| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Toshio Takahashi | + 3486 word(s) | 3486 | 2021-02-08 08:42:50 | | | |
| 2 | Rita Xu | -1807 word(s) | 1679 | 2021-03-11 03:51:07 | | |
Video Upload Options
Stem cells have extensive proliferative potential and the ability to differentiate into one or more mature cell types. The mechanisms by which stem cells accomplish self-renewal provide fundamental insight into the origin and design of multicellular organisms. These pathways allow the repair of damage and extend organismal life beyond that of component cells, and they probably preceded the evolution of complex metazoans.
1. Introduction
The appearance of the nervous system is considered to be an evolutionally epochal event that fundamentally changed how control is achieved within a multicellular body. Recent progress in genomics, molecular phylogenetics, developmental biology, and the study of simple nervous systems in living animals such as Cnidaria has provided a wealth of new empirical information about the earliest stages in neuronal evolution. Ancestral Cnidarians diverged over 500 million years ago in animal evolution [1]. Cnidaria such as Hydra, which is a descendant of ancestral Cnidarians, are composed of multiple cell types that represent the fundamental architecture of multicellular organisms. Hydra also have multipotent interstitial stem cells, which differentiate into nerve cells [2], nematocytes [2], gland cells [3], and germ cells [4]. The nervous system of Hydra is simple and is composed of a nerve net that extends throughout the animal. The cnidarian nervous system is mainly peptidergic [5]. It has been suggested that classical molecules such as acetylcholine (ACh) also contribute to the Hydra nervous system from the results of pharmacological experiments [6]. The membrane-bound ACh receptor and acetylcholinesterase have been demonstrated and confirmed by genome analysis [7][8]. Although ACh and other ACh receptor agonists function in neuronal and/or neuromuscular communication to regulate muscle contractions in Hydra, ACh itself has not been detected.
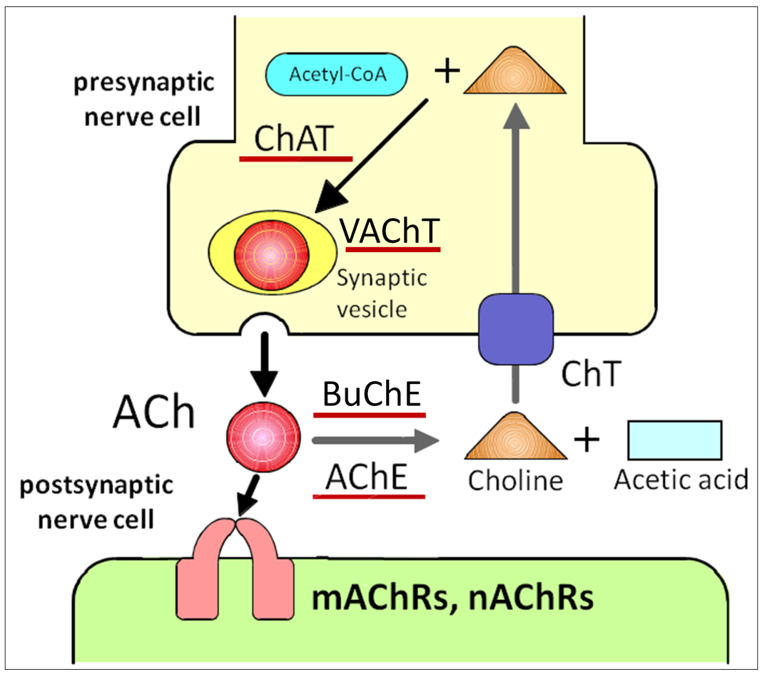
ACh is the first substance proven to be a neurotransmitter [9]. ACh is the major parasympathetic mediator and is synthesized by the catalytic conversion of acetyl-CoA and choline to CoA and ACh by choline acetyltransferase (ChAT) (Figure 1) [10][11]. In cholinergic neurons, ACh is transported into synaptic vesicles via the vesicular ACh transporter (VAChT) and stored there until released by exocytosis (Figure 1). VAChT was first cloned and characterized in Caenorhabditis elegans [12]. After the release of ACh into the synaptic cleft, the neurotransmitter evokes membrane action potentials by binding to ACh receptors (Figure 1). Then, ACh is rapidly and specifically degraded by acetylcholinesterase (AChE) and butyrylcholinesterase, which is a second, non-specific cholinesterase in mammals that also produces choline and acetic acid (Figure 1) [13][14]. By-products of choline are taken up into the presynaptic side of the synapse via the high-affinity choline transporter and reused for ACh synthesis (Figure 1) [15]. The organic cation, choline, is a substrate for carriers of organic cation transporters (OCTs). To date, three different OCTs (OCT1–3) that transport choline from the extracellular space into nerve cells have been identified [16]. ACh is stored at and released from VAChT in neurons [17]. Of interest, VAChT is expressed in a cell-type specific manner in non-neuronal cells [18]. The cells that do not express VAChT have no ability to store ACh but directly release ACh via OCTs [19][20]. Thus, OCTs are two-in-one choline and ACh transporters. These ACh synthetic pathways described above constitute the cholinergic system.
Figure 1. ACh release and receptor activation impacts neuronal activity. ACh is directly released into the synaptic cleft, followed by binding to nAChRs and mAChRs on the postsynaptic cell. Upon release, ACh is quickly degraded by extracellular AChE. ACh: acetylcholine, ChAT: choline acetyltransferase, VAChT: vesicular ACh transporter, mAChRs: muscarinic ACh receptors, nAChRs: nicotinic ACh receptors, AChE: acetylcholinesterase, BuChE: butyrylcholinesterase, ChT: choline transporter.
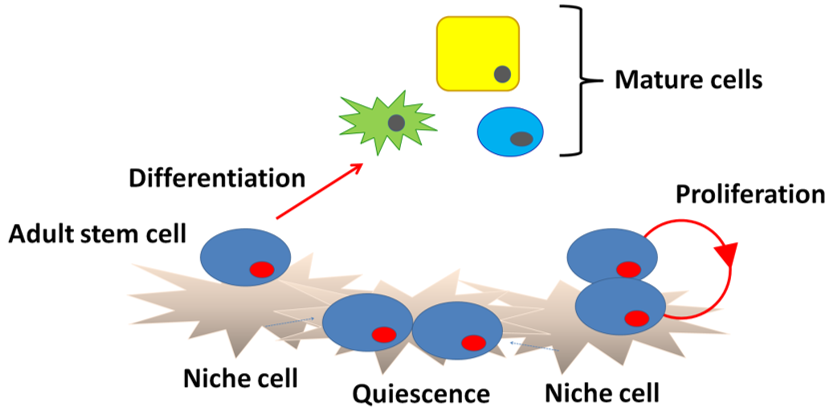
Schofield [21] originally hypothesized the existence of a microenvironment that is required for the maintenance of stem cells using hematopoietic stem cells and called such a region a “niche”. A niche is considered to be a subset of tissue cells and extracellular substrates that can indefinitely maintain stem cells and control their self-renewal and progenitor cell production in vivo (Figure 2). Specialized internal mechanisms and external signals confer the capacity of growth and differentiation to stem cells such as early embryonic cells in niches. Experimental evidence has revealed that ACh is widely distributed in biological systems beyond the nervous system. The widespread distribution suggests that ACh may be involved in regulation of stem cell functions such as proliferation, differentiation, and the establishment of cell–cell interactions [22]. Thus, the study of cholinergic mechanisms focusing on the regulation of proliferation, differentiation, and maintenance of stem cells is of great interest. Our previous pharmacological studies revealed that ACh is synthesized in intestinal epithelial cells and plays a role in cell division and the differentiation of Leucine-rich repeat-containing G-protein-coupled receptor 5-positive (Lgr5+) intestinal stem cells (ISCs) in the small intestine by binding to muscarinic ACh receptors (mAChRs) in crypt-villus organoids [23]. Furthermore, mAChRs and nicotinic ACh receptors (nAChRs) are involved in the proliferation of mouse embryonic and induced pluripotent stem cells [24][25][26]. This evidence leads us to propose the presence of a cholinergic niche that affects stem cell behavior.
