| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takanobu Yoshii | + 1781 word(s) | 1781 | 2021-02-22 08:56:18 |
Video Upload Options
Post-traumatic stress disorder is a common mental disorder, with high lifetime prevalence approximately 6–10% . The prevalence of PTSD in trauma-exposed people has been approximately 20%. PTSD is induced by traumatic stress including life threatening, actual or threatened severe injury, and sexual violence. In DSM-V criteria, PTSD has the following symptoms: intrusion of unwanted memory updates related to traumatic stress, avoidance for reminders, negative alterations in mood, and hyper-arousal. Conservatively, fear-conditioned learning involving the amygdala has been considered one of the causative factors.
1. Introduction
Volumetric neuroimaging studies of PTSD have identified different atrophic areas, such as the hippocampus, anterior cingulate cortex (ACC), posterior cingulate cortex [1], insular cortex [2], orbitofrontal cortex [3], ventromedial prefrontal cortex [4], occipital cortex [5], calcarine sulcus [6], or amygdala [7]. It is difficult to determine which region is most important because it is unclear whether the traumatic stress or the predisposition of the patient contributes more strongly to the pathology. Although brain volume reduction in thalamus has rarely been reported in research targeting PTSD, brain atrophy in the bilateral thalamus has been reported in pain study [8]. It was reported that psychological torture induced pain [9], and pain itself should be considered as one of the stress contents. It is difficult to rule out that thalamus has possibility of volumetric change induced by stress, and our group, in fact, observed stress-induced brain atrophy in the thalamus [10].
The thalamus was originally thought to act as a hub relaying sensory information to the cortices and occasionally playing a role in processing this information. However, this view has gradually changed, and the thalamus is now considered to have many functions, acting as a sensory hub between other subcortical nuclei and the cortices and contributing to sleep and wake awareness, motor control, and cognition. Thalamic functional abnormalities are thought to contribute to the dysregulation of sensory filtering, circadian rhythms, levels of alertness, and consciousness [11]. It has been mentioned that fearful stimulation activates the thalamus [12]. In the context of fear-related learning, sensory processing, including visual processing in the thalamus, has been investigated with respect to its impact on amygdala function and output, rather than as an important psychological or pathophysiological process that shapes the development of PTSD [13]. However, our animal voxel-based morphometry (VBM) study revealed the induction of brain atrophy in the thalamus by severe stress [14]. In addition, recent research works revealed the efficacies of interventions via visual technique: visual neurofeedback using implicit fear exposure [15][16] and visual game task [16].
2. Therapeutic Implications Targeting the Thalamus
In fact, a recent clinical study reported that trauma-exposed control patients without worsening PTSD exhibited depleted resting-state functional connectivity between the thalamus and the postcentral gyrus, while both healthy controls and PTSD patients did not exhibit such depletion [17]. A smaller pulvinar in similar traumatized control patients has been also reported [18]. Defective pulvinar functioning may thus result in a failure of fear processing [19], and I believe that reduced pulvinar function may contribute to preventing the development of PTSD. On the other hand, the functional amygdala seed connectivity study in PTSD indicates the enhancement of connectivity between centromedial amygdala and pulvinar, although the depletion between basolateral amygdala and SC was detected [20]. In addition, recovered PTSD patients showed that the lower tract strength of the amygdala–thalamus connection was normalized during recovery, while that of amygdala–hippocampus connection remained low [21]. PTSD patients also display disrupted regional activity of the thalamus, including the dorsal medial thalamus, and future research should investigate whether this may hinder overwriting traumatic memories with memories of ordinary daily life.
Taken together, I assume promising research: first, suppressing this pathway as a direction of preventing the progression of PTSD; second, activating retinotectal pathway followed with minimal stress exposure paradigm for desensitization of thalamus amygdala connectivity; and third, activating geniculovisual cortex pathways to reduce contribution of retinotectal pathway in visual fear processing.
In addition, I also would like to bridge the physiology of thalamus to therapeutic implications and consider the contribution of the physiology of thalamus to proposed therapeutic interventions.
