
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hisataka Shoji | + 2651 word(s) | 2651 | 2021-03-04 07:02:21 | | | |
| 2 | Vivi Li | Meta information modification | 2651 | 2021-03-10 10:44:25 | | |
Video Upload Options
Endotoxin removal therapy with polymyxin B immobilized fiber column (PMX) has been clinically applied for sepsis and septic shock patients since 1994. The effectiveness and usefulness of this therapy have been demonstrated for more than a quarter of a century. However, a documented survival benefit has not yet been demonstrable in a large, multicenter, randomized and controlled trial. Following the findings derived from a large sepsis clinical trial with PMX in North America, a new trial is ongoing to determine if PMX has a long-term survival benefit when administered to septic patients. Another approach to support a survival benefit from intervention with PMX is to utilize a detailed analysis available from a large clinical data base. The endotoxin adsorption capacity of PMX columns in vitro and the effectiveness of PMX columns can be further demonstrable in animal models. The capability of PMX and details of its mechanism of action to intervene in the sepsis cascade and impede organ dysfunction in septic patients is not fully understood. The surface antigen expression in monocytes and neutrophils are improved after PMX therapy. Immunomodulatory effects as a result of endotoxin removal and/or other mechanisms of action have been suggested. These effects and other potential immune effects may explain some of the improved effects upon organ dysfunction of sepsis and septic shock patients. Endotoxemia may be involved in the pathophysiology of other diseases than sepsis. A rapid diagnostic method to detect and target endotoxemia could allow us to practice precision medicine and expand the clinical indications of endotoxin removal therapy.
1. Introduction
Endotoxin, also known as lipopolysaccharide (LPS), is a central component of the cell wall component of Gram-negative bacteria. Sepsis is a major threat to human health in the elderly, the immune compromised, trauma patients and post-surgical patients. A recent international consensus conference has generated a new way to classify patients to fit into more strictly defined clinical groups.
This classification scheme recognizes the importance of sepsis as an end product of the pathogen-associated molecular patterns (PAMPs) to trigger the host response to infection. The host response is characterized by both proinflammatory and anti-inflammatory responses. A dysregulated host response causes organ dysfunction and leads to poor outcome [1]. Sepsis is now considered as: (1) an acute systemic inflammatory state, driven by a microbial pathogen (2) in which the dysfunctional host response is actually contributing to systemic inflammation and septic shock formation (sepsis 3) [2]. Sepsis 3 is further complicated by persistent hypotension requiring vasopressors and is accompanied by elevated blood lactate levels (>2 mmol/L). Unless these patients are rapidly resuscitated, they can enter a pathologic state where they develop multi-organ dysfunction that can be fatal.
Endotoxin has been considered as one of the therapeutic targets for the treatment of sepsis and septic shock for a long time. The neutralization of blood endotoxin with agents such as polyclonal or monoclonal antibodies has been investigated. However, these approaches did not become clinically available. To remove endotoxin from the blood circulation with a medical device was another approach.
In 1994, we developed a selective endotoxin removal column (PMX) which contains polymyxin B covalently bound fibrous adsorbents [3]. Polymyxin B is a polycationic antibiotic which binds the lipid A portion of endotoxin and neutralizes its toxicity. Lipid A is the toxic moiety of endotoxin with a conserved structure among Gram-negative bacterial species and strains. Therefore, polymyxin B as a ligand is expected to bind many kinds of endotoxin from Gram-negative bacteria. The intravenous use of polymyxin B is contraindicated due to its nephrotoxicity and neurotoxicity [4][5]. So, polymyxin B was covalently immobilized as a ligand on the surface of a fibrous material. Extracorporeal hemoperfusion with PMX (PMX-HP) was expected to act to adsorb endotoxin from blood circulation.
