
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hideaki Kaneto | + 1636 word(s) | 1636 | 2021-03-09 07:36:31 | | | |
| 2 | Vivi Li | Meta information modification | 1636 | 2021-03-09 08:43:21 | | |
Video Upload Options
While there are various kinds of drugs for type 2 diabetes mellitus at present, in this review article, we focus on metformin which is an insulin sensitizer and is often used as a first-choice drug worldwide. Metformin mainly activates adenosine monophosphate-activated protein kinase (AMPK) in the liver which leads to suppression of fatty acid synthesis and gluconeogenesis. Metformin activates AMPK in skeletal muscle as well, which increases translocation of glucose transporter 4 to the cell membrane and thereby increases glucose uptake. Further, metformin suppresses glucagon signaling in the liver by suppressing adenylate cyclase which leads to suppression of gluconeogenesis. In addition, metformin reduces autophagy failure observed in pancreatic β-cells under diabetic conditions. Furthermore, it is known that metformin alters the gut microbiome and facilitates the transport of glucose from the circulation into excrement. It is also known that metformin reduces food intake and lowers body weight by increasing circulating levels of the peptide hormone growth/differentiation factor 15 (GDF15). Furthermore, much attention has been drawn to the fact that the frequency of various cancers is lower in subjects taking metformin. Metformin suppresses the mechanistic target of rapamycin (mTOR) by activating AMPK in pre-neoplastic cells, which leads to suppression of cell growth and an increase in apoptosis in pre-neoplastic cells. It has been shown recently that metformin consumption potentially influences the mortality in patients with type 2 diabetes mellitus and coronavirus infectious disease (COVID-19). Taken together, metformin is an old drug, but multifaceted mechanisms of action of metformin have been unraveled one after another in its long history.
1. Introduction
Pancreatic β-cell dysfunction and insulin resistance in insulin target tissues such as the liver, skeletal muscle and adipose tissues are the two main characteristics of type 2 diabetes mellitus. The number of subjects with type 2 diabetes mellitus is markedly increasing all over the world due to changes in lifestyle such as overeating and lack of exercise. Such an increase in subjects with type 2 diabetes has become a financial burden in many countries. So far, various kinds of drugs for type 2 diabetes mellitus have been developed, and at present, there are many kinds of anti-diabetic drugs from which we can choose depending on each patient’s pathophysiological conditions. Incretin-related drugs (dipeptidyl peptidase-IV (DPP-IV) inhibitors and glucagon-like peptide-1 receptor activators (GLP-1RA)) and sodium-glucose cotransporter 2 (SGLT2) inhibitors are relatively new drugs and have been drawing much attention in various aspects. In contrast, metformin is an old drug, but its pleiotropic mechanisms of action have been gradually clarified in its long history. There were times when the reputation of metformin was not very high, but due to various discoveries about new mechanisms of action of metformin, the Association for the Study of Diabetes (the American Diabetes Association and the European Association for the Study of Diabetes) consensus guideline on the management of type 2 diabetes stipulates that metformin should be used as a first-choice drug for type 2 diabetes mellitus. Indeed, it is very often used as a first-choice drug in clinical practice all over the world. In addition, since metformin is quite cheap compared to other anti-diabetic drugs, usage of metformin reduces the financial burden on subjects with type 2 diabetes mellitus. In this review article, we focus on metformin which is an old but marvelous drug.
