
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pavla Bradáčová | + 2785 word(s) | 2785 | 2021-03-08 03:00:45 | | | |
| 2 | Peter Tang | -179 word(s) | 2606 | 2021-03-09 11:26:12 | | |
Video Upload Options
Antiphospholipid syndrome (APS) is a hypercoagulation condition associated with the incidence of heterogenic antiphospholipid antibodies (aPLs), which non-specifically affect hemostasis processes. APS is clinically manifested by recurrent arterial and venous thromboses and reproduction losses. The aPL antibodies, which may induce clinical manifestations of APS, include criteria antibodies anti-cardiolipin, anti-β2-glycoprotein-I, and lupus anticoagulant, but also non-criteria antibodies, for example anti-β2-glycoprotein-I domain I, anti-phosphatidylserine/prothrombin, anti-annexin V, and many others. APS occurs mostly in patients of younger and middle age, most frequently in females. Laboratory diagnostics of APS are quite difficult, as they include a wide spectrum of examining methods, which are based on various principles of detection and are performed using various laboratory techniques.
1. Introduction
Antiphospholipid syndrome (APS), also known as Hughes syndrome, was reported for the first time in 1983 by Dr. Graham Hughes [1]. APS is an autoimmune disease associated with persistent antiphospholipid antibodies (aPLs). The main target of the aPLs is binding to the phospholipid membranes of platelets with their subsequent activation. However, they also bind to endothelia, monocytes, and neutrophils with a procoagulation effect [2][3]. Antiphospholipid antibodies also interfere with the activation of the complement. All this may subsequently result in the development of thrombosis [4]. APS may be primary and also secondary. Primary APS is a condition in which the patient has no other autoimmune disease. Secondary APS occurs in relation with another autoimmune disease: systemic lupus erythematosus (SLE) [5][6][7].
Prevalence of aPLs in the population is approximately 1–5%, but only a minor part develops APS [8]. However, APS is considered to be the most common cause of acquired thrombophilia despite this fact. Clinical manifestations of APS are very variable. Venous thromboses may be manifested by phlebothrombosis of the lower or upper limbs, or by pulmonary embolism. Myocardial infarction or cerebrovascular accident is usually a consequence of arterial thromboses. In the group of pregnancy-related complications, APS is frequently a cause of preeclampsia, miscarriages, premature labor, growth retardation of the fetus due to an insufficient placenta, or death of the fetus. Migraine, immune thrombocytopenia, transient ischemic attack, livedo reticularis, autoimmune hemolytic anemia, and many others were observed as other non-criteria clinical manifestations of APS [9]. Progression of catastrophic antiphospholipid syndrome (CAPS) occurs in approximately 1% of patients with APS, whereby the patient is affected by thromboses mostly in small vessels, leading to multiorgan failure. CAPS is a very severe condition with high mortality [10][11].
Criteria for APS according to the Sydney classification are very strictly defined; at least one clinical and at least one laboratory criterion must be met. Clinical criteria of APS include the occurrence of arterial or venous thromboses and reproduction losses [12][13]. Up to 10–20% of recurrent reproduction losses and up to 20% of cerebrovascular accidents in patients below the age of 50 are caused by APS [14][15]. Laboratory criteria include positivity of at least one antibody of the anti-cardiolipin (aCL) IgG and IgM, anti-β2-glycoprotein-I (anti-β2GPI) IgG and IgM, and the lupus anticoagulant (LA) type [16]. In order to meet the laboratory criteria, the aPLs must be repeatedly positive in an interval of 12 weeks [17]. It is evaluated whether this is single, double, or triple positivity, since patients with triple positivity have the highest risk of thromboses and recurrent miscarriages [18][19][20]. It is required to avoid laboratory examination of APS during ongoing infection due to false positivity of the aPLs [21].
2. Antiphospholipid Antibodies
There is a wide range of antiphospholipid antibodies that interact with negatively charged phospholipid surfaces of many cells and tissues by various mechanisms. These aPLs, described on Figure 1, include APS criteria antibodies of the lupus anticoagulant, anti-cardiolipin, anti-β2-glycoprotein-I type, and APS non-criteria antibodies of the anti-β2-glycoprotein-I domain I (anti-DI), anti-annexin V, anti-annexin II, anti-prothrombin (anti-PT), anti-phosphatidylserine/prothrombin (anti-PS/PT), anti-cardiolipin/vimentin (aCL/Vim), anti-protein S/protein C (anti-PS/PC) type, and others.
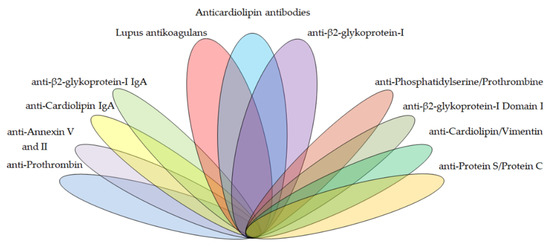
Figure 1. The spectrum potential antiphospholipid antibody targets in the diagnostics of antiphospholipid syndrome (APS).