PMX-HP has been clinically applied since 1994 in Japan. Currently, it is clinically available in some countries in Europe, Asia and North America. The first multicenter pilot-controlled study in six centers in Europe in 2005 confirmed the safety of PMX-HP and suggested the possibility of improving hemodynamic status and cardiac function [6]. After that, the results of three multicenter randomized controlled clinical trials (RCTs) in Italy, France and North America were published in 2009, 2015 and 2018, respectively. However, they failed to show a survival benefit in 28 days. Recently, a cohort study using a large clinical data base from the Japanese payment system DPC (diagnosis procedure combination) has suggested the improvement of mortality rate.
The endotoxin adsorption capacity in vitro and the effectiveness in animal experiments in vivo have been re-evaluated with PMX-HP. The immunostimulatory effects and the cellular elements inducing immunomodulatory effects and anti-apoptotic effects with PMX-HP have been demonstrated. However, the mechanism of action of PMX-HP is not fully understood.
2. Designing of Polymyxin B Immobilized Fiber Column (PMX)
Polymyxin B was covalently bonded on the surface of polystyrene-derivative fibers using primary amino group of diaminobutyric acid residue [3] (Figure 1). Immobilized polymyxin B molecules were expected to bind the lipid A portion of the endotoxin via ionic and hydrophobic interactions. Covalently fixed polymyxin B does not leak out into the blood stream. Thus, it enabled one to allow the clinical application without the known toxic effects of polymyxin B. The PMX-HP procedure is practiced through a whole blood circulation at a blood flow rate of 80 to 120 mL/min (Figure 2). As an anti-coagulant, unfractionated heparin is available. In Japan, the protease inhibitor Nafamostat mesilate is commonly used for its short half-life.
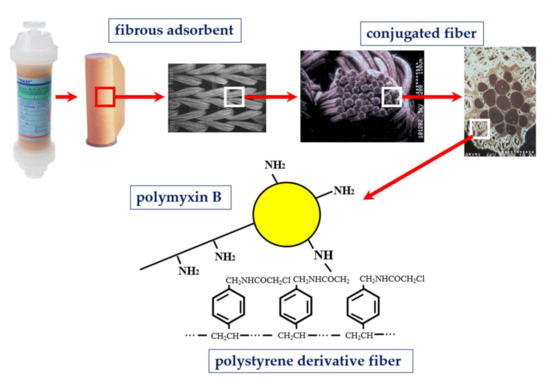
Figure 1. Structure of polymyxin B immobilized fiber column.
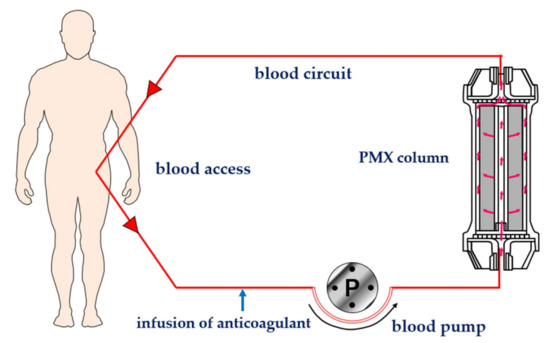
Figure 2. Schematic diagram of hemoperfusion with PMX (PMX-HP). PMX: polymyxin B immobilized fiber column.
3. Clinical Outcome with PMX Indication
3.1. Multicenter Randomized Controlled Study
The EUPHAS study in Italy was the first multicenter randomized controlled study with PMX-HP [7]. The targeted population was severe sepsis and/or septic shock patients who underwent emergency surgery for intra-abdominal Gram-negative infections. PMX-HP added to conventional therapy significantly improved mean arterial pressure and vasopressor requirement and reduced 28-day mortality by 32% (11/34 patients) in the PMX-HP group and 53% (16/30 patients) in the conventional therapy group. However, the number of the enrolled patients was small, and the study was terminated early because of a statistically significant reduction of mortality in PMX-HP group. It was declared unethical to deprive a potentially beneficial therapy to a group of patients that carry high mortality rates. This invited some criticism and failed to give a definitive answer.