2. Metformin Activates Adenosine Monophosphate-Activated Protein Kinase (AMPK) in the Liver and Skeletal Muscle Which Leads to Suppression of Gluconeogenesis in the Liver and Increase in Glucose Uptake into Skeletal Muscle
Metformin enhances insulin sensitivity and ameliorates glycemic control mainly through a reduction in hepatic glucose production and enhancement of glucose utilization. AMPK is one of the major cellular regulators for glucose and lipid metabolism. It was reported that metformin activated AMPK in the liver, leading to a reduction in acetyl-CoA carboxylase (ACC), enhancement of fatty acid oxidation and suppression of lipogenic enzyme expression [1][2][3][4]. Metformin-mediated AMPK activation suppresses expression of sterol regulatory element binding protein-1c (SREBP-1), an important lipogenic transcription factor, leading to suppression of fatty acid synthesis (Figure 1). Further, while phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase) are key gluconeogenic enzymes, metformin-mediated AMPK activation reduces both enzymes’ expression, leading to suppression of gluconeogenesis in the liver. Metformin also activates AMPK in skeletal muscle which increases translocation of glucose transporter 4 to the cell membrane and thereby increases glucose uptake. These effects finally ameliorate fatty liver and insulin resistance. It was reported recently that metformin inhibited mitochondrial respiratory complex I, leading to an increase in the ratio of adenosine monophosphate (AMP) to adenosine triphosphate (ATP). Such alteration likely leads to inactivation of AMPK [2]. It was also reported that metformin inactivated mitochondrial glycerol-3-phosphate dehydrogenase which was likely involved in suppression of gluconeogenesis in the liver [3].
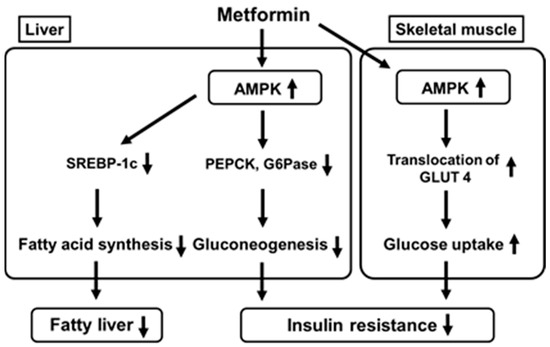
Figure 1. Metformin activates AMP-activated protein kinase (AMPK) in the liver which leads to suppression of fatty acid synthesis and gluconeogenesis. Metformin also activates AMPK in skeletal muscle which increases translocation of glucose transporter 4 to the cell membrane and thereby increases glucose uptake. SREBP-1c, sterol regulatory element binding protein-1c; PEPCK, phosphoenolpyruvate carboxykinase; GAPase, glucose 6-phosphatase; GLUT 4, glucose transporter 4.
3. Metformin Suppresses Glucagon Signaling in the Liver by Suppressing Adenylate Cyclase Which Leads to Suppression of Gluconeogenesis in the Liver
Glucagon is secreted from pancreatic α-cells and functions as one of the counter-regulatory hormones, leading to an increase in blood glucose levels. As one main mechanism of glucagon action, it is known that glucagon binds to the glucagon receptor in the liver, which activates adenylate cyclase and converts adenosine triphosphate (ATP) to cyclic AMP (cAMP). Increased cAMP activates protein kinase A (PKA), which facilitates gluconeogenesis. Thereby, glucagon leads to the aggravation of glycemic control. It was reported that metformin antagonized such action of glucagon, which led to amelioration of glycemic control. Metformin treatment led to the accumulation of AMP, which finally inhibited adenylate cyclase. Inactivation of adenylate cyclase reduced cyclic AMP levels and PKA activity and suppressed glucagon signaling, leading to suppression of gluconeogenesis (Figure 2) [5]. These findings clearly support the new mechanism of action of metformin as a suppressor of glucagon signaling in the liver. In addition, it was reported recently that metformin inhibited mitochondrial respiratory complex I, leading to an increase in the AMP/ATP ratio. Such alteration likely inactivates adenylate cyclase activity, leading to suppression of glucagon signaling in the liver [2]. Thereby, such inhibition of mitochondrial respiratory complex I suppresses gluconeogenesis through activation of AMPK, as well as suppressing glucagon signaling through inactivation of adenylate cyclase activity. Such alteration leads to amelioration of glucose metabolism and a reduction in insulin resistance in the liver, which finally leads to amelioration of glycemic control.
