
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Janette Iking | + 1417 word(s) | 1417 | 2021-03-02 10:18:19 | | | |
| 2 | Vicky Zhou | Meta information modification | 1417 | 2021-03-03 10:18:39 | | |
Video Upload Options
Inflammation Imaging means using imaging to provide insights into individual and temporospatial biology and grade of inflammation which can be of diagnostic, therapeutic, and prognostic value.
1. Introduction
Inflammation is a fundamental and well-balanced physiological process necessary for wound healing, protection against pathogens, and tissue homeostasis. Restrained or excessive inflammation, however, can have detrimental effects leading to pathological alterations that can worsen the outcome of patients or even form the basis of the disease itself. Consequently, the immune system and its response to pathological changes play a major role in virtually all diseases ranging from bacterial or viral infectious diseases, neurological disorders, cancer, autoimmune diseases, and cardiovascular diseases.
The adaptability of the human immune system is one of the reasons why it can react effectively and rapidly against pathogens; at the same time, it may render many novel therapies targeting inflammation or involving the immune system effective in some patients whereas other patients with the same condition do not respond at all. Accordingly, the immune response is being understood as a very individual process that demands customized therapies. Because inflammation is a very dynamic process that involves many immune cell subtypes, it can be challenging to identify the appropriate molecular target and timing for optimal intervention. In this context, molecular imaging has emerged as a helpful research tool to non-invasively visualize and study inflammation in vivo in a variety of diseases especially in a preclinical setting. However, molecular imaging may also provide insight into the individual biology of inflammation which can have diagnostic, therapeutic, and prognostic value for patients.
In recent years, many novel radiotracers and newly developed protocols for inflammation imaging have been particularly applied in the field of nuclear cardiology. Special emphasis is put on tracers that have already been successfully applied in the clinics (Table 1).
Table 1. Overview of radiotracers and their molecular targets for PET inflammation imaging.
| Target | PET Radiotracer | Cell Types Targeted by the Radiotracer | Evaluated Diseases | Advantages | Limitations | Approved for Use in Humans? |
|---|---|---|---|---|---|---|
| Glucose metabolism (predominantly glucose transporter 1 and 3 (GLUT1-3)) | 18F-FDG | High-glucose-using cells such as immune cells, cancer cells, cardiomyocytes, neurons, brown adipocytes, kidney cells | Myocardial infarction [1], cancer [2], atherosclerosis [3], sarcoidosis [4], endocarditis [5], IgG4-rel. diseases [6], arthritis [7], infection and others [8] | High sensitivity, fast technique completed in one session, broad availability [8] | High background signal, often need for non- physiological suppression techniques, not inflammation-specific, limited use in some clinical settings | Yes |
| Mannose receptor | 18F-FDM, 68Ga-NOTA-MSA, 68Ga-NOTA-anti-CD206 nanobody | Mainly expressed by macrophages (M2 > M1), immature dendritic cells, and liver sinusoidal endothelial cells | Mainly atherosclerosis [9][10][11] and cancer [12] | Higher cell specificity than 18F-FDG (M2 > M1 macrophages) | Correlation of mannose-directed PET signals with histology of leukocytes and distinction from 18F-FDG signal remains to be determined | No |