Figure 2. Niche structure. Niche cells under the basement membrane signal to stem cells to block differentiation and regulate division. Upon commitment, the stem cells differentiate into mature cells.
2. Neural Stem Cells (NSCs) in the Adult Mammalian Brain
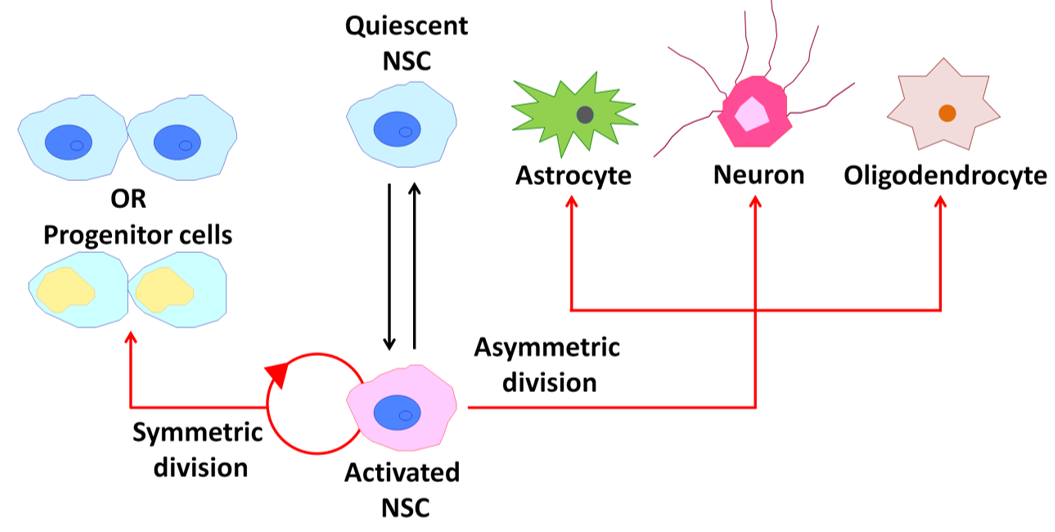
Adult mammalian neural stem cells (NSCs) contribute to brain plasticity via the generation of new neurons throughout life [27]. Adult NSCs also have fundamental properties of self-renewal, relative quiescence, differentiation capacity, and residence within a specific environmental niche similar to other adult somatic stem cells (Figure 3) [28]. New neurons are derived from NSCs that reside in two major neurogenic niches, the subventricular zone (SVZ) in the lateral ventricles and subgranular zone (SGZ) in the dentate gyrus of the hippocampus [28][29]. In the adult SVZ niche, NSCs give rise to neurons and oligodendrocytes [28]. On the other hand, neurons and astrocytes, but not oligodendrocytes, are generated from NSCs in the adult SGZ [30]. In this section, I review cholinergic signaling involved in postnatal/adult neurogenesis and how patterns of neuronal activity differentially and/or synergistically modulate downstream signaling in NSCs.
Figure 3. Behavior of neural stem cells (NSCs) within the adult mammalian brain. A schematic diagram illustrating the potential behavior of an NSC over its life cycle.
2.1. Cholinergic Activation of NSCs in the SVZ
Neurogenesis in the SVZ of the olfactory bulb (OB) continues throughout adulthood [31][32]. NSCs in the SVZ generate neuroblasts, which migrate tangentially through the rostral migratory stream toward the OB, and the neuroblasts finally differentiate into interneurons [33]. Within the dentate gyrus and OB, the interneurons abundantly express mAChRs and nAChRs [34], suggesting that the cholinergic system plays a role in regulating neurogenesis.