2.1. EMDR
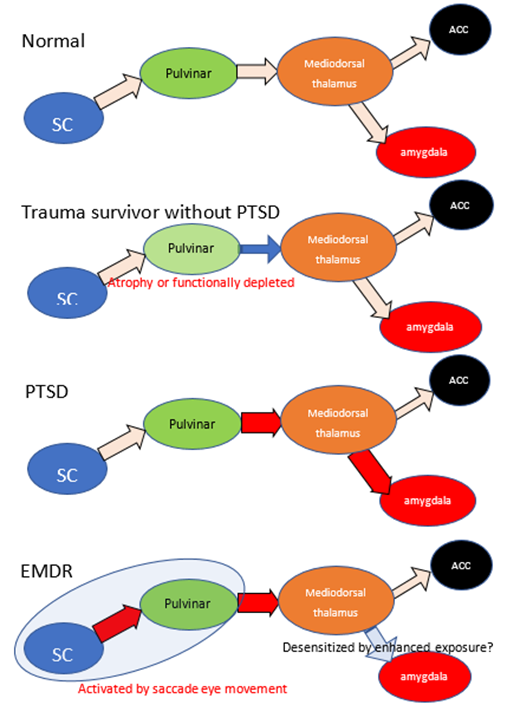
EMDR can be described as a methodology that extends PE. It is thought to take advantage of the fact that eye movements facilitate learning, but the mechanism has long been unclear, and many EMDR researchers themselves have relied on cognitive behavioral therapy as the basis for its effectiveness. However, a recent animal study revealed that projection from the SC to the mediodorsal thalamus may contribute to the processing of conditioned fear [22], and especially mediodorsal thalamus may contribute in reactions to fear memory [23][24][25]. Saccade eye movement is used for the desensitization of traumatic memory in EMDR therapy, and a recent animal study demonstrated that visual bilaterally alternating sensory stimulation, such as EMDR stimulation, provided a fear-reducing effect, with sustained activation from the SC to the mediodorsal thalamus [26]. This result may partly explain the therapeutic mechanism of EMDR. Despite the finding that limited pulvinar function may exert a preventative effect toward PTSD, the EMDR animal model exhibited enhanced functioning of the SC and mediodorsal thalamus. The SC and mediodorsal thalamus activation may have a therapeutic effect by promoting the exposure and desensitization of the thalamus–amygdala complex. Following EMDR treatment, patients also showed a significant reduction in gray matter volume in the left thalamus region [27], suggesting that the treatment may have modified the laterality that was induced by PTSD. I consider that the efficacy of EMDR for treating PTSD supports the idea that the thalamus may be the key site of PTSD pathogenesis. See Figure 1 as a chematic summery.
Figure 1: The schematic summary of retinotectal pathway and hypothetic mechanism of EMDR. Because of reports low morbidity rate of trauma survivors with smaller pulvinar, atrophy or dysfunction of pulvinar may have brought prevention of progression in PTSD. PTSD patients indicate enhanced connectivity between thalamus and amygdala, depleted between thalamus and ACC. EMDR intervention enhances the activity of retinotectal pathways and promotes the effects of exposure. Red arrow indicates enhanced connectivity and blue arrow indicate reduced. SC: superior colliculus, ACC: anterior cingulate cortex.
2.2. Functional MRI Neurofeedback Technique
A therapeutic approach targeting unconsciousness has been proposed [28], and one that attempts to reprogram the unconscious using an fMRI-based technique called decoded neurofeedback (DecNef) has been tried [15][16]. In conclusion, I could not find any literature which indicates contribution of thalamic change in this method. Tasks involving the unconscious are complicated: they require computational calculation of brain activity in the higher visual cortex during fear and control stimuli that attempt to stimulate brain activity in the higher visual cortex to approximate that observed during fear stimuli without using direct visual stimulation as non-explicit fear stimulation. This approach may have been inspired by prolonged exposure therapy, but the activity in the amygdala does not appear to be significantly altered in the learning phase. Only an acute BOLD signal response in the amygdala under a fear-target confrontation was demonstrated [14]. Based on our belief that the retinotectal pathway is more important than the geniculovisual cortex pathway in the processing of visual fears, I assume that this intervention achieves therapeutic benefits by reducing the contribution of the retinotectal pathway.