| Somatostatin receptors (SSTR) | 68Ga-DOTATOC, 68Ga- DOTATATE, 68Ga- DOTANOC | Overexpressed mainly on pro-inflammatory M1 macrophages | Atherosclerosis [13][14], sarcoidosis [15][16][17], other sources of myocardial inflammation (i.e., pericarditis, myocarditis, MI) [18], and others [19] (i.e., idiopathic pulmonary fibrosis, histiocytosis, tuberculosis, cardiac allograft rejection, and small vessel vasculitis) | higher cell specificity and improved signal-to-background-ratio of DOTA-peptides compared to 18F-FDG imaging (in particular advantageous for cardiac inflammation imaging) | Often labelled with gallium-68 (need for on-site generator) | Yes |
| C-X-C motif chemokine receptor 4 (CXCR4) | 68Ga-pentixafor, 64Cu-DOTA-FC131 |
Expressed on several pro-inflammatory immune cells, particularly overexpressed on macrophages and T cells | Cancer [20], atherosclerosis [21][22][23][24][25], myocardial infarction [26][27][28][29][30][31], osteomyelitis [32], urinary tract infections [33] and others | Potential theranostic target in atherosclerosis and MI; superiority over 18F-FDG in atherosclerosis; superior in chronic bone infections over granulocyte-directed 99mTc-besilesomab and 99mTc-labelled leukocytes | Not yet clinically approved, larger clinical trials needed to determine prognostic and diagnostic value in different inflammatory conditions; unspecific cellular source as various inflammatory cells express CXCR4 | No; several early phase I clinical trials for cancer imaging ongoing (i.e., NCT04504526) |
| C-C motif chemokine receptor 2 (CCR2) | 68Ga/64Cu-DOTA-ECL1i | Mainly expressed on pro-inflammatory monocytes, natural killer cells and T cells | Lung inflammation [34], cardiac injury [35], abdominal aortic aneurysm [36], pulmonary fibrosis [37] | Promising results regarding prognostic and therapy-monitoring abilities | unspecific cellular source as various inflammatory cells express CCR2; toxicity and biodistribution still need to be examined for a safe translation into the clinics | No; several phase I clinical trials ongoing, i.e., for imaging atherosclerosis (NCT04537403) and lung inflammation (NCT03492762) |
| Mitochondrial translocator protein (TSPO) | 11C-PK11195 and 2nd and 3rd generation TSPO tracers, such as 18F-flutriciclamide (18F-GE180) or 18F-DPA-714 |
Protein located in the outer mitochondrial membrane; upregulated in activated macrophages, particularly in microglia | Myocardial infarction [38], atherosclerosis [39], vascular inflammation [40][41], rheumatoid arthritis [42][43][44] | ability to visualize peripheral and central inflammatory networks; superiority over MRI regarding detection of subclinical synovitis | Limited use in detection of peripheral inflammation; multicellular receptor expression profile; presence of radiolabelled metabolites; variability between individuals regarding tracer binding affinity due to TSPO polymorphisms | No; several clinical trials are ongoing especially in the field of neuroinflammation (NCT03457493, NCT04412187, NCT03662750 and others) |
| αvβ3 integrin receptor | 18F-galacto-RGD, 68Ga-PRGD2, 18F-fluciclatide | Mediates cell adhesion; important role in angiogenesis, expressed on a variety of cells such as activated endothelial cells, solid tumor cells, immune cells | Atherosclerosis [45][46], myocardial infarction [47][48][49], rheumatoid arthritis [50] | Superiority over 18F-FDG regarding evaluation of disease severity in rheumatoid arthritis (68Ga-PRGD2) | Not yet clinically approved, larger clinical trials needed to determine prognostic and diagnostic value in different inflammatory conditions; unspecific cellular source as various cell types express integrins | No; clinical trials have been conducted in rheumatoid arthritis (NCT01940926) and MI (NCT01813045) |