Accordingly, an in vivo nicotinic exposure experiment was carried out in the SVZ in adult rats, but no effect on proliferation was seen [35][36], suggesting that nAChRs may not be involved in adult OB neurogenesis. However, Mechawar and coworkers [37] used knockout mice to answer the question of whether nAChRs are involved in events downstream of NSC proliferation in the SVZ. They undertook a study of OB neurogenesis using β2−/− mice that were subjected or not subjected to chronic nicotine exposure and found that β2-containing nAChRs are specifically involved in the survival of newborn granule cells in the OB local circuits. Unexpectedly, the behavior of β2−/− mice indicated a less robust short-term olfactory memory than their wild-type (WT) littermates. Furthermore, a pharmacological study using donepezil, a potent AChE inhibitor, revealed that cholinergic stimulation promotes the survival of newborn neurons in the adult OB [38]. Two interesting studies suggested that adult NSCs in the SVZ are regionally specified at an early embryonic stage and then remain largely quiescent until reactivation in the postnatal period [39][40]. The key molecule for postnatal reactivation of SVZ NSCs may be ACh via activation of nAChRs.
2.2. Cholinergic Activation of NSCs in the SGZ
Adult hippocampal neurogenesis is tightly controlled by NSCs located in the SGZ of the mammalian dentate gyrus that proliferate, differentiate, are maintained, and integrate into the local circuitry throughout life [41][42][43][44][45]. The cholinergic system is involved in the regulation of adult hippocampal neurogenesis. The dentate gyrus receives input from the basal forebrain through GABAergic and cholinergic projection neurons [46][47]. Injection of fibroblasts secreting ACh into the hippocampus reverses cognitive decline by increasing the proliferation of NSCs [48][49][50]. Furthermore, the administration of an AChE inhibitor increases NSC proliferation and promotes the survival of immature neurons through the α7 nAChR subtype [49][51][52][53].
In the SGZ, pharmacological activation of α7-subunit-containing nAChRs increases cellular proliferation [54]. Homomeric α7 nAChRs contribute to cognition, attention, learning, and memory through fast signal transduction [55][56]. α7 nAChRs have been implicated in diseases including epilepsy, autism, schizophrenia, and Alzheimer’s disease [57][58]. As these disorders have altered adult neurogenesis in the SGZ of the dentate gyrus, α7 nAChRs may control the normal maturation and integration of immature neurons and promote their survival [59][60][61][62]. Furthermore, Otto and Yakel found that blocking or removing α7 nAChRs increases neurogenesis overall but decreases NSC pools and special discrimination in adult males only, demonstrating the sexually dimorphic regulation of adult neurogenesis [63]. It is difficult to discern the impact of α7 nAChR activation on adult neurogenesis. The different and contradictory actions of this receptor may be due to the timing and location of its activation as well as a sexually dimorphic fashion [63][64]. Other α7 subunit-containing nAChRs, including the α7β2 subtype, are expressed in a diverse array of cells in the hippocampus, and their loss contributes to multiple neuropsychiatric and neurodegenerative disorders [65][66][67][68][69]. Thus, the regulation of adult neurogenesis via α7 subunit-containing nAChRs may provide a potential therapeutic strategy for treating neurodegenerative and neurological diseases. In the SGZ, immunohistochemical staining and functional analyses have revealed that type 1 and 4 mAChRs (M1 and M4) are expressed in immature hippocampal neurons [38][70]. Additionally, bromodeoxyuridine (BrdU) labeling analysis has shown that proliferating SGZ cells expressing M1 and M4 are also labeled with BrdU, suggesting the modification of NSC/progenitor cell populations [49].
mAChRs, which are metabotropic, seven-transmembrane proteins coupled to G proteins, activate various intracellular signaling pathways to control cellular function, including that of adult stem cells [23][70][71]. On the other hand, nAChRs are pentameric, ionotropic channels that mediate fast cholinergic transmission in the peripheral and central nervous systems [72]. Furthermore, the nAChR subtype, α2β4, is also involved in adult ISC function [73][74]. mAChR and nAChR signaling probably cooperates to fine-tune effects on cells including adult stem cells.