2.1. APS Criteria Antibodies
2.1.1. Lupus Anticoagulant
Lupus anticoagulants are a heterogenic group of immunoglobulins that specifically aim at epitopes of negatively charged protein binding phospholipids of the cellular membrane, prothrombin, and beta2-glycoprotein I, which in vitro prolongs the coagulation tests dependent on phospholipids when there is competition with coagulation factors for phospholipids [22].
Positivity of LA is a much more risky factor for the development of thromboembolism, cerebral ischemia, and recurrent reproduction losses in comparison with aCL and anti-β2GPI and even other non-criteria antibodies [23]. LA was demonstrated in 69% in a group of 192 patients with APS [24]. Choi et al. [25] carried out a retrospective study of 833 patients with a persistent presence of aPLs and they found that 46.9% of 96 patients with clinical manifestations of APS had positive LA vs. a group of 737 asymptomatic carriers, where the incidence of LA was only 25.6%. There were no significant differences between the two groups in other aPLs.
2.1.2. Anti-β2-Glycoprotein-I
β2-glycoprotein-I is anionic glycoprotein with five domains binding to phospholipids. Four domains have regular, conserved sequences, but the fifth domain is aberrant. This domain contains of the insertion of six residues, C-terminal extension of 19 residues, and another disulphide bond that includes the C-terminal cysteine. These additional amino acids in domain V are responsible for unique characteristics of this CUP domain because they form a large positively charged patch that determines affinity to anionic phospholipids [26].
The anti-β2GPI IgG and IgM antibody plays a major role in the pathogenesis of APS. Its presence is very strongly associated with thromboembolic complications. The β2-glycoprotein-I molecule consists of five homologous domains and occurs in two conformations, either in a closed circular form or in an open form. In the circular form, there is interaction with anti-β2GPI mainly between domains 1 and 5; in the open form, epitope is uncovered on domain 1, to which anti-β2GPI binds.
Detection of anti-β2GPI IgG (Figure 2) and IgM (Figure 3) is performed by the enzyme-linked imunosorbent assay (ELISA) method according to the international guideline of the Society of Thrombosis and Haemostasis Scientific and Standardization Committee ISTH SSC. The determined cut-off (99th percentile) in the enzyme-linked imunosorbent assay (ELISA) for positivity of anti-β2GPI is >40 IgG antiphospholipid units/mL (GPL), or IgM antiphospholipid units/mL (MPL) [16]. According to Liu et al., anti-β2GPI IgG is the best predictor of arterial thrombosis, with an odds ratio (OR) = 6.5 [24]. Demonstration of anti-β2GPI IgG has higher specificity for APS than aCL IgG, but lower sensitivity for APS than demonstration of aCL IgG at the same time [27]. However, the results of anti-β2GPI do not always significantly correlate with clinical manifestations of APS, which may be due to insufficient standardization of the ELISA method [28][29][30][31][32]. The modern method of anti-β2GPI detection is the chemiluminescence analysis (CLIA), in which the cut-off for positivity is >20 chemiluminescence unit (CU) (99th percentile) [33]. Multiline dot assay (MLDA) is also an available method.
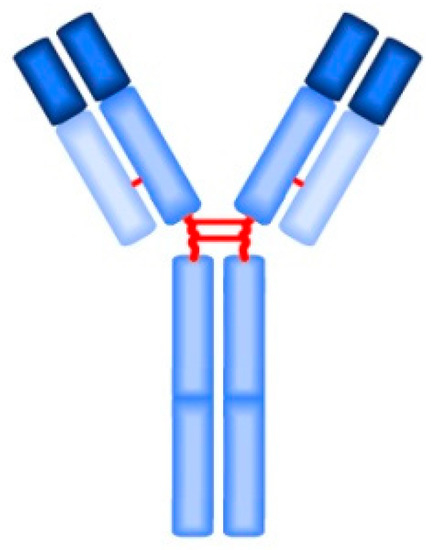
Figure 2. The IgG monomer structure.
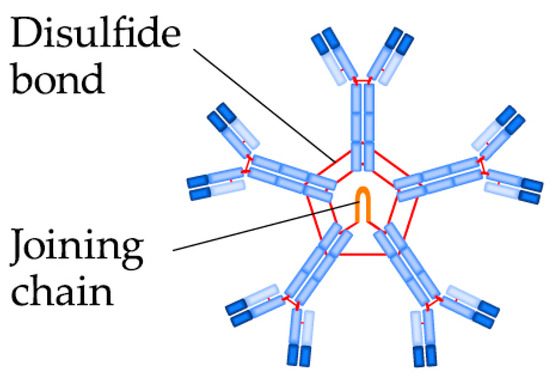
Figure 3. The IgM pentamer structure.