| Folate receptor (FR) (in particular the beta isoform (FR-β)) | 18F-Fluoro-PEG-folate;18F-AzaFol; 68Ga-Ga-NOTA-folate (68Ga-FOL) | High expression on cancer cells and activated M1-macrophages (and monocytes) with restricted FR expression in normal tissues | Rheumatoid arthritis [51][52][53][54][55], myocarditis [56], atherosclerosis [57], interstitial lung disease [58] | Important transport route for methotrexate making it an interesting target for rheumatoid arthritis; better target-to-background-ratio of 18F-fluoro-PEG-folate as compared to 11C-PK11195; significantly higher plaque-to-healthy vessel wall ratio of 68Ga-FOL as compared to 18F-FDG PET | Not yet clinically approved, larger clinical trials needed to determine prognostic and diagnostic value in different inflammatory conditions; | No; clinical trial for 18F-AzaFol in cancer imaging has been conducted (NCT03242993) |
| Fibroblast activation protein-α (FAP) | Various, mainly 68Ga-labelled FAP inhibitors such as 68Ga-FAPI-04; labelled antibodies directed to FAP | Fibroblasts and tumor cells | Cancer [59][60], rheumatoid arthritis [61][62][63], IgG4-related disease [64][65], myocardial infarction [66][67] | Excellent contrast due to due to low FAP expression in physiological tissues; theranostic properties since mainly FAP inhibitors are used; superiority over 18F-FDG in IgG4-rel. disease | Further studies are warranted to assess the prognostic and theranostic value | No; several clinical trials are ongoing, i.e., for rheumatoid arthritis(NCT04514614), IgG4-rel. disease (NCT04125511) and inflammatory bowel disease (NCT04507932) |
2. Imaging Inflammation with Positron Emission Tomography
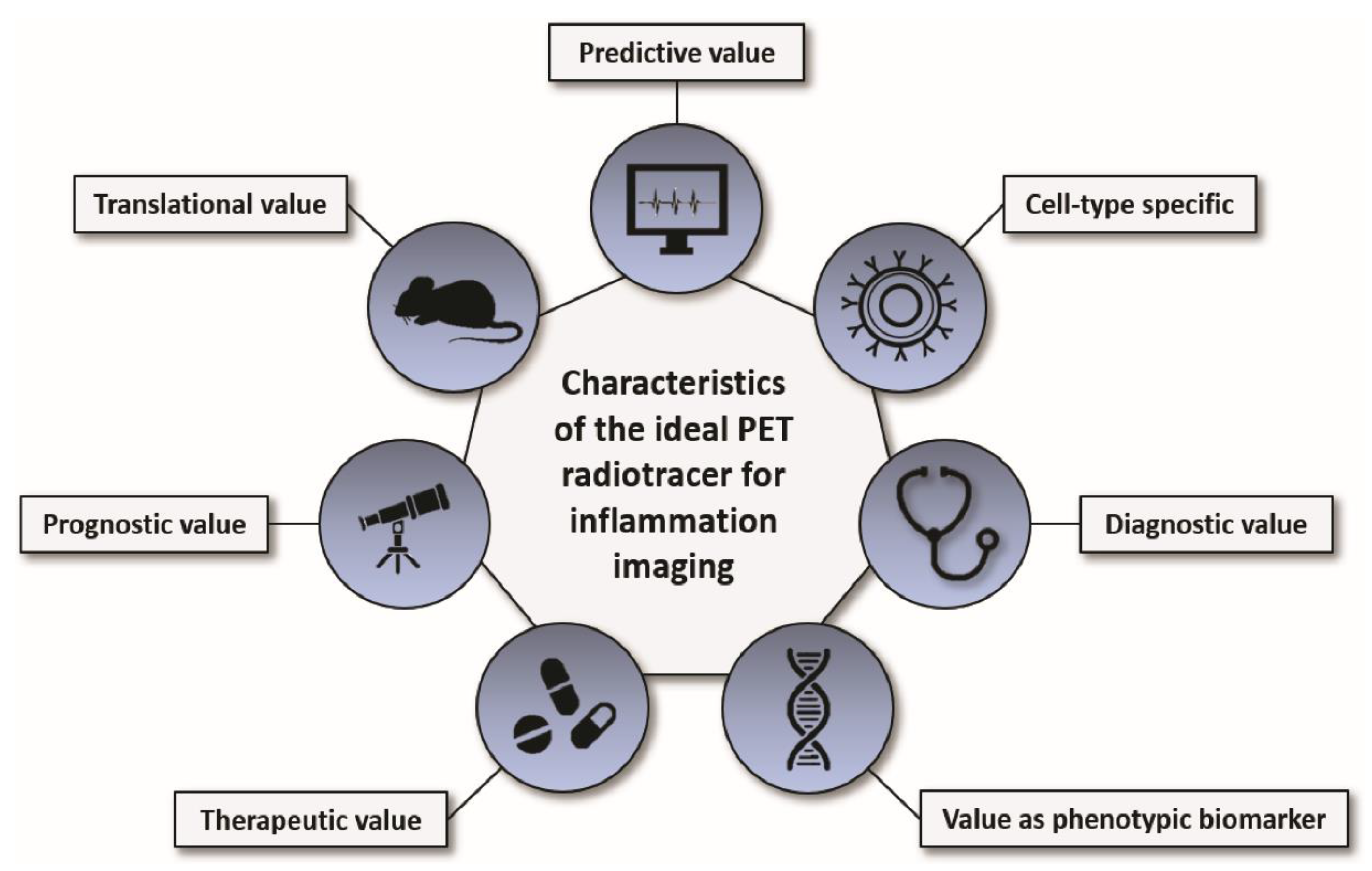
Inflammation plays a fundamental role in many medical conditions, but restrained or excessive inflammation can have detrimental effects that can worsen the outcome of patients. Molecular imaging of inflammation has emerged as a helpful tool to non-invasively visualize and study inflammation in vivo in a variety of diseases; it shows value as a strong clinical and preclinical research application and may provide insight into the individual biology of inflammation which can have diagnostic, therapeutic, and prognostic value. The perfect PET radiotracer for inflammation imaging has an excellent predictive value, is cell-type specific, shows a good target-to-background ratio (diagnostic value), has a value as phenotypic biomarker, responds to anti-inflammatory therapy (therapeutic value), has a good correlation with the functional outcome and/or progression of the disease (prognostic value), and is safe for its translation into patients (translational value; Figure 1). Despite promising preclinical and clinical results, none of the herein discussed radiotracers unites all of these desired characteristics, and several obstacles still need to be overcome to establish inflammation imaging in a routine clinical setting and for validated research. Improvement of PET radiotracers for imaging inflammation, accurate and standardized quantification of radiotracer uptake for interpretation and comparability of the results, comparable and reproducible imaging protocols and guidelines, further improvement of spatial resolution of PET devices (particularly important for inflammation imaging of small structures such as vessels), and a broader access to PET imaging facilities for physicians from different medical fields are just a few of the challenges that the community needs to address in the near future. Nonetheless, PET inflammation imaging may provide insight into the individual biology of inflammation which can be of great diagnostic, therapeutic, and prognostic value for patients.
References
- Rischpler, C.; Dirschinger, R.J.; Nekolla, S.G.; Kossmann, H.; Nicolosi, S.; Hanus, F.; Van Marwick, S.; Kunze, K.P.; Meinicke, A.; Gotze, K.; et al. Prospective Evaluation of 18F-Fluorodeoxyglucose Uptake in Postischemic Myocardium by Simultaneous Positron Emission Tomography/Magnetic Resonance Imaging as a Prognostic Marker of Functional Outcome. Circ. Cardiovasc. Imaging 2016, 9.