References
- Takahashi, T. Comparative Aspects of Structure and Function of Cnidarian Neuropeptides. Front. Endocrinol. 2020, 11, 339.
- David, C.N.; Gierer, A. A cell cycle kinetics and development of Hydra attenuate III. Nerve and nematocyte differentiation. J. Cell Sci. 1974, 16, 359–375.
- Schmidt, T.; David, C.N. Gland cells in Hydra: Cell cycle kinetics and development. J. Cell Sci. 1986, 85, 197–215.
- Bosch, T.C.G.; David, C.N. Stem cells of Hydra magnipapillata can differentiate into somatic cells and germ line cells. Dev. Biol. 1987, 121, 182–191.
- Grimmelikhuijzen, C.J.P.; Leviev, I.; Carstensen, K. Peptides in the nervous system of cnidrians: Structure, function and biosynthesis. Int. Rev. Cytol. 1996, 167, 37–89.
- Kass-Simon, G.; Pierobon, P. Cnidarian chemical neurotransmission, an updated overview. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 9–25.
- Chapman, J.A.; Kirkness, E.F.; Simakov, O.; Hampson, S.E.; Mitros, T.; Weinmaier, T.; Rattei, T.; Balasubramanian, P.G.; Borman, J.; Busam, D.; et al. The dynamic genome of Hydra. Nature 2010, 464, 592–596.
- Takahashi, T.; Hamaue, N. Molecular characterization of Hydra acetylcholinesterase and its catalytic activity. FEBS Lett. 2010, 584, 511–516.
- Loewi, O. Über humerole übertragbarkeit der herznervenwirkung. I. Mitt. Pflug. Arch. 1921, 189, 239–242.
- Hebb, C.O. Acetylcholine metabolism of nervous tissue. Pharmacol. Rev. 1954, 6, 39–43.
- Hebb, C.O.; Whittaker, V.P. Intracellular distributions of acetylcholine and choline acetylase. J. Physiol. 1958, 142, 187–196.
- Alfonso, A.; Grundahl, K.; Duerr, J.S.; Han, H.P.; Rand, J.B. The Caenorhabditis elegans unc-17 gene: A putative vesicular acetylcholine transporter. Science 1993, 261, 617–619.
- Massoulié, J.; Sussman, J.; Bon, S.; Silman, I. Structure and functions of acetylcholinesterase and butyrylcholinesterase. Prog. Brain Res. 1993, 98, 139–146.
- Li, B.; Stribley, J.A.; Ticu, A.; Xie, W.; Schopfer, L.M.; Hammond, P.; Brimijoin, S.; Hinrichs, S.H.; Lockridge, O. Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. J. Neurochem. 2000, 75, 1320–1331.
- Brandon, E.P.; Mellott, T.; Pizzo, D.P.; Coufal, N.; D’Amour, K.A.; Gobeske, K.; Lortie, M.; López-Coviella, I.; Berse, B.; Thal, L.J.; et al. Choline transporter 1 maintains cholinergic function in choline acetyltransferase haploinsufficiency. J. Neurosci. 2004, 24, 5459–5466.
- Uchida, Y.; Inazu, M.; Takeda, H.; Yamada, T.; Tajima, H.; Matsumiya, T. Expression and functional characterization of choline transporter in human keratinocytes. J. Pharmacol. Sci. 2009, 109, 102–109.
- Erickson, J.D.; Varoqui, H.; Schafer, M.K.; Modi, W.; Diebler, M.F.; Weihe, E.; Rand, J.; Eiden, L.E.; Bonner, T.I.; Usdin, T.B. Functional identification of a vesicular acetylcholine transporter and its expression from a ‘cholinergic’ gene locus. J. Biol. Chem. 1994, 269, 21929–21932.
