
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sona Ciernikova | + 1567 word(s) | 1567 | 2021-03-08 05:05:59 | | | |
| 2 | Vivi Li | Meta information modification | 1567 | 2021-03-10 02:30:11 | | | | |
| 3 | Vivi Li | + 5 word(s) | 1572 | 2021-03-11 03:52:59 | | |
Video Upload Options
While lifesaving achievements allowed for cancer cure in many patients, cancer survivors may suffer from long-term adverse effects substantially altering their quality of life and reintegration into normal life. Chemotherapy damages the intestinal mucosa and heavily disrupts the gut ecosystem leading to gastrointestinal toxicity. Increasing evidence from animal models and clinical studies demonstrated the associations between intestinal dysbiosis and depression, anxiety, pain, and impaired cognitive functions. Recently, the emerging role of the microbiome in chemotherapy-induced late effects affecting cognitive functions in cancer survivors has been proposed.
1. The Role of Gut Microbiome in Chemotherapy-related Cognitive Impairment and Psychoneurological Symptoms
Chemotherapeutic drugs affect brain functions, leading to numerous side effects in cognitive functioning. Some chemotherapeutics such as 5-fluorouracil, or cyclophosphamide, can directly cross the blood-brain barrier (BBB) resulting in oxidative stress, neuroinflammation, or damaging of neurovascular elements. However, many drugs, including paclitaxel and doxorubicin, cannot easily penetrate the BBB. Hence, indirect mechanisms via peripheral inflammatory mediators suggest inducing neurological changes and impaired cognitive functioning. Direct or indirect mechanisms of chemotherapeutics result in neuroinflammation, neuronal damage, and subsequent apoptosis [1].
Chemotherapy-related behavioral comorbidities and cognitive impairment might result from altered microbiota-gut-brain communication pathways including neuroinflammation and intestinal barrier integrity [2]. Importantly, a recent study in a mouse glioblastoma model suggests that changes in microbiota composition may contribute to a tumor microenvironment remodeling leading to tumor development. As the authors have shown, antibiotic treatment with vancomycin and gentamycin resulted in an early impairment of NK cells, changes in microglia phenotype, and increased growth of intracranial glioma in treated animals. The microbial analysis revealed the lower diversity with the absence of Prevotellaceae, Rikenellacaea, Helicobacteraceae, and increased abundance of Burkholderiales after the antibiotic administration [3]. Chemotherapy-induced cognitive impairment and psychological distress belong to the most frequent late effects in survivors including the patients with brain tumors such as glioma, glioblastoma, and primary central nervous system lymphoma [4].
Recent evidence highlights the ability of gut microbiota to modulate brain functions through the increasingly accepted concept of the microbiota-gut-brain axis [5]. Endocrine and metabolic signals together with neural connections form a bidirectional communication system between the central nervous system (CNS) and enteric nervous system (ENS), linking emotional and cognitive centers of the brain with peripheral intestinal functions. Microbiota-derived metabolites such as short-chain fatty acids (SCFA), neurotransmitters including gamma-aminobutyric acid (GABA), acetylcholine, and serotonin, as well as hormones, and immune system modulators contribute to the microbiota-gut-brain communication. Importantly, the existence of a possible relationship between the chemotherapy-modified microbiota-gut-brain axis and impaired cognitive functions in cancer survivors represents an emerging field of this research area. According to the available research findings, an immune-related pathway of cancer treatment-induced cognitive dysfunction via microbiota-gut-brain axis might represent a possible mechanism (Figure 1).