- Ferdinandus, J.; Barbato, F.; Chodyla, M.; Fendler, W.P.; Kessler, L.; Pomykala, K.L.; Metzenmacher, M.; Krefting, F.; Hager, T.; Umutlu, L.; et al. Volumetric PET response assessment outperforms conventional criteria in patients receiving high-dose pembrolizumab for malignant mesothelioma. J. Nucl. Med. 2021, 62, 191–194.
- Wenning, C.; Kloth, C.; Kuhlmann, M.T.; Jacobs, A.H.; Schober, O.; Hermann, S.; Schäfers, M.A. Serial F-18-FDG PET/CT distinguishes inflamed from stable plaque phenotypes in shear-stress induced murine atherosclerosis. Atherosclerosis 2014, 234, 276–282.
- Ahmadian, A.; Pawar, S.; Govender, P.; Berman, J.; Ruberg, F.L.; Miller, E.J. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J. Nucl. Cardiol. 2017, 24, 413–424.
- Pizzi, M.N.; Roque, A.; Fernández-Hidalgo, N.; Cuéllar-Calabria, H.; Ferreira-González, I.; Gonzàlez-Alujas, M.T.; Oristrell, G.; Gracia-Sánchez, L.; González, J.J.; Rodríguez-Palomares, J.; et al. Improving the Diagnosis of Infective Endocarditis in Prosthetic Valves and Intracardiac Devices with 18F-Fluordeoxyglucose Positron Emission Tomography/Computed Tomography Angiography: Initial Results at an Infective Endocarditis Referral Center. Circulation 2015, 132, 1113–1126.
- Luo, Y.; Pan, Q.; Zhang, W. IgG4-related disease revealed by 68Ga-FAPI and 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2625–2626.
- Watanabe, T.; Takase-Minegishi, K.; Ihata, A.; Kunishita, Y.; Kishimoto, D.; Kamiyama, R.; Hama, M.; Yoshimi, R.; Kirino, Y.; Asami, Y.; et al. 18F-FDG and 18F-NaF PET/CT demonstrate coupling of inflammation and accelerated bone turnover in rheumatoid arthritis. Mod. Rheumatol. 2016, 26, 180–187.
- Hess, S.; Hansson, S.H.; Pedersen, K.T.; Basu, S.; Høilund-Carlsen, P.F. FDG-PET/CT in infectious and inflammatory diseases. PET Clin. 2014, 9, 497–519.
- Tahara, N.; Mukherjee, J.; De Haas, H.J.; Petrov, A.D.; Tawakol, A.; Haider, N.; Tahara, A.; Constantinescu, C.C.; Zhou, J.; Boersma, H.H.; et al. 2-deoxy-2-[18F]fluoro-d-mannose positron emission tomography imaging in atherosclerosis. Nat. Med. 2014, 20, 215–219.
- Lee, S.P.; Im, H.J.; Kang, S.; Chung, S.J.; Cho, Y.S.; Kang, H.; Park, H.S.; Hwang, D.W.; Park, J.B.; Paeng, J.C.; et al. Noninvasive imaging of myocardial inflammation in myocarditis using 68Ga-tagged mannosylated human serum albumin positron emission tomography. Theranostics 2017, 7, 413–424.
- Kim, E.J.; Kim, S.; Seo, H.S.; Lee, Y.J.; Eo, J.S.; Jeong, J.M.; Lee, B.; Kim, J.Y.; Park, Y.M.; Jeong, M. Novel PET imaging of atherosclerosis with 68Ga-Labeled NOTA-Neomannosylated human serum albumin. J. Nucl. Med. 2016, 57, 1792–1797.
- Xavier, C.; Blykers, A.; Laoui, D.; Bolli, E.; Vaneyken, I.; Bridoux, J.; Baudhuin, H.; Raes, G.; Everaert, H.; Movahedi, K.; et al. Clinical Translation of [68Ga]Ga-NOTA-anti-MMR-sdAb for PET/CT Imaging of Protumorigenic Macrophages. Mol. Imaging Biol. 2019, 21, 898–906.
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of Atherosclerotic Inflammation by 68 Ga-DOTATATE PET Compared to [18 F]FDG PET Imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791.
- Tarkin, J.M.; Calcagno, C.; Dweck, M.R.; Evans, N.R.; Chowdhury, M.M.; Gopalan, D.; Newby, D.E.; Fayad, Z.A.; Bennett, M.R.; Rudd, J.H.F. 68Ga-DOTATATE PET Identifies Residual Myocardial Inflammation and Bone Marrow Activation After Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 2489–2491.
- Nobashi, T.; Nakamoto, Y.; Kubo, T.; Ishimori, T.; Handa, T.; Tanizawa, K.; Sano, K.; Mishima, M.; Togashi, K. The utility of PET/CT with 68Ga-DOTATOC in sarcoidosis: Comparison with 67Ga-scintigraphy. Ann. Nucl. Med. 2016, 30, 544–552.