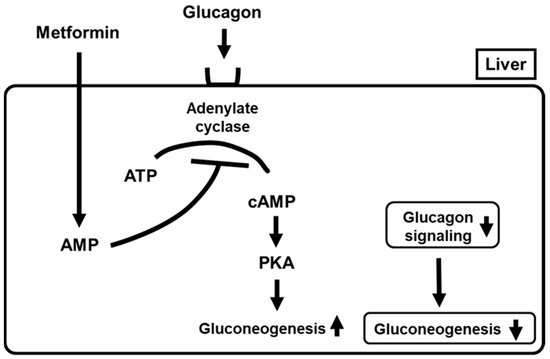
Figure 2. Metformin suppresses glucagon signaling in the liver by suppressing adenylate cyclase which leads to suppression of gluconeogenesis. PKA, protein kinase A.
4. Metformin Suppresses Mechanistic Target of Rapamycin (Mtor) by Activating AMPK in Pre-Neoplastic Cells and Thereby Suppresses the Onset and/or Development of Various Cancers
Much attention has been drawn recently to the fact that the frequency of various types of cancer in subjects with diabetes mellitus is higher compared to that in healthy subjects [6][7][8][9][10][11][12][13]. In particular, the frequency of hepatocellular carcinoma and colorectal cancer is higher under diabetic conditions compared to healthy conditions. Thus, malignancy has been recently regarded as one of diabetic complications in addition to acute and chronic complications such as microangiopathies (diabetic nephropathy, retinopathy and neuropathy) and macroangiopathies (ischemic heart diseases, stroke and arteriosclerosis obliterans). There are several possible reasons why the frequency of malignancy is increased under diabetic conditions. First, chronic hyperglycemia increases various inflammatory cytokines and thereby activates nuclear factor-kappa B (NF-kB) and/or signal transducer and activator of transcription 3 (STAT3), which finally leads to the onset of neoplastic cells. Second, hyperinsulinemia, which is often observed in obese subjects with type 2 diabetes mellitus, activates insulin receptors in pre-neoplastic cells, leading to the onset of neoplastic cells. In addition, hyperinsulinemia decreases expression of insulin-like growth factor binding proteins 1 and 2 (IGFBP1 and IGFBP2) and thereby activates insulin-like growth factor-1 (IGF-1), which could also lead to the onset of neoplastic cells.
Furthermore, attention has been drawn to the fact that the frequency of various cancers is lower in subjects taking metformin. Indeed, there is a large amount of clinical evidence showing the possibility that usage of metformin decreases the risk of neoplastic transformation and enhances the response to some chemotherapies [14][15][16][17][18][19][20]. Metformin suppresses mTOR by activating AMPK in pre-neoplastic cells which leads to suppression of cell growth and an increase in apoptosis in pre-neoplastic cells (Figure 3) [14][15]. It seems that metformin exerts potential anti-tumorigenic effects independently of its hypoglycemic effects. In general, insulin and IGF-1 activate PI3-K, Akt and mTOR, which finally leads to enhancement of cell growth and suppression of apoptotic cell death in pre-neoplastic cells. There are several potential mechanisms concerning how metformin can suppress the development of neoplastic cells. First, metformin activates the AMPK pathway in pre-neoplastic cells which leads to suppression of mTOR activation. Such a pathway finally leads to suppression of cell growth and an increase in apoptosis in pre-neoplastic cells. Second, since metformin is an insulin sensitizer, it reduces circulating insulin levels, which is also, at least in part, involved in the anti-tumorigenic effects of metformin. Inhibition of protein synthesis, inhibition of the unfolded protein response (UPR), activation of the immune system and eradication of cancer stem cells are also possibly involved in the anti-tumorigenic effects of metformin.
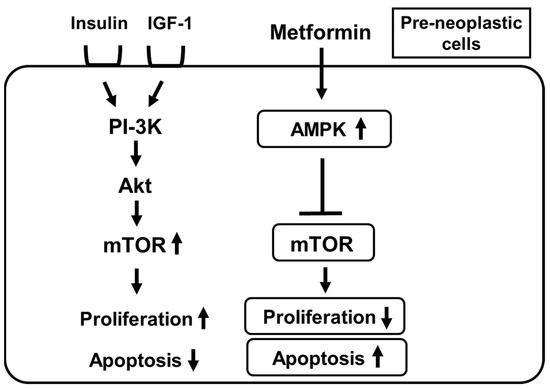
Figure 3. Metformin suppresses the mechanistic target of rapamycin (mTOR) by activating AMPK in pre-neoplastic cells which leads to suppression of cell growth and an increase in apoptosis in pre-neoplastic cells. IGF-1, insulin-like growth factor; PI-3K, phosphatidylinositol-3 kinase; Akt, protein kinase B.