2.1.3. Anti-Cardiolipin
Anti-cardiolipin antibodies include a group of antibodies against the cardiolipin part of the VDRL (venereal disease research laboratory) antigen, which are the antibodies that react with phospholipids of the prothrombin activator complex and antibodies that can react with cardiolipin in the fixed phase [34].
aCL IgG is much more associated with cerebral thromboses and myocardial infarctions than aCL IgM. Detection of aCL may be performed by ELISA, CLIA, and MLDA. The determined cut-off (99th percentile) in ELISA for positivity of aCL is >40 GPL/MPL [16]. The cut-off recommended by the manufacturer in CLIA for positivity of aCL is >20 CU (99th percentile) [24].
2.2. APS Non-Criteria Antibodies
2.2.1. Anti-β2-Glycoprotein-I Domain I
The presence of APS anti-DI antibodies correlates more significantly with the incidence of thromboses and reproduction losses against other aPLs [35]. The occurrence of anti-DI together with LA is significantly associated with patients with APS and venous thrombosis [27]. Sensitivity of anti-DI after APS of 85% and specificity of 99.5% point to quite great usefulness of anti-DI for APS diagnostics, however, more studies are still needed [36]. Radin et al. [37] analyzed 11 studies involving 1218 patients with APS, where positivity of anti-DI was demonstrated in 45.4%. Tonello et al. [38] carried out a study of 105 patients with APS and persistent presence of the aPL criteria and they demonstrated anti-DI in 41.9%. Positivity of anti-DI was significantly associated with triple positivity. On the contrary, anti-DI negativity was significant in patients with an isolated presence of other aPL criteria. The cut-off recommended by the manufacturer for positivity of anti-DI in CLIA is >20 CU (99th percentile) [24][33]. Serrano et al. specified their own cut-off of >23.8 units (99th percentile) in ELISA for anti-DI in a measurement of 321 healthy volunteers [39]. Slavík et al. [40] examined 74 patients with APS who had positivity at least in one aCL and anti-β2GPI class at the same time. They demonstrated positivity of anti-DI in 21 samples, of which 57% had clinical manifestations of APS. They increased the predictive value for thrombosis from 25% to 68% in anti-DI positive patients by an examination of anti-DI.
2.2.2. Anti-β2-Glycoprotein-I IgA
Antibodies of the IgA class are produced by B-lymphocytes, which may be found in the mucosae, therefore, IgA are also called mucosal antibodies; they are the most common antibodies in the body. IgA antibodies are structurally similar to IgF, but IgA more frequently occur as dimers (Figure 4). The basic function of IgA is to block bacterial adhesion molecules and their opsonization. IgA do not active the complement.
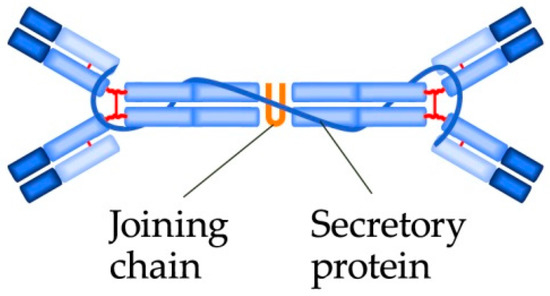
Figure 4. The IgA dimer structure.
Positivity of the anti-β2GPI IgA class, but with LA negativity at the same time, may be a cause of recurrent unexplainable reproduction losses in females [27][41]. Positivity is put in relation with thrombocytopenia, livedo reticularis, and pulmonary hypertension, and it increases the risk of fatal graft rejection in patients after kidney transplantation [42]. Anti-β2GPI IgA antibodies are more associated with APS than with anti-β2GPI IgM [43]. Ruiz-Garcia et al. performed ELISA measurement of anti-β2GPI IgA in 156 patients with clinical criteria of APS and they demonstrated isolated positivity of anti-β2GPI IgA in 22.4% [44]. Vlagea et al. [45] carried out a study for the presence of anti-β2GPI IgA (cut-off >20 U/mL 99th percentile, 100 healthy follow-ups) in 314 patients with APS and SLE. The presence of isolated positivity of anti-β2GPI IgA in the group of APS was detected only in 7.2%, whereas the presence was detected in 76.2% in the SLE group. Chayoua et al. [46] analyzed a multicentric study of aPL detection in 1068 patients from 8 sites by 4 various methods (CLIA, ELISA, multiplex fluorescence flow immunoassay (MFFIA), fluorescence enzyme immunoassay (EliA)) and they determined isolated positivity of anti-β2GPI IgA in patients with clinical manifestations of APS in 0.3–5% dependent on the device used.
2.2.3. Anti-Cardiolipin IgA
The significance of aCL IgA for the development of thrombotic complications has also been of much interest recently [47]. Using CLIA (cut-off recommended by the manufacturer >20 CU), Liu et al. detected aCL IgA in 192 samples of APS in 42%, in 90 samples of seronegative APS (SN-APS) in 12%, and in healthy donors in 0% [24].