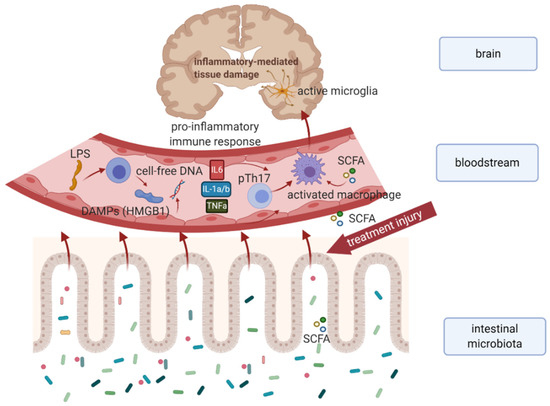
Figure 1. Hypothetical model explaining the immune-related mechanism of cancer treatment-induced cognitive dysfunction in survivors. Chemo- or radiotherapy-related dysbiosis, and intestinal barrier disruption result in an increased level of microbiota-derived metabolites (e.g. SCFA), bacterial LPS, DAMPs, as well as cell-free DNA in systemic circulation leading to proinflammatory immune response. Subsequent activation of microglial cells results in neuroinflammation and neuronal apoptosis associated with cognitive impairment. Abbreviations: DAMPs, damage-associated molecular patterns; HMGB 1, high-mobility group box 1; IL-1a/b, interleukin 1a, and 1b; IL6, interleukin 6; LPS, intestinal microbiota associated lipopolysaccharide; SCFA, short-chain fatty acids produced by intestinal microbiota; TNFa, tumor necrosis factor-alpha
Disruption of intestinal microbiota and subsequent dysbiosis result in the gut pathogenic microbiome, and the generation of lipopolysaccharides (LPS) derived from the cell wall of gram-negative bacteria. This bacterial endotoxin may disrupt intestinal integrity and LPS efflux from the gut contributes to neuroinflammation and oxidative stress followed by glial activation in the hippocampus [6] [7]. It has been reported that LPS induces inflammation by binding to microglial toll-like receptors (TLRs) and evoking M1 microglial activation associated with a reduction in neurogenesis [8]. In particular, binding of LPS to microglial TLR4 suggests activation of the inflammatory cascade by NF-κB and proinflammatory cytokines (TNF-α, IL-1β, and COX2) resulting in elevated neuroinflammation [9].
Chemotherapy-induced dysbiosis leads to a dysregulation of the microbiota-gut-brain axis at distinct stages. In a recent preclinical study, a correlation between chemotherapy-induced changes in the intestinal microflora and neuroinflammatory changes in the brain was observed in mice after paclitaxel treatment. Results confirmed that elevated circulating cytokine levels and neuroinflammation were associated with cognitive impairment, anxiety, and mood disorders. Moreover, enhanced neuroinflammation and whole-body immune response were detected after the treatment. The intestinal microbiome of treated animals changed towards the reduction in butyrate-producing bacteria (e.g. Lachnospiraceae) compared to the control group. Taken together, the results showed chemotherapy-induced anorexia, slowed growth, cognitive impairment, and an increase in central and peripheral inflammatory processes, as well as increased endotoxin levels in the bloodstream. Hence, modulation of the intestinal microbiota might represent a potential therapeutic strategy not only for reducing the gastrointestinal side effects of chemotherapy but also for mitigating the impact on neurological functions [10].
The results from available clinical studies indicated that cancer treatment-related psychoneurological symptoms and toxicities can be mediated by the microbiota-gut-brain axis. A cross-sectional study by Okubo et al. provided the first evidence that chemotherapy-induced changes in gut microbiota influenced the fear of cancer recurrence (FCR) among breast cancer survivors. The metagenomic analysis found a link between a higher relative abundance of Bacteroides and higher FCR. On the other hand, a lower FCR was associated with a higher relative abundance of Lachnospiraceae and Ruminococcus. In addition, lower bacterial diversity was significantly associated with higher FCR [11]. The link between the gut microbiome changes and alterations in psychosocial factors including anxiety, depression, fatigue, sleep quality, and cardiorespiratory fitness was found in a proof-of-concept study on a cohort of 12 breast cancer survivors. Several bacterial taxa as Bacteroides, Roseburia, Prevotella were significantly associated with changes in psychosocial symptoms. The change in fatigue interference correlated with the frequency of genera Faecalibacterium and Prevotella whereas the change in anxiety was associated with the frequency of genera Coprococcus and Bacteroides [12]. Very recently, Bai et al. demonstrated pre- and post-radiotherapy correlations between microbial diversity and psychoneurological symptom (PNS) cluster in a pilot study comprising 13 patients with head and neck cancers [13]. PNS cluster has been previously defined as a set of symptoms including pain, fatigue, sleep disturbance, depressive symptoms, and cognitive dysfunction [14], reliably associated with reduced quality of life. Microbial analysis of stool samples from cancer patients showed higher abundances of Bacteroidetes, Ruminiclostridium9, Tyzzerella, Eubacterium_fissicatena, and DTU089 in patients with the high PNS cluster. On the other hand, patients with the low PNS cluster displayed higher abundances in Lactococcus, Phascolarctobacterium, and Desulfovibrio. Importantly, significant differences in both glycan and vitamin metabolism between the high and low PNS clusters pre- and post-radiotherapeutic treatment were observed [13].