- Kummer, W.; Lips, K.S.; Pfeil, U. The epithelial cholinergic system of the airways. Histochem. Cell Biol. 2008, 130, 219–234.
- Lips, K.S.; Volk, C.; Schmitt, B.M.; Pfeil, U.; Arndt, P.; Miska, D.; Ermert, L.; Kummer, W.; Koepsell, H. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am. J. Respir. Cell Mol. Biol. 2005, 33, 79–88.
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 2007, 24, 1227–1251.
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25.
- Wessler, I.; Kirkpatrick, C.J.; Racké, K. The cholinergic ‘pitfall’: Acetylcholine, a universal cell molecule in biological systems, including humans. Clin. Exp. Pharmacol. Physiol. 1999, 26, 198–205.
- Takahashi, T.; Ohnishi, H.; Sugiura, Y.; Honda, K.; Suematsu, M.; Kawasaki, T.; Deguchi, T.; Fujii, T.; Orihashi, K.; Hippo, Y.; et al. Non-neuronal acetylcholine as an endogenous regulator of proliferation and differentiation of Lgr5-positive stem cells in mice. FEBS J. 2014, 281, 4672–4690.
- Paraoanu, L.E.; Steinert, G.; Koehler, A.; Wessler, I.; Layer, P.G. Expression and possible functions of the cholinergic system in a murine embryonic stem cell line. Life Sci. 2007, 80, 2375–2379.
- Landgraf, D.; Barth, M.; Layer, P.G.; Sperling, L.E. Acetylcholine as a possible signaling molecule in embryonic stem cells: Studies on survival, proliferation and death. Chem. Biol. Interact. 2010, 187, 115–119.
- Ishizuka, T.; Ozawa, A.; Goshima, H.; Watanabe, Y. Involvement of nicotinic acetylcholine receptor in the proliferation of mouse induced pluripotent stem cells. Life Sci. 2012, 90, 637–648.
- Kempermann, G.; Gage, F.H. New nerve cells for the adult brain. Sci. Am. 1999, 280, 48–53.
- Bond, A.M.; Ming, G.-I.; Song, H. Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell 2015, 17, 385–395.
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452.
- Bontaguidi, M.A.; Wheeler, M.A.; Shapiro, J.S.; Stadel, R.P.; Sun, G.J.; Ming, G.L.; Song, H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 2011, 145, 1142–1155.
- Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002, 22, 629–634.
- Gritti, A.; Bonfanti, L.; Doetsch, F.; Caille, I.; Alvarez-Buylla, A.; Lim, D.A.; Galli, R.; Garcia-Verdugo, J.M.; Herrera, D.G.; Vescovi, A.L. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J. Neurosci. 2002, 22, 437–445.
- Carleton, A.; Petreanu, L.T.; Lansford, R.; Alvarez-Buylla, A.; Lledo, P.-M. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 2003, 6, 507–518.
- Castillo, P.E.; Carleton, A.; Vincent, J.-D.; Lledo, P.-M. Multiple and opposing roles of cholinergic transmission in the main olfactory bulb. J. Neurosci. 1999, 19, 9180–9191.
- Abrous, D.N.; Adriani, W.; Montaron, M.-F.; Aurousseau, C.; Rougon, G.; Le Moal, M.; Piazza, P.V. Nicotine self-administration impares hippocampal plasticity. J. Neurosci. 2002, 22, 3656–3662.
- Jang, M.H.; Shin, M.C.; Jung, S.B.; Lee, T.H.; Bahn, G.H.; Kwon, Y.K.; Kim, E.H.; Kim, C.J. Alcohol and nicotine reduce cell proliferation and enhance apoptosis in dentate gyrus. Neuroreport 2002, 13, 1509–1513.