2.2.4. Anti-Prothrombin and Anti-Phosphatidylserine/Prothrombin Complex
The anti-PT IgG antibody may be a very useful predictive factor for the development of thrombosis in patients with SLE [48]. Anti-PT is capable of a bond even to the PS/PT complex. Positivity of anti-PS/PT IgG, IgM with positivity of LA at the same time is very significantly associated with arterial and also venous thromboses and pregnancy complaints [49][50][51] and sensitivity, and specificity for APS is also higher than during positivity of aCL [52]. Using ELISA (cut-off >30 [53]), Liu et al. detected anti-PS/PT IgG, IgM in samples of APS in 72%, in SN-APS in 36%, and in healthy donors in 0%. Anti-PS/PT was more commonly detected in the group of APS and SN-APS than aCL IgG and IgM and anti-β2GPI IgG and IgM. They further found out that particularly anti-PS/PT IgG is the best predictor for deep vein thrombosis, OR = 9.2 [24]. Hui shi et al. found in a study of 186 samples with APS + SN-APS that if LA is positive together with anti-PS/PT, then the OR for the development of thrombosis is 101.6 [54].
2.2.5. Anti-Annexin V and Anti-Annexin II
Annexins are in the group of Ca2+-dependent proteins binding phospholipids. Annexin V is the main part of trophoblast and vascular endothelia. Annexin V binds phospholipids with anticoagulation activity; it serves as a so-called protective shield. This shield may be impaired in case of the interaction of annexin V with antibodies, causing thrombosis and reproduction losses [55]. However, the correlation of anti-annexin V with pregnancy complications is not completely significant and more studies are needed [56]. Annexin II is important for the bonding of β2GPI to endothelium and to monocytes. Using the ELISA method, Canas et al. [57] found that demonstration of anti-annexin II is significantly higher in patients with APS than in healthy donors and patients with SLE without thrombosis. However, sensitivity is quite low despite this fact, since anti-annexin II was demonstrated only in 25% of patients with APS.
2.2.6. Anti-Cardiolipin/Vimentin
Vimentin is a part of endothelial cells and may be present even on the surface of apoptotic neutrophils, T-lymphocytes, activated macrophages, and platelets. Vimentin and cardiolipin act on the surface of apoptotic cells as immunogens and may induce the production of antibodies. The presence aCL/Vim is strongly associated with recurrent thrombosis and pregnancy morbidity [52][58]. Ortona et al. demonstrated the presence of aCL/Vim by the ELISA method in patients with APS in 92.5%, in patients with SN-APS in 55.2%, and in patients with SLE in 43.3%. Positivity of aCL/Vim was not demonstrated in any case in a group of healthy donors [59].
2.2.7. Anti-Protein S/Protein C
The mechanism of action of anti-PS/PC is their bond to complexes of phospholipids with coagulation inhibitors protein S and protein C; this results in blocking their activity and subsequently the development of thrombosis. Anti-PS/PC is usually a frequent cause of pregnancy complications and preeclampsia. However, positivity of anti-PC/PS has lower sensitivity and also specificity for APS in comparison with aCL IgG [59].
2.2.8. Antibodies Against Phospholipid Antigens
This group of antiphospholipid antibodies includes antibodies against phosphatidic acid (anti-PA), phosphatidylserine (anti-PS), phosphatidyletanolamine (anti-PE), phosphatidylinositol (anti-PI), phosphatidylcholine (anti-PC), phosphatidylglycerol (anti-PG), lyso-bis-phosphatidic acid (anti-LBPA), and a mixture of phospholipids (APhL). Natural IgG antibodies to the above-mentioned types of lipids are ubiquitously distributed in sera of healthy humans and are believed to serve beneficial functions. Although natural antibodies to lipids generally exhibit germ line or near germ line binding specificities, the antibodies commonly increase transiently in the acute phases of most, if not all, infectious diseases and may serve as a first line of defense [60]. Some studies show that anti-PE may be a cause of fetal loss. Even anti-PS, which inhibits production of choriogonadotropin hormone (HCG), may act similarly [27][61]. Korematsu et al. [62] reported increased levels of anti-PC and anti-PE in three children with cerebral infarction. The anti-LBPA antibodies were demonstrated in a significant number of patients with APS, however, sensitivity and specificity were lower than in aCL and anti-β2GPI [63]. Castanon et al. [64] examined various aPL IgMs and IgGs in 548 serum samples using the ELISA method. Comparison of two groups of APS/healthy donors demonstrated the presence of APhL in 89.7/0%, anti-PI in 89.7/32.1%, anti-PS in 86.2/7.1%, aCL in 93.1/32.1%, and anti-β2GPI in 86.2/0%. Park et al. [65] demonstrated by line immunoassay (LIA) detection that single positivity of anti-PS (OR 16.5) and anti-PA (OR 9.6) is a better predictive factor for thrombosis than positivity of anti-β2GPI (OR 5.5).