2. Gut Microbiome and Chemotherapy-Induced Peripheral Neuropathy
A key role of intestinal microbiota in the development of inflammatory pain has been detected by measuring the hypernociceptive responses in germ-free and conventional mice [15]. Recent evidence supports the significant impact of the gut microbiome on neuropathic pain providing the potential for novel therapeutic strategies [16]. Chemotherapy-induced peripheral neuropathy (CIPN) and the gut microbiome may be linked via the immune-nervous-endocrine axis [17]. CIPN, characterized by pain, muscle weakness, numbness, burning, or tingling, is the long-lasting toxic side-effect of cancer treatment with taxanes, platinum compounds, and other commonly used anti-cancer drugs [18]. Neurotoxic effects of chemotherapeutics leading to the production of ROS, and activation of pain receptors, together with neuroinflammation represent the possible mechanisms underlying CIPN. The results showed that cognitive impairment and distinct psychological disorders are often linked to CIPN. Interestingly, a striking association between gut microbiota and CIPN has been uncovered, showing reduced oxaliplatin-induced mechanical hyperalgesia in germ-free (GF) mice, or animals with temporarily eradicated gut bacteria by antibiotics [19]. Accordingly, Ramakrishna et al. observed a crucial role of gut bacteria in paclitaxel-induced pain sensitivity and resistance when comparing the microbiota composition of C57BL/6 (B6) and 129SvEv (129) mice. In their study, microglia were found to be causally involved in paclitaxel-induced pain symptoms, and the possible interplay of several bacterial taxa was identified. From initial microbiota (before paclitaxel administration), Lactobacillus intestinalis and Eubacterium siraeum were suggested to be inhibitors of the pain phenotype. In addition, the pain inhibiting phenotype after paclitaxel administration was supposed to be driven by the members of Porphyromonadaceae. Since paclitaxel decreased the abundance of Akkermansia muciniphila, altered brain functions might have resulted from changes in communication via the microbiota-gut-brain axis [20]. Recently, an association between increased levels of circulating butyrate and neuropathic pain improvement following fecal microbiota transplantation (FMT) in obese mice has highlighted the novel approaches for neuropathy prevention, or pain relief [21].
Due to the lack of specificity of most chemotherapeutics to target only malignant cells, cancer patients experience numerous acute, and long-term side-effects throughout the body including gastrointestinal toxicity and cognitive and behavioral impairment. The increasing number of long-term survivors highlights the need for elucidation of underlying mechanisms in chemotherapy-induced late effects. Recently, animal models and clinical studies have uncovered the significant association between changes in the intestinal microbiota and treatment-related comorbidities. A deep understanding of the microbiota-gut-brain axis in cancer and elucidating the impact of an altered intestinal microbiome on immune, metabolic, psychological, and cognitive pathways is crucial for improving the physical and psychosocial health of survivors.
Acknowledgment: The research was funded by the Slovak Research and Development Agency (APVV), grant number APVV-19-0411 and APVV-15-0086, and by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and Slovak Academy of Sciences (VEGA), contract No. 2/0052/18 and 1/0327/19.
The entry is from 10.3390/cancers13040782
References
- Subramaniam, C.B.; Bowen, J.M.; Gladman, M.A.; Lustberg, M.B.; Mayo, S.J.; Wardill, H.R; The microbiota-gut-brain axis: An emerging therapeutic target in chemotherapy-induced cognitive impairment. Neurosci Biobehav Rev 2020, 116, 470-479, doi:10.1016/j.neubiorev.2020.07.002.
- Jordan, K.R.; Loman, B.R.; Bailey, M.T.; Pyter, L.M; Gut microbiota-immune-brain interactions in chemotherapy-associated behavioral comorbidities. Cancer 2018, 124, 3990-3999, doi:10.1002/cncr.31584.
- D'Alessandro, G.; Antonangeli, F.; Marrocco, F.; Porzia, A.; Lauro, C.; Santoni, A.; Limatola, C; Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur J Immunol 2020, 50, 705-711, doi:10.1002/eji.201948354.
- Abrey, L.E; The impact of chemotherapy on cognitive outcomes in adults with primary brain tumors. J Neurooncol 2012, 108, 285-290, doi:10.1007/s11060-012-0807-6.