The ABDO-MIX study in France was a prospective, multicenter, randomized controlled trial to test whether PMX-HP reduces mortality and organ failure in peritonitis-induced septic shock from abdominal infections. The primary outcome was 28-day mortality [8]. The mortality was 27.7% (33/119) in the PMX-HP group and 19.5% (22/113) in the conventional group (p = 0.14). It demonstrated a non-significant increase in mortality and no improvement in organ failure with PMX-HP. The same result as EUPHAS by enrolling a similar patient population was expected, but ABDO-MIX gave a conflicting result.
Some speculations were pointed out why ABDO-MIX could not demonstrate the same result as EUPHAS. The observed 28-day mortality rate in the control group was 19.5% in ABDO-MIX and 53.3% in EUPHAS. ABDO-MIX may not have enrolled a critically sick patient for that sample size. Secondly, only 81 of the 119 treated patients (68%) completed the two scheduled sessions of PMX-HP for the column clotting and hemodynamic instability. All patients in EUPHAS had completed the two planned sessions. These two studies could not give a definitive answer to prove the effectiveness of PMX-HP for the treatment of septic shock. Further well-designed multicenter randomized controlled studies would be needed.
The EUPHRATES trial is the most recent clinical study with PMX-HP in North America [9]. The objective of this multicenter, randomized, blinded, sham-controlled trial was to test whether adding PMX-HP to conventional medical therapy improves survival compared with conventional therapy alone among patients with septic shock and high endotoxin activity value (EA value) with endotoxin activity assay (EAA).
They enrolled 450 adult critically ill patients with septic shock and EA value of 0.60 or higher. The primary outcome was mortality at 28 days among all patients randomized (all participants) and among patients randomized with a multiple organ dysfunction score (MODS) of more than 9. Among eligible 450 patients, the survival rate of the PMX treated group was 37.7% (84/223) and the control group was 34.5% (78/226). There was no significant difference in mortality at 28 days, and in the population with a MODS of more than 9, the PMX group was 44.5% (65/146) and the control group was 43.9% (65/148).
Regarding the secondary and exploratory end point analyses, the change of mean arterial pressure (MAP) in day 3 was significantly higher than the control group both in all patients’ population and in patients with MODS more than 9.0 (p = 0.02). Mechanical ventilation free days (VFD) to day 28 was significantly longer in PMX-HP group than the control group in patients with MODS more than 9.0 (p = 0.02). Investigators reported the possible reasons PMX-HP failed to improve survival. For the patients who have overwhelming blood endotoxin burden, the dose and duration of PMX-HP as applied in this trial may have been insufficient to significantly reduce the endotoxin burden.
Klein et al. did a post hoc analysis and evaluated the impact of PMX-HP in patients with septic shock and an EA value measured between 0.6–0.89 [10]. At 28 days, 26.1% patients (23/88) in the PMX-HP group died versus 36.8% (39/106) in the control group. The absolute mortality reduction was 10.7%. The 28-day survival time in the PMX-HP group was significantly longer than for the control group. PMX-HP group compared with the control group showed greater change in mean arterial pressure (MAP) and ventilator free days (VFD).
The study by Romashin et al. gave the theoretical reasons for this post hoc analysis [11]. When EA values are greater than 0.9, endotoxin mass concentrations can be much greater than 4 ng/mL. This means that in a total whole blood volume of 5 L with endotoxin equally distributed between cells and plasma, a total blood load greater than 20 μg could be achieved. If endotoxin is distributed additionally into the extracellular space (10 L), then the total extracellular endotoxin load could be significantly higher than the adsorption capacity of a single PMX-HP. In the dose–response curve of endotoxin burden (LPS in ng/mL, y axis) and EA value (x axis), it was shown that above an EA value of 0.9 (corresponding to >4 ng/mL of LPS) the curve exhibits an asymptotic behavior and thus cannot be used to quantitate LPS levels in this range. The patients with an EA value more than 0.9 may have high burden of endotoxin and may not be adequate to enroll in the current EUPHRATES protocol. Post hoc analysis gave the hypothesis-generating results. The TIGRIS multicenter randomized controlled trial in the US is ongoing to prove this hypothesis.