Taken together, while the frequency of various types of malignancies in subjects with diabetes mellitus is higher compared to that in healthy subjects, much attention has been drawn to the fact that the frequency of various cancers is lower in subjects taking metformin.
References
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174.
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585.
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546.
- Minamii, T.; Nogami, M.; Ogawa, W. Mechanisms of metformin action: In and out of the gut. J. Diabetes Investig. 2018, 9, 701–703.
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260.
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004, 4, 579–591.
- Mayor, S. High glucose and diabetes increase cancer risk. Lancet Oncol. 2005, 6, 71.
- Noto, H.; Goto, S.; Tsujimoto, T.; Noda, M. Cancer risk in diabetic patients treated with metformin: A systematic review and meta-analysis. PLoS ONE 2012, 7, e33411.
- Noto, H.; Goto, A.; Tsujimoto, T.; Osame, K.; Noda, M. Latest insights into the risk of cancer in diabetes. J. Diabetes. Investig. 2013, 4, 225–232.
- Walker, J.J.; Johnson, J.A.; Wild, S.H. Diabetes treatments and cancer risk: The importance of considering aspects of drug exposure. Lancet Diabetes Endocrinol. 2013, 1, 132–139.
- Shi, Y.; Hu, F.B. The global implications of diabetes and cancer. Lancet 2014, 383, 1947–1948.
- Rahman, A. Type 2 diabetes and risk of pancreatic adenocarcinoma. Lancet Oncol. 2014, 15, e420.
- Gregg, E.W.; Cheng, Y.J.; Srinivasan, M.; Lin, J.; Geiss, L.S.; Albright, A.L.; Imperatore, G. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: An epidemiological analysis of linked national survey and vital statistics data. Lancet 2018, 391, 2430–2440.
- Dowling, R.J.; Zakikhani, M.; Fantus, I.G.; Pollak, M.; Sonenberg, N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007, 67, 10804–10812.
- Kourelis, T.V.; Siegel, R.D. Metformin and cancer: New application for an old drug. Med. Oncol. 2012, 29, 1314–1327.
- Chan, A.T. Metformin for cancer prevention: A reason for optimism. Lancet Oncol. 2016, 17, 407–409.
- Demb, J.; Yaseyyedi, A.; Liu, L.; Bustamante, R.; Earles, A.; Ghosh, P.; Gutkind, J.S.; Gawron, A.J.; Kaltenbach, T.R.; Martinez, M.E.; et al. Metformin is associated with reduced odds for colorectal cancer among persons with dDiabetes. Clin. Trans. Gastroenterol. 2019, 10, e00092.
- Shi, Y.Q.; Zhou, X.C.; Du, P.; Yin, M.Y.; Xu, L.; Chen, W.J.; Xu, C.F. Relationships are between metformin use and survival in pancreatic cancer patients concurrent with diabetes: A systematic review and meta-analysis. Medicine 2020, 99, e21687.
- Kim, Y.S.; Choi, E.A.; Lee, J.W.; Kim, Y.; You, H.S.; Han, Y.E.; Kim, H.S.; Bae, Y.J.; Kang, H.T.; Kim, J. Metformin use reduced the overall risk of cancer in diabetic patients: A study based on the Korean NHIS-HEALS cohort. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1714–1722.
- Lee, J.-W.; Choi, E.-A.; Kim, Y.-S.; Kim, Y.; You, H.-S.; Han, Y.-E.; Kim, H.-P.; Bae, Y.-J.; Kim, J.; Kang, H.-T. Metformin usage and the risk of colorectal cancer: A national cohort study. Int. J. Colorectal Dis. 2021, 36, 303–310.