- Gormsen, L.C.; Haraldsen, A.; Kramer, S.; Dias, A.H.; Kim, W.Y.; Borghammer, P. A dual tracer 68Ga-DOTANOC PET/CT and 18F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res. 2016, 6.
- Lapa, C.; Reiter, T.; Kircher, M.; Schirbel, A.; Werner, R.A.; Pelzer, T.; Pizarro, C.; Skowasch, D.; Thomas, L.; Schlesinger-Irsch, U.; et al. Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: An initial comparison to cardiac MRI. Oncotarget 2016, 7, 77807–77814.
- Lapa, C.; Reiter, T.; Li, X.; Werner, R.A.; Samnick, S.; Jahns, R.; Buck, A.K.; Ertl, G.; Bauer, W.R. Imaging of myocardial inflammation with somatostatin receptor based PET/CT—A comparison to cardiac MRI. Int. J. Cardiol. 2015, 194, 44–49.
- Anzola, L.K.; Glaudemans, A.W.J.M.; Dierckx, R.A.J.O.; Martinez, F.A.; Moreno, S.; Signore, A. Somatostatin receptor imaging by SPECT and PET in patients with chronic inflammatory disorders: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2496–2513.
- Herrmann, K.; Lapa, C.; Wester, H.J.; Schottelius, M.; Schiepers, C.; Eberlein, U.; Bluemel, C.; Keller, U.; Knop, S.; Kropf, S.; et al. Biodistribution and radiation dosimetry for the chemokine receptor CXCR4-targeting probe 68Ga-pentixafor. J. Nucl. Med. 2015, 56, 410–416.
- Hyafil, F.; Pelisek, J.; Laitinen, I.; Schottelius, M.; Mohring, M.; Döring, Y.; Van Der Vorst, E.P.C.; Kallmayer, M.; Steiger, K.; Poschenrieder, A.; et al. Imaging the Cytokine Receptor CXCR4 in atherosclerotic plaques with the radiotracer 68Ga-Pentixafor for PET. J. Nucl. Med. 2017, 58, 499–506.
- Kircher, M.; Tran-Gia, J.; Kemmer, L.; Zhang, X.; Schirbel, A.; Werner, R.A.; Buck, A.K.; Wester, H.J.; Hacker, M.; Lapa, C.; et al. Imaging Inflammation in Atherosclerosis with CXCR4-Directed 68Ga-Pentixafor PET/CT: Correlation with 18F-FDG PET/CT. J. Nucl. Med. 2020, 61, 751–756.
- Derlin, T.; Sedding, D.G.; Dutzmann, J.; Haghikia, A.; König, T.; Napp, L.C.; Schütze, C.; Owsianski-Hille, N.; Wester, H.J.; Kropf, S.; et al. Imaging of chemokine receptor CXCR4 expression in culprit and nonculprit coronary atherosclerotic plaque using motion-corrected [68Ga]pentixafor PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1934–1944.
- Li, X.; Yu, W.; Wollenweber, T.; Lu, X.; Wei, Y.; Beitzke, D.; Wadsak, W.; Kropf, S.; Wester, H.J.; Haug, A.R.; et al. [68Ga]Pentixafor PET/MR imaging of chemokine receptor 4 expression in the human carotid artery. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1616–1625.
- Li, X.; Kemmer, L.; Zhang, X.; Kircher, M.; Buck, A.K.; Wester, H.J.; Hacker, M.; Lapa, C. Anti-Inflammatory Effects on Atherosclerotic Lesions Induced by CXCR4-Directed Endoradiotherapy. J. Am. Coll. Cardiol. 2018, 72, 122–123.
- Lapa, C.; Reiter, T.; Werner, R.A.; Ertl, G.; Wester, H.J.; Buck, A.K.; Bauer, W.R.; Herrmann, K. [68Ga]Pentixafor-PET/CT for Imaging of Chemokine Receptor 4 Expression after Myocardial Infarction. JACC Cardiovasc. Imaging 2015, 8, 1466–1468.
- Rischpler, C.; Nekolla, S.G.; Kossmann, H.; Dirschinger, R.J.; Schottelius, M.; Hyafil, F.; Wester, H.J.; Laugwitz, K.L.; Schwaiger, M. Upregulated myocardial CXCR4-expression after myocardial infarction assessed by simultaneous GA-68 pentixafor PET/MRI. J. Nucl. Cardiol. 2016, 23, 131–133.
- Thackeray, J.T.; Derlin, T.; Haghikia, A.; Napp, L.C.; Wang, Y.; Ross, T.L.; Schäfer, A.; Tillmanns, J.; Wester, H.J.; Wollert, K.C.; et al. Molecular Imaging of the Chemokine Receptor CXCR4 After Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2015, 8, 1417–1426.