- Cryan, J.F.; O'Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V; et al. The Microbiota-Gut-Brain Axis. Physiol Rev 2019, 99, 1877-2013, doi:10.1152/physrev.00018.2018.
- Catorce, M.N.; Gevorkian, G; LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Curr Neuropharmacol 2016, 14, 155-164, doi:10.2174/1570159x14666151204122017.
- Chen, J.; Buchanan, J.B.; Sparkman, N.L.; Godbout, J.P.; Freund, G.G.; Johnson, R.W; Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun 2008, 22, 301-311, doi:10.1016/j.bbi.2007.08.014.
- Tang, Y.; Le, W; Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol 2016, 53, 1181-1194, doi:10.1007/s12035-014-9070-5.
- Cerovic, M.; Forloni, G.; Balducci, C; Neuroinflammation and the Gut Microbiota: Possible Alternative Therapeutic Targets to Counteract Alzheimer's Disease?. Front Aging Neurosci 2019, 11, 284, doi:10.3389/fnagi.2019.00284.
- Loman, B.R.; Jordan, K.R.; Haynes, B.; Bailey, M.T.; Pyter, L.M; Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice. Sci Rep 2019, 9, 16490, doi:10.1038/s41598-019-52893-0.
- Okubo, R.; Kinoshita, T.; Katsumata, N.; Uezono, Y.; Xiao, J.; Matsuoka, Y.J; Impact of chemotherapy on the association between fear of cancer recurrence and the gut microbiota in breast cancer survivors. Brain Behav Immun 2020, 85, 186-191, doi:10.1016/j.bbi.2019.02.025.
- Paulsen, J.A.; Ptacek, T.S.; Carter, S.J.; Liu, N.; Kumar, R.; Hyndman, L.; Lefkowitz, E.J.; Morrow, C.D.; Rogers, L.Q; Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Support Care Cancer 2017, 25, 1563-1570, doi:10.1007/s00520-016-3568-5 .
- Bai, J.; Bruner, D.W.; Fedirko, V.; Beitler, J.J.; Zhou, C.; Gu, J.; Zhao, H.; Lin, I.H.; Chico, C.E.; Higgins, K.A; et al. Gut Microbiome Associated with the Psychoneurological Symptom Cluster in Patients with Head and Neck Cancers. Cancers (Basel) 2020, 12, 2531, doi:10.3390/cancers12092531.
- Kim, E.; Jahan, T.; Aouizerat, B.E.; Dodd, M.J.; Cooper, B.A.; Paul, S.M.; West, C.; Lee, K.; Swift, P.S.; Wara, W; et al. Differences in symptom clusters identified using occurrence rates versus symptom severity ratings in patients at the end of radiation therapy. Cancer Nurs 2009, 32, 429-436, doi:10.1097/NCC.0b013e3181b046ad.
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci U S A 2008, 105, 2193-2197, doi:10.1073/pnas.0711891105.
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G; Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J Headache Pain 2020, 21, 103, doi:10.1186/s10194-020-01170-x.
- Zhong, S.; Zhou, Z.; Liang, Y.; Cheng, X.; Li, Y.; Teng, W.; Zhao, M.; Liu, C.; Guan, M.; Zhao, C; et al. Targeting strategies for chemotherapy-induced peripheral neuropathy: does gut microbiota play a role? . Crit Rev Microbiol 2019, 45, 369-393, doi:10.1080/1040841X.2019.1608905.
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C; Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 2013, 63, 419-437, doi:10.3322/caac.21204.
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci 2017, 20, 1213-1216, doi:10.1038/nn.4606.
- Ramakrishna, C.; Corleto, J.; Ruegger, P.M.; Logan, G.D.; Peacock, B.B.; Mendonca, S.; Yamaki, S.; Adamson, T.; Ermel, R.; McKemy, D; et al. Dominant Role of the Gut Microbiota in Chemotherapy Induced Neuropathic Pain. Sci Rep 2019, 9, 20324, doi:10.1038/s41598-019-56832-x.
- Bonomo, R.R.; Cook, T.M.; Gavini, C.K.; White, C.R.; Jones, J.R.; Bovo, E.; Zima, A.V.; Brown, I.A.; Dugas, L.R.; Zakharian, E; et al. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc Natl Acad Sci U S A 2020, 117, 26482-26493, doi:10.1073/pnas.2006065117.