2.3. Visual Task Games
The visiospatial game task followed with minimal exposure has been applied for prevention of PTSD via proof randomized control trial [16]. There is a debate that the therapeutic mechanisms via competition for limited working memory resources by the task [29] and I also could not find any literature describing contribution of thalamic change for this intervention. Although these studies were not designed for action on the thalamus, it seems difficult to rule out that it is undergoing some action on the thalamus via visual task. At present, there is no evidence that the thalamus is affected by this task; however, it is certainly a task including discrimination and spatial cognition, and it can be reasonably speculated that the geniculovisual pathway would be used. It is interesting whether this intervention might produce preventative effect via reducing contribution of retinotectal pathway in the phase of PTSD progression.
2.4. Hyperbaric Oxygen Therapy (HBOT)
I would like to introduce an intervention method that is neither medications nor exposure. It has been shown in a randomized control trial that treating patients with a history of childhood sexual abuse with HBOT results in an improvement of PTSD symptoms and psychological distress, as well as an improved FA value (based on MRI-DTI) in the anterior thalamic radiation, left thalamus, and left insula [30]. This treatment was originally designed to treat fibromyalgia; however, it was subsequently suspected to have activated the entire thalamus, and may thus have additional therapeutic effects for PTSD. HBOT may also promote the exposure and desensitization of thalamus–amygdala complex. This treatment is expected to have few serious side effects, and I consider that HBOT is thus a promising intervention for PTSD, which requires further research.
2.5. Oxytocin Administration
Oxytocin is a neurochemical agent that is thought to improve human interaction and has been experimentally applied to PTSD therapy. Oxytocin administration enhanced the activity of the left thalamus in both patients and controls during a distraction task (resetting negative feelings with a working-memory task) and was negatively correlated with error rates in this task in PTSD patients [31]. Oxytocin administration also decreased the functional connectivity between the left thalamus and the amygdala in male PTSD patients and trauma-exposed controls, although female patients indicated enhanced connectivity between left thalamus and the amygdala [31]. The mechanism of influencing on thalamus amygdala connectivity with sexual variation has been unknown and the potential efficacy of oxytocin administration should be further investigated.
2.6. Other Medications
Conventionally used medications may have some effect on the thalamus, but these interventions do not yield satisfactory therapeutic effects. Several studies have thus indicated a relationship between the thalamus and PTSD. These findings provide only a seed for future research, and are not sufficient to establish any new interventions. Selective serotonin reuptake inhibitors (SSRIs) have been established as a treatment option for PTSD, and the mediodorsal thalamus is a serotonin-rich area. Although one animal study showed that chronic predator-scent exposure altered serotonin and dopamine levels in the thalamus [32], another did not show a noticeable effect in the thalamus [33]. Prazosin: α -1 adrenergic antagonist treatment has been already established as intervention to hyper arousal and nightmares in PTSD [34]. Oral propranolol administration (1 mg/kg) improved PTSD symptoms following the enhancement of activation of the thalamus and amygdala [35]. Noradrenergic system is intimately connected to the thalamus, and it is an interesting and promising area for future research seeking the relationship between the noradrenergic modulation and alteration of the thalamus in PTSD patients. A significant correlation between re-experiencing and thalamic β2 nicotinic acetylcholine receptor binding using single-photon emission computed tomography (SPECT) has already been described [36], The micro-opioid system in the dorsomedial thalamus has also been found to contribute to fear extinction [37]. However, these have not yet been targeted in medication research.
References
- Nardo, D.; Hogberg, G.; Looi, J.C.; Larsson, S.; Hallstrom, T.; Pagani, M. Gray matter density in limbic and paralimbic corti-ces is associated with trauma load and EMDR outcome in PTSD patients. J. Psychiatr. Res. 2010, 44, 477–485, doi:10.1016/j.jpsychires.2009.10.014.