- Reiter, T.; Kircher, M.; Schirbel, A.; Werner, R.A.; Kropf, S.; Ertl, G.; Buck, A.K.; Wester, H.J.; Bauer, W.R.; Lapa, C. Imaging of C-X-C Motif Chemokine Receptor CXCR4 Expression After Myocardial Infarction with [68 Ga]Pentixafor-PET/CT in Correlation With Cardiac MRI. JACC Cardiovasc. Imaging 2018, 11, 1541–1543.
- Wang, Y.; Dembowsky, K.; Chevalier, E.; Stüve, P.; Korf-Klingebiel, M.; Lochner, M.; Napp, L.C.; Frank, H.; Brinkmann, E.; Kanwischer, A.; et al. C-X-C Motif Chemokine Receptor 4 Blockade Promotes Tissue Repair after Myocardial Infarction by Enhancing Regulatory T Cell Mobilization and Immune-Regulatory Function. Circulation 2019, 139, 1798–1812.
- Hess, A.; Derlin, T.; Koenig, T.; Diekmann, J.; Wittneben, A.; Wang, Y.; Wester, H.J.; Ross, T.L.; Wollert, K.C.; Bauersachs, J.; et al. Molecular imaging-guided repair after acute myocardial infarction by targeting the chemokine receptor CXCR4. Eur. Heart J. 2020, 41, 3564–3575.
- Bouter, C.; Meller, B.; Sahlmann, C.O.; Staab, W.; Wester, H.J.; Kropf, S.; Meller, J. 68 Ga-pentixafor PET/CT imaging of chemokine receptor CXCR4 in chronic infection of the bone: First insights. J. Nucl. Med. 2018, 59, 320–326.
- Derlin, T.; Gueler, F.; Bräsen, J.H.; Schmitz, J.; Hartung, D.; Herrmann, T.R.; Ross, T.L.; Wacker, F.; Wester, H.J.; Hiss, M.; et al. Integrating MRI and chemokine receptor CXCR4-targeted PET for detection of leukocyte infiltration in complicated urinary tract infections after kidney transplantation. J. Nucl. Med. 2017, 58, 1831–1837.
- Liu, Y.; Gunsten, S.P.; Sultan, D.H.; Luehmann, H.P.; Zhao, Y.; Blackwell, T.S.; Bollermann-Nowlis, Z.; Pan, J.H.; Byers, D.E.; Atkinson, J.J.; et al. PET-based imaging of chemokine receptor 2 in experimental and disease-related lung inflammation. Radiology 2017, 283, 758–768.
- Heo, G.S.; Kopecky, B.; Sultan, D.; Ou, M.; Feng, G.; Bajpai, G.; Zhang, X.; Luehmann, H.; Detering, L.; Su, Y.; et al. Molecular Imaging Visualizes Recruitment of Inflammatory Monocytes and Macrophages to the Injured Heart. Circ. Res. 2019, 124, 881–890.
- English, S.J.; Sastriques, S.E.; Detering, L.; Sultan, D.; Luehmann, H.; Arif, B.; Heo, G.S.; Zhang, X.; Laforest, R.; Zheng, J.; et al. CCR2 positron emission tomography for the assessment of abdominal aortic aneurysm inflammation and rupture prediction. Circ. Cardiovasc. Imaging 2020, e009889.
- Brody, S.; Gunsten, S.; Luehmann, H.; Sultan, D.; Hoelscher, M.; Heo, G.S.; Pan, J.; Koenitzer, J.; Lee, E.; Huang, T.; et al. Chemokine Receptor 2-targeted Molecular Imaging in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 78–89.
- Thackeray, J.T.; Hupe, H.C.; Wang, Y.; Bankstahl, J.P.; Berding, G.; Ross, T.L.; Bauersachs, J.; Wollert, K.C.; Bengel, F.M. Myocardial Inflammation Predicts Remodeling and Neuroinflammation After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 263–275.
- Gaemperli, O.; Shalhoub, J.; Owen, D.R.J.; Lamare, F.; Johansson, S.; Fouladi, N.; Davies, A.H.; Rimoldi, O.E.; Camici, P.G. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur. Heart J. 2012, 33, 1902–1910.
- Pugliese, F.; Gaemperli, O.; Kinderlerer, A.R.; Lamare, F.; Shalhoub, J.; Davies, A.H.; Rimoldi, O.E.; Mason, J.C.; Camici, P.G. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. J. Am. Coll. Cardiol. 2010, 56, 653–661.
- Lamare, F.; Hinz, R.; Gaemperli, O.; Pugliese, F.; Mason, J.C.; Spinks, T.; Camici, P.G.; Rimoldi, O.E. Detection and quantification of large-vessel inflammation with 11C-(R)-PK11195 PET/CT. J. Nucl. Med. 2011, 52, 33–39.