3.2. Systematic Review with Meta-Analysis for PMX-HP
Several studies have been undertaken in the past 10 years to examine the efficacy of PMX-HP. Chang T et al. included a total of 17 trials [12]. The pooled risk ratio for overall mortality was 0.81 (95% (CI), 0.70–0.95), favoring the PMX-HP group (p = 0.007). The included studies were stratified into three groups based on the mortality rates of the conventional treatment group: low-risk group (mortality rate < 0.3), intermediate-risk group (0.3–0.6), and high-risk group (>0.6). Subgroup meta-analysis depending upon the risk stratification revealed a significant reduction of mortality in the intermediate-risk group (risk ratio, 0.84; 95% (CI), 0.77–0.92) and high-risk group (risk ratio, 0.64; 95% (CI), 0.52–0.78), but not in the low-risk group (risk ratio, 1.278; 95% (CI), 0.888–1.839). They concluded that PMX-HP may reduce mortality in patients with severe sepsis and septic shock in specific disease severity subgroups.
Li et al. included 13 studies in the meta-analysis [13]. The use of PMX-HP could reduce overall mortality (relative risk (RR) 0.68, 95% confidence interval (CI) 0.51–0.91, p = 0.01). Subgroup analysis also suggested the mortality rate of patients in Acute Physiology and Chronic Health Evaluation (APACHE II) scores < 25 group (RR 0.64, 95% (CI) 0.52–0.78, p < 0.0001) and sepsis group (RR 0.48, 95% (CI) 0.32–0.72, p = 0.0003) significantly decreased after PMX-HP treatment. They found a beneficial effect of PMX-HP for non-sicker patients stratified by APACHE II, as opposed to the Chang T et al. study. They insisted it might be more reasonable to stratify disease severity subgroups by APACHE II scores rather than the mortality rates of the conventional treatment group.
Terayama et al. selected seven RCTs comparing PMX-HP with conventional therapy on the outcome of mortality in patients with severe sepsis or septic shock [14]. PMX-HP therapy was associated with lower mortality (risk ratio, 0.65; 95% confidence interval (CI), 0.47–0.89; p = 0.007; I2 = 72%). Meta-regression analysis revealed a significant negative slope between effect size of PMX-DHP therapy and baseline mortality rate in individual studies (p = 0.003), suggesting the probability of a beneficial effect with PMX-HP increased with increasing baseline risk.
Fujii et al. included six RCTs and derived a precisely opposite conclusion [15]. The pooled risk ratio (RR) for 28-day mortality associated with PMX-HP was 1.03 (95% confidence interval (CI) 0.78–1.36; I2 = 25%; n = 797). They concluded that there is currently insufficient evidence to support the routine use of PMX-HP to treat patients with sepsis or septic shock. They included three same studies as Terayama et al. study and newly included three studies, in which two studies were positive and one was a negative study of the EUPHRATES trial [9]. So, it is estimated that the negative result of large scale RCTs had a much greater impact.
The results of systematic review with meta-analysis could not give a definitive answer. The conclusion derived was changed depending on the studies they could include for the analysis. Further rigorous RCTs targeting the pre-defined adequate patients who are likely to benefit from PMX-HP are warranted to define the clinical role of PMX-HP.
3.3. Cohort Study Using a Large Clinical Database
Iwagami et al. utilized the DPC database from 2007–2012 and assessed the survival benefit of PMX-HP in septic shock patients who received vasopressor infusion and continuous renal replacement therapy (CRRT) in the ICU [16]. Acute kidney injury (AKI) often occurrs as a complication of sepsis and is associated with high mortality in the ICU. They hypothesized that septic shock patients who required renal replacement therapy for AKI were sufficiently ill to represent the proper target population for PMX-HP. Of 3759 eligible patients, 1068 received PMX-HP. After propensity score matching, they produced a matched cohort of 978 pairs. The 28-day mortality was 40.2% (393/978) in the PMX-HP group and 46.8% (458/978) in the control group (p = 0.003). This large retrospective study using the DPC data base suggested that septic shock patients starting CRRT might benefit from PMX-HP.