3. Methods
Table 1 summarizes the available methods for detecting antibodies in the diagnostis of APS based on the principle and technique of the procedure.
Table 1. Overview of the methods available for the examination of biomarkers.
|
Methods |
Assay |
Determination |
|---|---|---|
|
Dilute Russell’s viper venom time (DRVVT) Activated partial thromboplastin time (aPTT) |
Liquid-phase |
Quantitative |
|
Enzyme-linked immunosorbent assay (ELISA) |
Solid-phase |
Quantitative |
|
Fluorescence enzyme immunoassay (EliA) |
Quantitative |
|
|
Chemiluminescence immunoassay (CLIA) |
Quantitative |
|
|
Multiplex flow fluorescence immunoassay (MFFIA) |
Quantitative |
|
|
Multiline dot assay (MLDA) |
Semi-quantitative |
|
|
Line immunoassay (LIA) |
Qualitative |
|
|
Thin-layer chromatography (TLC) |
Qualitative |
References
- Hughes, G.R. Thrombosis, abortion, cerebral disease, and the lupus anticoagulant. Br. Med. J. 1983, 287, 1088–1089.
- López-Pedrera, C.; Barbarroja, N.; Patiño-Trives, A.M.; Collantes, E.; Aguirre, M.A.; Perez-Sanchez, C. New Biomarkers for Atherothrombosis in Antiphospholipid Syndrome: Genomics and Epigenetics Approaches. Front. Immunol. 2019, 10, 764.
- Radic, M.; Pattanaik, D. Cellular and Molecular Mechanisms of Anti-Phospholipid Syndrome. Front. Immunol. 2018, 9, 969.
- Schreiber, K.; Sciascia, S.; de Groot, P.G.; Devreese, K.; Jacobsen, S.; Ruiz-Irastorza, G.; Salmon, J.E.; Shoenfeld, Y.; Shovman, O.; Hunt, B.J. Antiphospholipid syndrome. Nat. Rev. Dis. Primers 2018, 4, 17103.
- Abeysekera, R.A.; Wazil, A.W.M.; Nanayakkara, N.; Ratnatunga, N.V.I.; Fernando, K.M.; Thinnarachchi, J. Primary antiphospholipid syndrome presenting as antiphospholipid syndrome nephropathy: A case report. J. Med. Case Rep. 2015, 9, 28.
- Rand, J.H. The antiphospholipid syndrome. Hematol. Am. Soc. Hematol. Educ. Program 2007, 2007, 136–142.
- Meroni, P.L.; Toubi, E.; Shoenfeld, Y. Are Anti-Phospholipid Syndrome and Systemic Lupus Erythematosus Two Different Diseases? A 10-Year Late Remake. Isr. Med. Assoc. J. 2019, 21, 491–493.
- Cervera, R. Antiphospholipid syndrome. Thromb. Res. 2017, 151, S43–S47.
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; de Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre pro-spective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018.
- Garcia, D.; Erkan, D. Diagnosis and Management of the Antiphospholipid Syndrome. N. Engl. J. Med. 2018, 378, 2010–2021.
- Rodríguez-Pintó, I.; Moitinho, M.; Santacreu, I.; Shoenfeld, Y.; Erkan, D.; Espinosa, G.; Cervera, R. Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of 500 patients from the International CAPS Registry. Autoimmun. Rev. 2016, 15, 1120–1124.
- Liu, L.; Sun, D. Pregnancy outcomes in patients with primary antiphospholipid syndrome: A systematic review and meta-analysis. Medicine 2019, 98, e15733.
- Whitaker, K.L. Antiphospholipid antibody syndrome: The difficulties of diagnosis. JAAPA 2017, 30, 10–14.
- Khamashta, M.A. Management of thrombosis and pregnancy loss in the antiphospholipid syndrome. Lupus 1998, 7, S162–S165.
- Ruiz-Irastorza, G.; Crowther, M.; Branch, W.; Khamashta, M.A. Antiphospholipid syndrome. Lancet 2010, 376, 1498–1509.
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306.
- Devreese, K.M.J.; Ortel, T.L.; Pengo, V.; De Laat, B.; Antibodies, T.S.O.L.A. Laboratory criteria for antiphospholipid syndrome: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 809–813.
- Pengo, V.; Banzato, A.; Bison, E.; Bracco, A.; Denas, G.; Ruffatti, A. What have we learned about antiphospholipid syndrome from patients and antiphospholipid carrier co-horts? Semin. Thromb. Hemost. 2012, 38, 322–327.
- Pengo, V.; Ruffatti, A.; Del Ross, T.; Tonello, M.; Cuffaro, S.; Hoxha, A.; Banzato, A.; Bison, E.; Denas, G.; Bracco, A.; et al. Confirmation of initial antiphospholipid antibody positivity depends on the antiphospholipid antibody profile. J. Thromb. Haemost. 2013, 11, 1527–1531.