- Gent, Y.Y.J.; Voskuyl, A.E.; Kloet, R.W.; Van Schaardenburg, D.; Hoekstra, O.S.; Dijkmans, B.A.C.; Lammertsma, A.A.; Van Der Laken, C.J. Macrophage positron emission tomography imaging as a biomarker for preclinical rheumatoid arthritis: Findings of a prospective pilot study. Arthritis Rheum. 2012, 64, 62–66.
- Gent, Y.Y.J.; ter Wee, M.M.; Voskuyl, A.E.; den Uyl, D.; Ahmadi, N.; Dowling, C.; van Kuijk, C.; Hoekstra, O.S.; Boers, M.; Lems, W.F.; et al. Subclinical synovitis detected by macrophage PET, but not MRI, is related to short-term flare of clinical disease activity in early RA patients: An exploratory study. Arthritis Res. Ther. 2015, 17, 1–6.
- Van Der Laken, C.J.; Elzinga, E.H.; Kropholler, M.A.; Molthoff, C.F.M.; Van Der Heijden, J.W.; Maruyama, K.; Boellaard, R.; Dijkmans, B.A.C.; Lammertsma, A.A.; Voskuyl, A.E. Noninvasive imaging of macrophages in rheumatoid synovitis using 11C-(R)-PK11195 and positron emission tomography. Arthritis Rheum. 2008, 58, 3350–3355.
- Beer, A.J.; Pelisek, J.; Heider, P.; Saraste, A.; Reeps, C.; Metz, S.; Seidl, S.; Kessler, H.; Wester, H.J.; Eckstein, H.H.; et al. PET/CT imaging of integrin αvβ3 expression in human carotid atherosclerosis. JACC Cardiovasc. Imaging 2014, 7, 178–187.
- Jenkins, W.S.; Vesey, A.T.; Vickers, A.; Neale, A.; Moles, C.; Connell, M.; Joshi, N.V.; Lucatelli, C.; Fletcher, A.M.; Spratt, J.C.; et al. In vivo alpha-V beta-3 integrin expression in human aortic atherosclerosis. Heart 2019, 105, 1868–1875.
- Jenkins, W.S.A.; Vesey, A.T.; Stirrat, C.; Connell, M.; Lucatelli, C.; Neale, A.; Moles, C.; Vickers, A.; Fletcher, A.; Pawade, T.; et al. Cardiac αVβ3 integrin expression following acute myocardial infarction in humans. Heart 2017, 103, 607–615.
- Higuchi, T.; Bengel, F.M.; Seidl, S.; Watzlowik, P.; Kessler, H.; Hegenloh, R.; Reder, S.; Nekolla, S.G.; Wester, H.J.; Schwaiger, M. Assessment of αvβ3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc. Res. 2008, 78, 395–403.
- Sun, Y.; Zeng, Y.; Zhu, Y.; Feng, F.; Xu, W.; Wu, C.; Xing, B.; Zhang, W.; Wu, P.; Cui, L.; et al. Application of 68Ga-PRGD2 PET/CT for αvβ3-integrin imaging of myocardial infarction and stroke. Theranostics 2014, 4, 778–786.
- Zhu, Z.; Yin, Y.; Zheng, K.; Li, F.; Chen, X.; Zhang, F.; Zhang, X. Evaluation of synovial angiogenesis in patients with rheumatoid arthritis using 68Ga-PRGD2 PET/CT: A prospective proof-of-concept cohort study. Ann. Rheum. Dis. 2014, 73, 1269–1272.
- Chandrupatla, D.M.S.H.; Jansen, G.; Vos, R.; Verlaan, M.; Chen, Q.; Low, P.S.; Windhorst, A.D.; Lammertsma, A.A.; van der Laken, C.J.; Molthoff, C.F.M. In-vivo monitoring of anti-folate therapy in arthritic rats using [18F]fluoro-PEG-folate and positron emission tomography. Arthritis Res. Ther. 2017, 19, 1–9.
- Chandrupatla, D.M.S.H.; Molthoff, C.F.M.; Lammertsma, A.A.; van der Laken, C.J.; Jansen, G. The folate receptor β as a macrophage-mediated imaging and therapeutic target in rheumatoid arthritis. Drug Deliv. Transl. Res. 2019, 9, 366–378.
- Gent, Y.Y.J.; Weijers, K.; Molthoff, C.F.M.; Windhorst, A.D.; Huisman, M.C.; Smith, D.E.C.; Kularatne, S.A.; Jansen, G.; Low, P.S.; Lammertsma, A.A.; et al. Evaluation of the novel folate receptor ligand [18F]fluoro-PEG-folate for macrophage targeting in a rat model of arthritis. Arthritis Res. Ther. 2013, 15, 1–11.
- Chandrupatla, D.M.S.H.; Jansen, G.; Mantel, E.; Low, P.S.; Matsuyama, T.; Musters, R.P.; Windhorst, A.D.; Lammertsma, A.A.; Molthoff, C.F.M.; Van Der Laken, C.J. Imaging and Methotrexate Response Monitoring of Systemic Inflammation in Arthritic Rats Employing the Macrophage PET Tracer [18F]Fluoro-PEG-Folate. Contrast Media Mol. Imaging 2018, 2018, 8092781.