Nakamura et al. used the dataset of the Japan Septic Disseminated Intravascular Coagulation (JSEPTIC DIC) study conducted in 40 institutions, which aimed to evaluate anti-DIC drugs in patients with severe sepsis or septic shock [17]. They investigated the potential survival benefit of PMX-HP retrospectively in this patient’s population. Of 1723 eligible patients, 522 had received PMX-HP. After propensity score matching, 262 matched pairs were obtained. The proportion of all-cause hospital mortality was significantly lower in the PMX-HP group than in the non-PMX-HP group (32.8% vs. 41.2%, p = 0.042).
3.4. Registry Study after EUPHAS Trial in Italy
The EUPHAS 2 study is a multicenter registry study for PMX-HP, and the aim was to verify the application of PMX-HP in the daily clinical practice (https://www.euphas2.eu accessed on 18 February 2021. Phase 1 in EUPHAS 2 involved 57 centers between January 2010 and December 2014, collecting retrospective data of 357 patients (297 in Europe and 60 in Asia) suffering from severe sepsis and septic shock caused by proved or suspected Gram-negative infection [18]. Septic shock was diagnosed in 305 (85.4%) patients and severe sepsis in 52 (14.6%). The most common source of infection was abdominal (44.0%) followed by pulmonary (17.6%). Gram-negative bacteria represented 60.6% of the pathogens responsible for infection. The survival rate of 28-days was 54.5% (60.4% in abdominal and 47.5% in pulmonary infection). Patients with abdominal infection treated with PMX-HP within 24 h from the diagnosis of septic shock had a 28-day survival rate of 64.5%. This number was comparable with 68% observed in the EUPHAS study [7]. There were no life-threatening adverse events related to PMX-HP and the feasibility of PMX-HP application was confirmed. The blood endotoxin (EA value) was measured in 132 out of 357 patients (37.0%). The measurement was possible in 18 out of 24 centers. A median EA value at the time 0 was 0.77 (0.69–0.90). The EA value of ≥0.6 was in 120 patients and less than 0.6 in 12 patients. This means that 90% (120/132) of the patients whose EA value was measured had a high value. Endotoxemia may be accompanied frequently in this patient’s population. Phase 2 in the EUPHAS 2 registry has been ongoing since 2015.
References
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851.
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810.
- Shoji, H.; Tani, T.; Hanasawa, K.; Kodama, M. Extracorporeal endotoxin removal by polymyxin B immobilized fiber cartridge: Designing and antiendotoxin efficacy in the clinical application. Ther. Apher. 1998, 2, 3–12.
- Justo, J.A.; Bosso, J.A. Adverse Reactions Associated with Systemic Polymyxin Therapy. Pharmacotherapy 2015, 35, 28–33.
- Velkov, T.; Dai, C.; Ciccotosto, G.D.; Cappai, R.; Hoyer, D.; Li, J. Polymyxins for CNS infections: Pharmacology and neurotoxicity. Pharmacol. Ther. 2018, 181, 85–90.
- Vincent, J.L.; Laterre, P.F.; Cohen, J.; Burchardi, H.; Bruining, H.; Lerma, F.A.; Wittebole, X.; Backer, D.D.; Brett, S.; Marzo, D.; et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock 2005, 23, 400–405.
- Cruz, D.N.; Antonelli, M.; Fumagalli, R.; Foltran, F.; Brienza, N.; Donati, A.; Malcangi, V.; Petrini, F.; Volta, G.; Pallavicini, F.M.B.; et al. Early use of polymyxin B hemoperfusion in abdominal septic shock The EUPHAS randomized controlled trial. JAMA 2009, 301, 2445–2452.