- Pengo, V.; Ruffatti, A.; Legnani, C.; Gresele, P.; Barcellona, D.; Erba, N.; Testa, S.; Marongiu, F.; Bison, E.; Denas, G.; et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J. Thromb. Haemost. 2010, 8, 237–242.
- Pengo, V.; Denas, G. Diagnostics and treatment of thrombotic antiphospholipid syndrome (APS): A personal perspec-tive. Thromb. Res. 2018, 169, 35–40.
- Tripodi, A. Laboratory Testing for Lupus Anticoagulants: A Review of Issues Affecting Results. Clin. Chem. 2007, 53, 1629–1635.
- Chaturvedi, S.; McCrae, K.R. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. 2017, 31, 406–417.
- Liu, T.; Gu, J.; Wan, L.; Hu, Q.; Teng, J.; Liu, H.; Cheng, X.; Ye, J.; Su, Y.; Sun, Y.; et al. “Non-criteria” antiphospholipid antibodies add value to antiphospholipid syndrome diagnoses in a large Chinese cohort. Arthritis Res. 2020, 22, 1–11.
- Choi, H.; Ahn, S.S.; Song, J.J.; Park, Y.; Song, J.; Lee, S.-W. Anti-phospholipid antibody syndrome occurrence in patients with persistent anti-phospholipid antibodies. Rheumatol. Int. 2019, 39, 1359–1367.
- De Groot, P.G.; Meijers, J.C. β(2)-Glycoprotein I: Evolution, structure and function. J. Thromb. Haemost. 2011, 9, 1275–1284.
- Misasi, R.; Capozzi, A.; Longo, A.; Recalchi, S.; Lococo, E.; Alessandri, C.; Conti, F.; Valesini, G.; Sorice, M. “New” antigenic targets and methodological approaches for refining laboratory diagnosis of antiphospho-lipid syndrome. J. Immunol. Res. 2015, 2015, 858542.
- Chayoua, W.; Kelchtermans, H.; Moore, G.W.; Musiał, J.; Wahl, D.; De Laat, B.; Devreese, K.M.J. Identification of high thrombotic risk triple-positive antiphospholipid syndrome patients is dependent on anti-cardiolipin and anti-β2glycoprotein I antibody detection assays. J. Thromb. Haemost. 2018, 16, 2016–2023.
- Decavele, A.S.; Schouwers, S.; Devreese, K.M. Evaluation of three commercial ELISA kits for anticardiolipin and anti-β2-glycoprotein I antibodies in the laboratory diagnosis of the antiphospholipid syndrome. Int. J. Lab. Hematol. 2011, 33, 97–108.
- Gebhart, J.; Posch, F.; Koder, S.; Quehenberger, P.; Perkmann, T.; Kuessel, L.; Ay, C.; Pabinger, I. High risk of adverse pregnancy outcomes in women with a persistent lupus anticoagulant. Blood Adv. 2019, 3, 769–776.
- Pierangeli, S.S.; Favaloro, E.J.; Lakos, G.; Meroni, P.L.; Tincani, A.; Wong, R.C.; Harris, E.N. Standards and reference materials for the anticardiolipin and anti-β2glycoprotein I assays: A report of recommendations from the APL Task Force at the 13th International Congress on Antiphospholipid Antibodies. Clin. Chim. Acta 2012, 413, 358–360.
- Willis, R.; Lakos, G.; Harris, E.N. Standardization of Antiphospholipid Antibody Testing—Historical Perspectives and Ongoing Initiatives. Semin. Thromb. Hemost. 2014, 40, 172–177.
- Janek, D.; Slavik, L.; Ulehlova, J.; Krcova, V.; Hlusi, A.; Prochazkova, J. Validation of a New Panel of Automated Chemiluminescence Assays for Anticardiolipin Antibodies in the Screening for Antiphospholipid Syndrome. Clin. Lab. 2016, 62, 1309–1315.
- Meroni, P.L.; Tincani, A.; Harris, E.N.; Valesini, G.; Hughes, G.R.; Balestrieri, G. The pathophysiology of anti-phospholipid antibodies. Clin. Exp. Rheumatol. 1989, 7, 81–84.
- Yin, D.; Chayoua, W.; Kelchtermans, H.; de Groot, P.G.; Moore, G.W.; Gris, J.C.; Zuily, S.; Musial, J.; de Laat, B.; Devreese, K.M.J. Detection of anti-domain I antibodies by chemiluminescence enables the identification of high-risk an-tiphospholipid syndrome patients: A multicenter multiplatform study. J. Thromb. Haemost. 2020, 18, 463–478.
- Mahler, M.; Norman, G.L.; Meroni, P.L.; Khamashta, M. Autoantibodies to domain 1 of β2 glycoprotein 1: A promising candidate biomarker for risk manage-ment in antiphospholipid syndrome. Autoimmun. Rev. 2012, 12, 313–317.