- Chen, S.; Xia, W.; Li, L.; Liu, J.; He, Z.; Zhang, Z.; Yan, L.; Zhang, J.; Hu, D. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: A voxel-based morphometric study. Psychiatry Res. 2006, 146, 65–72, doi:10.1016/j.pscychresns.2005.09.006.
- Hakamata, Y.; Matsuoka, Y.; Inagaki, M.; Nagamine, M.; Hara, E.; Imoto, S.; Murakami, K.; Kim, Y.; Uchitomi, Y. Structure of orbitofrontal cortex and its longitudinal course in cancer-related post-traumatic stress disorder. Neurosci. Res. 2007, 59, 383–389, doi:10.1016/j.neures.2007.08.012.
- Carrion, V.G.; Weems, C.F.; Watson, C.; Eliez, S.; Menon, V.; Reiss, A.L. Converging evidence for abnormalities of the pre-frontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: An MRI study. Psychiatry Res. 2009, 172, 226–234, doi:10.1016/j.pscychresns.2008.07.008.
- Tavanti, M.; Battaglini, M.; Borgogni, F.; Bossini, L.; Calossi, S.; Marino, D.; Vatti, G.; Pieraccini, F.; Federico, A.; Castrogio-vanni, P.; et al. Evidence of diffuse damage in frontal and occipital cortex in the brain of patients with post-traumatic stress disorder. Neurol. Sci. 2012, 33, 59–68, doi:10.1007/s10072-011-0659-4.
- Zhang, J.; Tan, Q.; Yin, H.; Zhang, X.; Huan, Y.; Tang, L.; Wang, H.; Xu, J.; Li, L. Decreased gray matter volume in the left hippocampus and bilateral calcarine cortex in coal mine flood disaster survivors with recent onset PTSD. Psychiatry Res. 2011, 192, 84–90, doi:10.1016/j.pscychresns.2010.09.001.
- Rogers, M.A.; Yamasue, H.; Abe, O.; Yamada, H.; Ohtani, T.; Iwanami, A.; Aoki, S.; Kato, N.; Kasai, K. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychia-try Res. 2009, 174, 210–216, doi:10.1016/j.pscychresns.2009.06.001.
- Pan, P.L.; Zhong, J.G.; Shang, H.F.; Zhu, Y.L.; Xiao, P.R.; Dai, Z.Y.; Shi, H.C. Quantitative meta-analysis of grey matter anomalies in neuropathic pain. Eur. J. Pain 2015, 19, 1224–1231, doi:10.1002/ejp.670.
- Tsur, N.; Defrin, R.; Ginzburg, K. PTSD, Orientation to Pain, and Pain Perception in Ex-prisoners of War who Underwent Torture. Psychosom. Med. 2017, doi:10.1097/PSY.0000000000000461.
- Yoshii, T.; Oishi, N.; Ikoma, K.; Nishimura, I.; Sakai, Y.; Matsuda, K.; Yamada, S.; Tanaka, M.; Kawata, M.; Narumoto, J.; et al. Brain atrophy in the visual cortex and thalamus induced by severe stress in animal model. Sci. Rep. 2017, 7, 12731, doi:10.1038/s41598-017-12917-z.
- Yin, Y.; Jin, C.; Hu, X.; Duan, L.; Li, Z.; Song, M.; Chen, H.; Feng, B.; Jiang, T.; Jin, H.; et al. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: A functional magnetic resonance imaging study. Brain Res 2011, 1411, 98–107, doi:10.1016/j.brainres.2011.07.016.
- Burra, N.; Hervais-Adelman, A.; Celeghin, A.; de Gelder, B.; Pegna, A.J. Affective blindsight relies on low spatial frequencies. Neuropsychologia 2019, 128, 44–49, doi:10.1016/j.neuropsychologia.2017.10.009.
- Liberzon, I.; Abelson, J.L. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 2016, 92, 14–30, doi:10.1016/j.neuron.2016.09.039.
- Koizumi, A.; Amano, K.; Cortese, A.; Shibata, K.; Yoshida, W.; Seymour, B.; Kawato, M.; Lau, H. Fear reduction without fear through reinforcement of neural activity that bypasses conscious exposure. Nat. Human Behav. 2016, 1, 6, doi:10.1038/s41562-016-0006.