- Mechawar, N.; Saghatelyan, A.; Grailhe, R.; Scoriels, L.; Gheusi, G.; Gabellec, M.-M.; Lledo, P.-M.; Changeux, J.-P. Nicotinic receptors regulate the survival of newborn neurons in the adult olfactory bulb. Proc. Natl. Acad. Sci. USA 2004, 101, 9822–9826.
- Kaneko, N.; Okano, H.; Sawamoto, K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells 2006, 11, 1145–1159.
- Fuentealba, R.N.; Rompani, S.B.; Parraguez, J.I.; Obernier, K.; Romero, R.; Cepko, C.L.; Alvarez-Buylla, A. Embryonic origin of postnatal neural stem cells. Cell 2015, 161, 1644–1655.
- Furutachi, S.; Miya, H.; Watanabe, T.; Kawai, H.; Yamasaki, N.; Harada, Y.; Imayoshi, I.; Nelson, M.; Nakayama, K.I.; Hirabayashi, Y.; et al. Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat. Neurosci. 2015, 18, 657–665.
- Cameron, H.A.; McEwen, B.S.; Gould, E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J. Neurosci. 1995, 15, 4687–4692.
- Tozuka, Y.; Fukuda, S.; Namba, T.; Seki, T.; Hisatsune, T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 2005, 47, 803–815.
- Ge, S.; Goh, E.L.; Sailor, K.A.; Kitabatake, Y.; Ming, G.L.; Song, H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 2006, 439, 589–593.
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Boström, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013, 153, 1219–1227.
- Goncalves, J.T.; Schafer, S.T.; Gage, F.H. Adult neurogenesis in the hippocampus: From stem cells to behavior. Cell 2016, 167, 897–914.
- Cooper-Kuhn, C.M.; Winkler, J.; Kuhn, H.G. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J. Neurosci. Res. 2004, 77, 155–165.
- Bao, H.; Asrican, B.; Li, W.; Gu, B.; Wen, Z.; Lim, S.-A.; Haniff, I.; Ramakrishnan, C.; Deisseroth, K.; Philpot, B.; et al. Long-range GABAergic inputs regulate neural stem cell quiescence and control adult hippocampal neurogenesis. Cell Stem Cell 2017, 21, 604–617.
- Dickinson-Anson, H.; Winkler, J.; Fisher, L.J.; Song, H.J.; Poo, M.; Gage, F.H. Acetylcholine-secreting cells improve age-induced memory deficits. Mol. Ther. 2003, 8, 51–61.
- Mohapel, P.; Leanza, G.; Kokaia, M.; Lindvall, O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol. Aging 2005, 26, 939–946.
- Van der Borght, K.; Mulder, J.; Keijser, J.N.; Eggen, B.J.; Luiten, P.G.; Van der Zee, E.A. Input from the medial septum regulates adult hippocampal neurogenesis. Brain Res. Bull. 2005, 67, 117–125.
- Kotani, S.; Yamauchi, T.; Teramoto, T.; Ogura, H. Pharmacological evidence to cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience 2006, 142, 505–514.
- Kita, Y.; Ago, Y.; Higashino, K.; Asada, K.; Takano, E.; Takuma, K.; Matsuda, T. Galantamine promotes adult hippocampal neurogenesis via M(1) muscarinic and alpha7 nictinic receptors in mice. Int. J. Neuropsychopharmacol. 2014, 17, 1957–1968.
- Narimatsu, N.; Harada, N.; Kurihara, H.; Nakagawa, N.; Sobue, K.; Okajima, K. Donepezil improves cognitive function in mice by increasing the production of insulin-like growth factor-I in the hippocampus. J. Pharmacol. Exp. Ther. 2009, 330, 2–12.
- Narla, S.; Klejbor, I.; Birkaya, B.; Lee, Y.W.; Morys, J.; Stachowiak, E.K.; Terranova, C.; Bencherif, M.; Stachowiak, M.K. Alpha7 nicitinic receptor agonist reactivates neurogenesis in adult brain. Biochem. Pharmacol. 2013, 86, 1099–1104.