- Radin, M.; Cecchi, I.; Roccatello, D.; Meroni, P.L.; Sciascia, S. Prevalence and Thrombotic Risk Assessment of Anti-β2 Glycoprotein I Domain I Antibodies: A Systematic Review. Semin. Thromb. Hemost. 2017, 44, 466–474.
- Tonello, M.; Mattia, E.; Del Ross, T.; Favaro, M.; Calligaro, A.; Hoxha, A.; Bison, E.; Pengo, V.; Ruffatti, A. Clinical value of anti-domain I-β2Glycoprotein 1 antibodies in antiphospholipid antibody carriers. A single centre, prospective observational follow-up study. Clin. Chim. Acta 2018, 485, 74–78.
- Serrano, M.; Martinez-Flores, J.A.; Norman, G.L.; Naranjo, L.; Morales, J.M.; Serrano, A. The IgA Isotype of Anti-β2 Glycoprotein I Antibodies Recognizes Epitopes in Domains 3, 4, and 5 That Are Located in a Lateral Zone of the Molecule (L-Shaped). Front. Immunol. 2019, 10, 1031.
- Slavik, L.; Janek, D.; Ulehlova, J.; Krcova, V.; Hlusi, A. Detection of Anti-Domain I β-2 Glycoprotein I Antibodies as New Potential Target in Antiphospholipid Syndrome Diagnosis. J. Hematol. Thrombo. Dis. 2017, 5, 276.
- Pérez, D.; Tincani, A.; Serrano, M.; Shoenfeld, Y.; Serrano, A. Antiphospholipid syndrome and IgA anti-β2-glycoprotein I antibodies: When Cinderella becomes a princess. Lupus 2017, 27, 177–178.
- Morales, J.M.; Serrano, M.; Martinez-Flores, J.A.; Gainza, F.J.; Marcen, R.; Arias, M.; Escuin, F.; Pérez, D.; Andres, A.; Martínez, M.A.; et al. Pretransplant IgA-Anti-Beta 2 Glycoprotein I Antibodies as a Predictor of Early Graft Thrombosis after Renal Transplantation in the Clinical Practice: A Multicenter and Prospective Study. Front. Immunol. 2018, 9, 468.
- Pericleous, C.; Ferreira, I.; Borghi, O.; Pregnolato, F.; McDonnell, T.; Garza-Garcia, A.; Driscoll, P.; Pierangeli, S.; Isenberg, D.; Ioannou, Y.; et al. Measuring IgA Anti-β2-Glycoprotein I and IgG/IgA Anti-Domain I Antibodies Adds Value to Current Serological Assays for the Antiphospholipid Syndrome. PLoS ONE 2016, 11, e0156407.
- Ruiz-García, R.; Serrano, M.; Martínez-Flores, J.Á.; Mora, S.; Morillas, L.; Martín-Mola, M.Á.; Morales, J.M.; Paz-Artal, E.; Serrano, A. Isolated IgA Anti-β2 Glycoprotein I Antibodies in Patients with Clinical Criteria for Antiphospholipid Syndrome. J. Immunol. Res. 2014, 2014, 1–8.
- Vlagea, A.; Pascual-Salcedo, D.; Doforno, R.Á.; Lavilla, P.; Diez, J.; Merlano, B.P.; Cuesta, M.V.; Gil, A. IgA anti-β2 glycoprotein I antibodies: Experience from a large center. Thromb. Res. 2018, 162, 38–43.
- Chayoua, W.; Yin, D.-M.; Kelchtermans, H.; Moore, G.W.; Gris, J.-C.; Musiał, J.; Zuily, S.; Cate, H.T.; De Laat, B.; Devreese, K.M.J. Is There an Additional Value in Detecting Anticardiolipin and Anti-β2 glycoprotein I IgA Antibodies in the Antiphospholipid Syndrome? Thromb. Haemost. 2020, 120, 1557–1568.
- Devreese, K.M.J. Testing for antiphospholipid antibodies: Advances and best practices. Int. J. Lab. Hematol. 2020, 42, 49–58.
- Bizzaro, N.; Ghirardello, A.; Zampieri, S.; Iaccarino, L.; Tozzoli, R.; Ruffatti, A.; Villalta, D.; Tonutti, E.; Doria, A. Anti-prothrombin antibodies predict thrombosis in patients with systemic lupus erythematosus: A 15-year longitudinal study. J. Thromb. Haemost. 2007, 5, 1158–1164.
- Sciascia, S.; Sanna, G.; Murru, V.; Roccatello, D.; Khamashta, M.A.; Bertolaccini, M.L. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. Thromb. Haemost. 2014, 111, 354–364.
- Cifu’, A.; Domenis, R.; Pistis, C.; Curcio, F.; Fabris, M. Anti-β2-glycoprotein I and anti-phosphatidylserine/prothrombin antibodies exert similar pro-thrombotic effects in peripheral blood monocytes and endothelial cells. Autoimmun. Highlights 2019, 10, 1–8.