- Verweij, N.J.F.; Yaqub, M.; Bruijnen, S.T.G.; Pieplenbosch, S.; ter Wee, M.M.; Jansen, G.; Chen, Q.; Low, P.S.; Windhorst, A.D.; Lammertsma, A.A.; et al. First in man study of [18F]fluoro-PEG-folate PET: A novel macrophage imaging technique to visualize rheumatoid arthritis. Sci. Rep. 2020, 10, 1–10.
- Jahandideh, A.; Uotila, S.; Ståhle, M.; Virta, J.; Li, X.G.; Kytö, V.; Marjamäki, P.; Liljenbäck, H.; Taimen, P.; Oikonen, V.; et al. Folate Receptor β-Targeted PET Imaging of Macrophages in Autoimmune Myocarditis. J. Nucl. Med. 2020, 61, 1643–1649.
- Moisio, O.; Palani, S.; Virta, J.; Elo, P.; Liljenbäck, H.; Tolvanen, T.; Käkelä, M.; Miner, M.G.; Herre, E.A.; Marjamäki, P.; et al. Radiosynthesis and preclinical evaluation of [68Ga]Ga-NOTA-folate for PET imaging of folate receptor β-positive macrophages. Sci. Rep. 2020, 10, 1–10.
- Schniering, J.; Benešová, M.; Brunner, M.; Haller, S.; Cohrs, S.; Frauenfelder, T.; Vrugt, B.; Feghali-Bostwick, C.; Schibli, R.; Distler, O.; et al. 18F-AzaFol for Detection of Folate Receptor-β Positive Macrophages in Experimental Interstitial Lung Disease—A Proof-of-Concept Study. Front. Immunol. 2019, 10, 2724.
- Henry, L.R.; Lee, H.O.; Lee, J.S.; Klein-Szanto, A.; Watts, P.; Ross, E.A.; Chen, W.T.; Cheng, J.D. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin. Cancer Res. 2007, 13, 1736–1741.
- Cohen, S.J.; Alpaugh, R.K.; Palazzo, I.; Meropol, N.J.; Rogatko, A.; Xu, Z.; Hoffman, J.P.; Weiner, L.M.; Cheng, J.D. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas 2008, 37, 154–158.
- Laverman, P.; Van Der Geest, T.; Terry, S.Y.A.; Gerrits, D.; Walgreen, B.; Helsen, M.M.; Nayak, T.K.; Freimoser-Grundschober, A.; Waldhauer, I.; Hosse, R.J.; et al. Immuno-PET and immuno-SPECT of rheumatoid arthritis with radiolabeled anti-fibroblast activation protein antibody correlates with severity of arthritis. J. Nucl. Med. 2015, 56, 778–783.
- Terry, S.Y.A.; Koenders, M.I.; Franssen, G.M.; Nayak, T.K.; Freimoser-Grundschober, A.; Klein, C.; Oyen, W.J.; Boerman, O.C.; Laverman, P. Monitoring therapy response of experimental arthritis with radiolabeled tracers targeting fibroblasts, macrophages, or integrin αvβ3. J. Nucl. Med. 2016, 57, 467–472.
- Van Der Geest, T.; Laverman, P.; Gerrits, D.; Walgreen, B.; Helsen, M.M.; Klein, C.; Nayak, T.K.; Storm, G.; Metselaar, J.M.; Koenders, M.I.; et al. Liposomal treatment of experimental arthritis can be monitored noninvasively with a radiolabeled anti-fibroblast activation protein antibody. J. Nucl. Med. 2017, 58, 151–155.
- Luo, Y.; Pan, Q.; Yang, H.; Peng, L.; Zhang, W.; Li, F. Fibroblast activation protein targeted PET/CT with 68 Ga-FAPI for imaging IgG4-related disease: Comparison to 18 F-FDG PET/CT. J. Nucl. Med. 2021, 62, 266–271.
- Schmidkonz, C.; Rauber, S.; Atzinger, A.; Agarwal, R.; Götz, T.I.; Soare, A.; Cordes, M.; Prante, O.; Bergmann, C.; Kleyer, A.; et al. Disentangling inflammatory from fibrotic disease activity by fibroblast activation protein imaging. Ann. Rheum. Dis. 2020, 79, 1485–1491.
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular imaging of fibroblast activity after myocardial infarction using a 68Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749.
- Siebermair, J.; Köhler, M.I.; Kupusovic, J.; Nekolla, S.G.; Kessler, L.; Ferdinandus, J.; Guberina, N.; Stuschke, M.; Grafe, H.; Siveke, J.T.; et al. Cardiac fibroblast activation detected by Ga-68 FAPI PET imaging as a potential novel biomarker of cardiac injury/remodeling. J. Nucl. Cardiol. 2020.