- Taschereau-Dumouchel, V.; Cortese, A.; Chiba, T.; Knotts, J.D.; Kawato, M.; Lau, H. Towards an unconscious neural rein-forcement intervention for common fears. Proc. Natl. Acad. Sci. USA 2018, 115, 3470–3475, doi:10.1073/pnas.1721572115.
- Iyadurai, L.; Blackwell, S.E.; Meiser-Stedman, R.; Watson, P.C.; Bonsall, M.B.; Geddes, J.R.; Nobre, A.C.; Holmes, E.A. Pre-venting intrusive memories after trauma via a brief intervention involving Tetris computer game play in the emergency de-partment: A proof-of-concept randomized controlled trial. Mol. Psychiatry 2017, 10.1038/mp.2017.23, doi:10.1038/mp.2017.23.
- Jeon, S.; Lee, Y.J.; Park, I.; Kim, N.; Kim, S.; Jun, J.Y.; Yoo, S.Y.; Lee, S.H.; Kim, S.J. Resting State Functional Connectivity of the Thalamus in North Korean Refugees with and without Posttraumatic Stress Disorder. Sci. Rep. 2020, 10, 3194, doi:10.1038/s41598-020-59815-5.
- Chen, Y.; Fu, K.; Feng, C.; Tang, L.; Zhang, J.; Huan, Y.; Cui, J.; Mu, Y.; Qi, S.; Xiong, L.; et al. Different regional gray matter loss in recent onset PTSD and non PTSD after a single prolonged trauma exposure. PLoS ONE 2012, 7, e48298, doi:10.1371/journal.pone.0048298.
- Bertini, C.; Pietrelli, M.; Braghittoni, D.; Ladavas, E. Pulvinar Lesions Disrupt Fear-Related Implicit Visual Processing in Hemianopic Patients. Front. Psychol. 2018, 9, 2329.
- Rabellino, D.; Densmore, M.; Frewen, P.A.; Théberge, J.; McKinnon, M.C.; Lanius, R.A. Aberrant Functional Connectivity of the Amygdala Complexes in PTSD during Conscious and Subconscious Processing of Trauma-Related Stimuli. PLoS ONE 2016, 11, e0163097, doi:10.1371/journal.pone.0163097.
- Yoon, S.; Kim, J.E.; Hwang, J.; Kang, I.; Jeon, S.; Im, J.J.; Kim, B.R.; Lee, S.; Kim, G.H.; Rhim, H.; et al. Recovery from Post-traumatic Stress Requires Dynamic and Sequential Shifts in Amygdalar Connectivities. Neuropsychopharmacology 2017, 42, 454–461, doi:10.1038/npp.2016.136.
- Wei, P.; Liu, N.; Zhang, Z.; Liu, X.; Tang, Y.; He, X.; Wu, B.; Zhou, Z.; Liu, Y.; Li, J.; et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun. 2015, 6, 6756.
- Padilla-Coreano, N.; Do-Monte, F.H.; Quirk, G.J. A time-dependent role of midline thalamic nuclei in the retrieval of fear memory. Neuropharmacology 2012, 62, 457–463, doi:10.1016/j.neuropharm.2011.08.037.
- Paydar, A.; Lee, B.; Gangadharan, G.; Lee, S.; Hwang, E.M.; Shin, H.S. Extrasynaptic GABAA receptors in mediodorsal tha-lamic nucleus modulate fear extinction learning. Mol. Brain 2014, 7, 39, doi:10.1186/1756-6606-7-39.
- Salay, L.D.; Ishiko, N.; Huberman, A.D. A midline thalamic circuit determines reactions to visual threat. Nature 2018, 557, 183–189, doi:10.1038/s41586-018-0078-2.
- Baek, J.; Lee, S.; Cho, T.; Kim, S.W.; Kim, M.; Yoon, Y.; Kim, K.K.; Byun, J.; Kim, S.J.; Jeong, J.; et al. Neural circuits underlying a psychotherapeutic regimen for fear disorders. Nature 2019, 566, 339–343.