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715.
- Levin, E.D. Alpha7-nicotinic receptors and cognition. Curr. Drug Targets 2012, 13, 602–606.
- Adams, C.E.; Yonchek, J.C.; Schulz, K.M.; Graw, S.L.; Stitzel, J.; Teschke, P.U.; Stevens, K.E. Reduced Chrna7 expression in mice is associated with decreases in hippocampal markers of inhibitory function: Implications for neuropsychiatric diseases. Neuroscience 2012, 207, 274–282.
- Dineley, K.T.; Pandya, A.A.; Yakel, J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015, 36, 96–108.
- Campbell, N.R.; Fernandes, C.C.; Halff, A.W.; Berg, D.K. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation of adult-born neurons in the hippocampus. J. Neurosci. 2010, 30, 8734–8744.
- Hollands, C.; Bartolotti, N.; Lazarov, O. Alzheimer’s disease and hippocampal at neurogenesis exploring shared mechanisms. Front. Neurosci. 2016, 10, 178.
- Jessberger, S.; Parent, J.M. Epilepsy and adult neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, e020677.
- Kang, E.; Wen, Z.; Song, H.; Christian, K.M.; Ming, G.L. Adult neurogenesis and psychiatric disorders. Cold Spring Harb. Perspect. Biol. 2015, 8, a019026.
- Otto, S.L.; Yakei, J.L. The α7 nicotinic acetylcholine receptors regulate hippocampal adult-neurogenesis in a sexually dimorphic fashion. Brain Struct. Funct. 2019, 224, 829–846.
- Gu, Z.; Yakel, J.L. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron 2011, 71, 155–165.
- Rubboli, F.; Court, J.A.; Sala, C.; Morris, C.; Chini, B.; Perry, E.; Clementi, F. Distribution of nicotinic receptors in the human hippocampus and thalamus. Eur. J. Neurosci. 1994, 6, 1596–1604.
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Schrattenholz, A.; Maelicke, A. Nicotinic acetylcholine receptors on hippocampal neurons: Distribution on the neuronal surface and modulation of receptor activity. J. Recept. Signal Transduct. Res. 1997, 17, 243–266.
- Graham, A.J.; Matin-Ruiz, C.M.; Teaktong, T.; Ray, M.A.; Court, J.A. Human brain nicotinic receptors, their distribution and participation in neuropsychiatric disorders. Curr. Drug Targets CNS Neurol. Disord. 2002, 1, 387–397.
- Gillentine, M.A.; Yin, J.; Bajic, A.; Zhang, P.; Cummock, S.; Kim, J.J.; Schaaf, C.P. Functional consequences of CHRNA7 copy-number alternations in induced pluripotent stem cells and neural progenitor cells. Am. J. Hum. Genet. 2017, 101, 874–887.
- John, D.; Shelukhina, I.; Yanagawa, Y.; Deuchars, J.; Henderson, Z. Functional alpha7 nicotinic receptors are expressed on immature granule cells of the postnatal dentate gyrus. Brain Res. 2015, 1601, 15–30.
- Dutar, P.; Nicoll, R.A. Classification of muscarinic receptors in hippocampus in terms of receptor subtypes and second-messenger systems: Electrophysiological studies in vitro. J. Neurosci. 1988, 8, 4214–4224.
- Caulfield, M.P. Muscarinic receptors-characterization, coupling and function. Pharmacol. Ther. 1993, 58, 319–379.
- Dani, J.A. Overview of nicotinic receptors and their roles in the central nervous system. Biol. Psychiatry 2001, 49, 166–174.
- Takahashi, T.; Shiraishi, A.; Murata, J. The coordinated activities of nAChR and Wnt signaling regulate intestinal stem cell function in mice. Int. J. Mol. Sci. 2018, 19, 738.
- Takahashi, T. Roles of nAChR and Wnt signaling in intestinal stem cell function and inflammation. Int. Immunopharmacol. 2020, 81, 106260.