-1 cardioprotective properties [27][28].
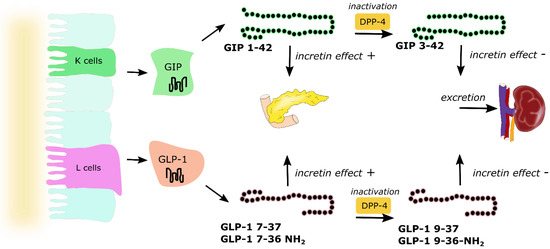
Figure 3. Secretion and metabolism of glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide 1 (GLP-1). GIP is secreted by K cells of the upper intestine, whereas GLP-1 is secreted by L cells of the lower intestine. Active forms of both incretins GIP(1–42), GLP-1(7–37) and amidated GLP-1(7–36) act directly on the pancreas and are responsible for the incretin effect. Both GIP and GLP-1 are inactivated by dipeptidyl peptidase 4 (DPP-4) to GIP(3–42), GLP-1(9–37) and GLP-1(9–36), which are excreted by the kidneys. Arrows represent pathways of incretins metabolism.
References
- Chia, C.W.; Egan, J.M. Incretins in obesity and diabetes. Ann. N. Y. Acad. Sci. 2020, 1461, 104–126.
- João, A.L.; Reis, F.; Fernandes, R. The incretin system ABCs in obesity and diabetes—Novel therapeutic strategies for weight loss and beyond. Obes. Rev. 2016, 17, 553–572.
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20, 5–21.
- Opinto, G.; Natalicchio, A.; Marchetti, P. Physiology of incretins and loss of incretin effect in type 2 diabetes and obesity. Arch. Physiol. Biochem. 2013, 119, 170–178.
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536.
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987.
- Kalra, S.; Das, A.K.; Sahay, R.K.; Baruah, M.P.; Tiwaskar, M.; Das, S.; Chatterjee, S.; Saboo, B.; Bantwal, G.; Bhattacharya, S.; et al. Consensus Recommendations on GLP-1 RA Use in the Management of Type 2 Diabetes Mellitus: South Asian Task Force. Diabetes Ther. 2019, 10, 1645–1717.
- Dogruel, H.; Balci, M.K. Development of therapeutic options on type 2 diabetes in years: Glucagon-like peptide-1 receptor agonist’s role intreatment; from the past to future. World J. Diabetes 2019, 10, 446–453.
- Nolen-Doerr, E.; Stockman, M.-C.; Rizo, I. Mechanism of Glucagon-Like Peptide 1 Improvements in Type 2 Diabetes Mellitus and Obesity. Curr. Obes. Rep. 2019, 8, 284–291.
- Dahiya, L.; Kaur, R.; Kumar, R.; Kumar, M.; Palta, K. GLP-1 Receptor Agonists in Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2020, 16, 279–292.
- Davies, M.J.; Bianchi, C.; Del Prato, S. Use of incretin-based medications: What do current international recommendations suggest with respect to GLP-1 receptor agonists and DPP-4 inhibitors? Metabolism 2020, 107, 154242.
- Muscelli, E.; Mari, A.; Casolaro, A.; Camastra, S.; Seghieri, G.; Gastaldelli, A.; Holst, J.J.; Ferrannini, E. Separate Impact of Obesity and Glucose Tolerance on the Incretin Effect in Normal Subjects and Type 2 Diabetic Patients. Diabetes 2007, 57, 1340–1348.
- Knop, F.K.; Aaboe, K.; Vilsbøll, T.; Vølund, A.; Holst, J.J.; Krarup, T.; Madsbad, S. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes. Metab. 2011, 14, 500–510.
- Rehfeld, J.F. The Origin and Understanding of the Incretin Concept. Front. Endocrinol. 2018, 9, 387.
- Brown, J.C.; Mutt, V.; Pederson, R.A. Further purification of a polypeptide demonstrating enterogastrone activity. J. Physiol. 1970, 209, 57–64.
- Brown, J.C.; Dryburgh, J.R. A Gastric Inhibitory Polypeptide II: The Complete Amino Acid Sequence. Can. J. Biochem. 1971, 49, 867–872.
- Dupre, J.; Ross, S.; Watson, D.; Brown, J. Stimulation Of Insulin Secretion By Gastric Inhibitory Polypeptide In Man. J. Clin. Endocrinol. Metab. 1973, 37, 826–828.
- Creutzfeldt, W. The [Pre-] History of the Incretin Concept. Regul. Pept. 2005, 128, 87–91.
- Campbell, J.E.; Drucker, D.J. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metab. 2013, 17, 819–837.
- Nyberg, J.; Jacobsson, C.; Anderson, M.F.; Eriksson, P.S. Immunohistochemical distribution of glucose-dependent insulinotropic polypeptide in the adult rat brain. J. Neurosci. Res. 2007, 85, 2099–2119.
- Adriaenssens, A.E.; Biggs, E.K.; Darwish, T.; Tadross, J.; Sukthankar, T.; Girish, M.; Polex-Wolf, J.; Lam, B.Y.; Zvetkova, I.; Pan, W.; et al. Glucose-Dependent Insulinotropic Polypeptide Receptor-Expressing Cells in the Hypothalamus Regulate Food Intake. Cell Metab. 2019, 30, 987–996.e6.
- Fujita, Y.; Asadi, A.; Yang, G.K.; Kwok, Y.N.; Kieffer, T.J. Differential processing of pro-glucose-dependent insulinotropic polypeptide in gut. Am. J. Physiol. Liver Physiol. 2010, 298, G608–G614.
- Gabe, M.B.N.; Van Der Velden, W.J.; Smit, F.X.; Gasbjerg, L.S.; Rosenkilde, M.M. Molecular interactions of full-length and truncated GIP peptides with the GIP receptor—A comprehensive review. Peptides 2020, 125, 170224.
- Sandoval, D.A.; D’Alessio, D.A. Physiology of Proglucagon Peptides: Role of Glucagon and GLP-1 in Health and Dis-ease. Physiol. Rev. 2015, 95, 36.
- Parker, H.E.; Reimann, F.; Gribble, F.M. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev. Mol. Med. 2010, 12, e1.
- Holst, J.J.; Gromada, J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am. J. Physiol. Metab. 2004, 287, E199–E206.
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences: Similarities and Differences of GIP and GLP-1. J. Diabetes Investig. 2010, 1, 8–23.
- Tasyurek, H.M.; Altunbas, H.A.; Balci, M.K.; Sanlioglu, S. Incretins: Their physiology and application in the treatment of diabetes mellitus. Diabetes/Metab. Res. Rev. 2014, 30, 354–371.