- Payen, D.M.; Guilhot, J.; Launey, Y.; Lukaszewicz, A.C.; Kaaki, M.; Veber, B.; Pottecher, J.; Joannes-Boyau, O.; Martin-Lefevre, L.; Jabaudon, M.; et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: A multicenter randomized control trial. Intensiv. Care Med. 2015, 41, 975–984.
- Dellinger, R.P.; Bagshaw, S.M.; Antonelli, M.; Foster, D.M.; Klein, D.J.; Marshall, J.C.; Palevsky, P.M.; Weisberg, L.S.; Schorr, C.A.; Trzeciak, S.; et al. Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients with Septic Shock and Elevated Endotoxin Level the EUPHRATES Randomized Clinical Trial. JAMA 2018, 320, 1455–1463.
- Klein, D.J.; Foster,D. ; Walker, P.M.; Bagshaw, S.M.; Mekonnen, H.; Antonelli, M. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: A post hoc analysis of the EUPHRATES trial. Intensiv. Care Med. 2018, 44, 2205–2212.
- Romaschin, A.D.; Obiezu-Forster, C.D.; Shoji, H.; Klein, D.C. Novel insights into the direct removal of endotoxin by polymyxin B hemoperfusion. Blood Purif. 2017, 44, 193–197.
- Chang, T.; Tu, Y.K.; Lee, C.T.; Chao, A.; Huang, C.H.; Wang, M.J.; Yeh, Y.C. Effects of Polymyxin B Hemoperfusion on Mortality in Patients With Severe Sepsis and Septic Shock: A Systemic Review, Meta-Analysis Update, and Disease Severity Subgroup Meta-Analysis. Crit. Care Med. 2017, 45, e858–e864.
- Li, X.; Liu, C.; Mao, Z.; Qi, S.; Song, R.; Zhou, F. Effectiveness of polymyxin B-immobilized hemoperfusion against sepsis and septic shock: A systematic review and meta-analysis. J. Crit. Care 2020.
- Terayama, T.; Yamakawa, K.; Umemura, Y.; Aihara, M.; Fujimi, S. Polymyxin B Hemoperfusion for Sepsis and Septic Shock: A Systematic Review and Meta-Analysis. Surg. Infect. 2017, 18, 225–233.
- Fujii, T.; Ganeko, R.; Kataoka, Y.; Furukawa, T.A.; Featherstone, R.; Doi, K.; Vincent, J.-L.; Pasero, D.; Robert, R.; Ronco, C.; et al. Polymyxin B‑immobilized hemoperfusion and mortality in critically ill adult patients with sepsis/septic shock: A systematic review with meta‑analysis and trial sequential analysis. Intensiv. Care Med. 2017, 44, 167–178.
- Iwagami, M.; Yasunaga, H.; Noiri, E.; Horiguchi, H.; Fushimi,K. ; Matsubara, T.; Yahagi, N.; Nangaku, M.; Doi, K. Potential survival benefit of polymyxin B hemoperfusion in septic shock patients on continuous renal replacement therapy: A propensity-matched analysis. Blood Purif. 2016, 42, 9–17.
- Nakamura, Y.; Japan Septic Disseminated Intravascular Coagulation (JSEPTIC DIC) study group; Kitamura, T. ; Kiyomi, F.; Hayakawa, M.; Hoshino, K.; Kawano, Y.; Yamasaki, R.; Nishida, T.; Mizunuma, M.; et al. Potential survival benefit of polymyxin B hemoperfusion in patients with septic shock: A propensity-matched cohort study. Crit. Care 2017, 21, 1–9.
- Cutuli, S.L.; Artigas, A.; Fumagalli, R.; Monti, G.; Ranieri, V.M.; Ronco, C.; Antonelli, M.; The EUPHAS 2 Collaborative Group. Polymyxin‑B hemoperfusion in septic patients: Analysis of a multicenter registry. Ann. Intensiv. Care 2016, 6, 77.