- Bossini, L.; Santarnecchi, E.; Casolaro, I.; Koukouna, D.; Caterini, C.; Cecchini, F.; Fortini, V.; Vatti, G.; Marino, D.; Fernandez, I.; et al. Morphovolumetric changes after EMDR treatment in drug-naïve PTSD patients. Riv. Psichiatr. 2017, 52, 24–31.
- Sohn, E. Decoding the neuroscience of consciousness. Nature 2019, 571, S2–S5, doi:10.1038/d41586-019-02207-1.
- Kessler, H.; Schmidt, A.C.; James, E.L.; Blackwell, S.E.; von Rauchhaupt, M.; Harren, K.; Kehyayan, A.; Clark, I.A.; Sauvage, M.; Herpertz, S.; et al. Visuospatial computer game play after memory reminder delivered three days after a traumatic film reduces the number of intrusive memories of the experimental trauma. J. Behav. Ther. Exp. Psychiatry 2020, 67, 101454.
- Hadanny, A.; Bechor, Y.; Catalogna, M.; Daphna-Tekoah, S.; Sigal, T.; Cohenpour, M.; Lev-Wiesel, R.; Efrati, S. Hyperbaric Oxygen Therapy Can Induce Neuroplasticity and Significant Clinical Improvement in Patients Suffering From Fibromyalgia With a History of Childhood Sexual Abuse-Randomized Controlled Trial. Front. Psychol. 2018, 9, 2495.
- Koch, S.B.J.; van Zuiden, M.; Nawijn, L.; Frijling, J.L.; Veltman, D.J.; Olff, M. Effects of intranasal oxytocin on distraction as emotion regulation strategy in patients with post-traumatic stress disorder. Eur. Neuropsychopharmacol. 2019, 29, 266–277.
- Dremencov, E.; Lapshin, M.; Komelkova, M.; Alliluev, A.; Tseilikman, O.; Karpenko, M.; Pestereva, N.; Manukhina, E.; Downey, H.F.; Tseilikman, V. Chronic predator scent stress alters serotonin and dopamine levels in the rat thalamus and hypothalamus, respectively. Gen. Physiol. Biophys. 2019, 38, 187–190, doi:10.4149/gpb_2019003.
- Han, F.; Xiao, B.; Wen, L.; Shi, Y. Effects of fluoxetine on the amygdala and the hippocampus after administration of a single prolonged stress to male Wistar rates: In vivo proton magnetic resonance spectroscopy findings. Psychiatry Res. 2015, 232, 154–161, doi:10.1016/j.pscychresns.2015.02.011.
- Mahabir, M.; Tucholka, A.; Shin, L.M.; Etienne, P.; Brunet, A. Emotional face processing in post-traumatic stress disorder after reconsolidation impairment using propranolol: A pilot fMRI study. J. Anxiety Disord. 2015, 36, 127–133, doi:10.1016/j.janxdis.2015.10.004.
- De Berardis, D.; Marini, S.; Serroni, N.; Iasevoli, F.; Tomasetti, C.; de Bartolomeis, A.; Mazza, M.; Tempesta, D.; Valchera, A.; Fornaro, M.; et al. Targeting the Noradrenergic System in Posttraumatic Stress Disorder: A Systematic Review and Me-ta-Analysis of Prazosin Trials. Curr. Drug Targets 2015, 16, 1094–1106, doi:10.2174/1389450116666150506114108.
- Czermak, C.; Staley, J.K.; Kasserman, S.; Bois, F.; Young, T.; Henry, S.; Tamagnan, G.D.; Seibyl, J.P.; Krystal, J.H.; Neumeis-ter, A. beta2 Nicotinic acetylcholine receptor availability in post-traumatic stress disorder. Int. J. Neuropsychopharmacol. 2008, 11, 419–424, doi:10.1017/s1461145707008152.
- Bengoetxea, X.; Goedecke, L.; Blaesse, P.; Pape, H.C.; Jungling, K. The micro-opioid system in midline thalamic nuclei modulates defence strategies towards a conditioned fear stimulus in male mice. J. Psychopharmacol. 2020.