- Sciascia, S.; Radin, M.; Sanna, G.; Cecchi, I.; Roccatello, D.; Bertolaccini, M.L. Clinical utility of the global anti-phospholipid syndrome score for risk stratification: A pooled analysis. Rheumatology 2018, 57, 661–665.
- Conti, F.; Capozzi, A.; Truglia, S.; Lococo, E.; Longo, A.; Misasi, R.; Alessandri, C.; Valesini, G.; Sorice, M. The mosaic of “seronegative” antiphospholipid syndrome. J. Immunol. Res. 2014, 2014, 389601.
- Ho, W.K.; Rigano, J. Prevalence of autoantibodies directed against prothrombin in unprovoked venous thromboembo-lism. J. Thromb. Thrombolysis 2020, 49, 446–450.
- Shi, H.; Zheng, H.; Yin, Y.; Hu, Q.; Teng, J.; Sun, Y.; Liu, H.-L.; Cheng, X.; Ye, J.; Su, Y.; et al. Antiphosphatidylserine/prothrombin antibodies (aPS/PT) as potential diagnostic markers and risk predictors of venous thrombosis and obstetric complications in antiphospholipid syndrome. Clin. Chem. Lab. Med. 2017, 56, 614–624.
- Rand, J.H. Antiphospholipid Antibody-mediated Disruption of the Annexin-V Antithrombotic Shield: A Thrombogenic Mechanism for the Antiphospholipid Syndrome. J. Autoimmun. 2000, 15, 107–111.
- Bertolaccini, M.L.; Amengual, O.; Atsumi, T.; Binder, W.L.; de Laat, B.; Forastiero, R.; Kutteh, W.H.; Lambert, M.; Matsubayashi, H.; Murthy, V.; et al. ‘Non-criteria’ aPL tests: Report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus 2011, 20, 191–205.
- Cañas, F.; Simonin, L.; Couturaud, F.; Renaudineau, Y. Annexin A2 autoantibodies in thrombosis and autoimmune diseases. Thromb. Res. 2015, 135, 226–230.
- Ortona, E.; Capozzi, A.; Colasanti, T.; Conti, F.; Alessandri, C.; Longo, A.; Garofalo, T.; Margutti, P.; Misasi, R.; Khamashta, M.A.; et al. Vimentin/cardiolipin complex as a new antigenic target of the antiphospholipid syndrome. Blood 2010, 116, 2960–2967.
- Arachchillage, D.R.J.; Efthymiou, M.; Mackie, I.J.; Lawrie, A.S.; Machin, S.J.; Cohen, H. Anti-protein C antibodies are associated with resistance to endogenous protein C activation and a severe thrombotic phenotype in antiphospholipid syndrome. J. Thromb. Haemost. 2014, 12, 1801–1809.
- Matyas, G.R.; Alving, C.R. Antigen-specific enhancement of natural human IgG antibodies to phosphatidylcholine, phosphatidylglycerol, phosphatidylinositol-4-phosphate, cholesterol, and lipid A by a liposomal vaccine containing lipid A. Vaccine 2011, 29, 5137–5144.
- Pignatelli, P.; Ettorre, E.; Menichelli, D.; Pani, A.; Violi, F.; Pastori, D. Seronegative antiphospholipid syndrome: Refining the value of “non-criteria” antibodies for di-agnosis and clinical management. Haematologica 2020, 105, 562–572.
- Korematsu, S.; Yamada, H.; Miyahara, H.; Ihara, K. Increased levels of anti-phosphatidylcholine and anti-phosphatidylethanolamine antibodies in pediatric patients with cerebral infarction. Brain Dev. 2017, 39, 542–546.
- Alessandri, C.; Bombardieri, M.; Di Prospero, L.; Conigliaro, P.; Conti, F.; Labbadia, G.; Misasi, R.; Sorice, M.; Valesini, G. Anti-lysobisphosphatidic acid antibodies in patients with antiphospholipid syndrome and systemic lupus erythematosus. Clin. Exp. Immunol. 2005, 140, 173–180.
- Castanon, A.; Pierre, G.; Willis, R.; Harris, E.N.; Papalardo, E.; Romay-Penabad, Z.; Schleh, A.; Jajoria, P.; Smikle, M.; DeCeulaer, K.; et al. Performance Evaluation and Clinical Associations of Immunoassays That Detect Antibodies to Nega-tively Charged Phospholipids Other Than Cardiolipin. Am. J. Clin. Pathol. 2018, 149, 401–411.
- Park, H.S.; Gu, J.Y.; Jung, H.S.; Kim, H.K. Thrombotic Risk of Non-Criteria Anti-Phospholipid Antibodies Measured by Line Immunoassay: Superi-ority of Anti-Phosphatidylserine and Anti-Phosphatidic Acid Antibodies. Clin. Lab. 2019, 65, 171207.




