
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hans Gregersen | + 2400 word(s) | 2400 | 2020-12-09 06:58:43 | | | |
| 2 | Peter Tang | -177 word(s) | 2223 | 2021-03-06 12:43:46 | | |
Video Upload Options
Recently, a simulated stool named Fecobionics was developed. It has the consistency and shape of normal stool. Fecobionics records a variety of parameters including pressures, bending, and shape changes. It has been used to study defecation patterns in large animals and humans, including patients with symptoms of obstructed defecation and fecal incontinence. Recently, it was applied in a canine colon model where it revealed patterns consistent with shallow waves originating from slow waves generated by the interstitial Cells of Cajal.
1. Introduction
Biomechatronics (often abbreviated bionics) is an applied science that aims to tie interdisciplinary knots between biology and engineering (mechanical, electrical, and electronics engineering). The term bionic was coined by Steele in 1958 and popularized in science fiction films by the movie industry with humans given supernatural powers by electromechanical implants. It mimics how the human body works. Bionics is probably best known in development of prosthetic limbs, vision aids, robotics, and neuroscience. The bionics term overlaps with electrical medicine.
The gastrointestinal (GI) tract has always imposed challenges for studies of its function. This is due to the difficult access to remote parts of the GI tract that basically is a long tube with multiple compartments. The control of GI function is complex due to the layered structure and neural regulation.
During the last decades, bionics advancements have facilitated design and development of devices that can be ingested. Ingestible capsules contain cameras (Pillcam), pressure and pH sensors (Smartpill), or magnets that can be tracked. This has provided an excellent opportunity to study remote parts of the GI tract without apparent disturbance of the function. A recently introduced technology is the magnetic tracking system, which can measure motility, direction of movement, and transit time from the stomach, the small bowel and the colon [1]. Colon anatomy is well suited for 3D modeling of transit. A recent analysis has been utilized for classification of different motility patterns based on the velocity and length of magnetic capsule movements [2][3][4]. Insight into colonic motility offers many possibilities for classification of dysmotility symptoms or interventional testing of pharmacological treatments. However, despite the clinical need for new technology for the many patients with lower GI tract symptoms, current technology is limited, e.g., the magnetic tracking system is not commercially available, and often results differ between the technologies in use [5].
Within the past years a simulated stool named Fecobionics with the shape and properties of normal stool was developed [6][7][8][9][10][11]. Fecobionics records, in a single experiment, numerous parameters including pressures, bending, and shape changes during colonic transport and defecation of the device. Fecobionics has been used to study defecation patterns in large animals as well as humans (normal subjects and select patients groups including patients with symptoms of obstructed defecation and fecal incontinence). Very recently, it was applied in a canine colon model for evaluation of neuromuscular function and transit patterns [12]. Novel analysis such as the rear-front pressure (preload-afterload) diagrams and quantification of defecation indices have been developed. Furthermore, papers have been published on modeling of Fecobionics data [10][11].
Modeling of GI function is becoming more important than ever and is necessary for deeper understanding of the mechanical aspects or organ function, e.g., to predict progression of diseases. Advanced high-resolution clinical measurement technologies and rapid advances in computational power now allow construction of comprehensive mechanical models of GI organs [13][14][15][16][17][18][19][20].
2. Lower GI Tract Physiology and Functional Disorders
Colon serves to absorb water and salts from luminal content and pushes the content towards the rectum where it is stored before defecation. Normal colonic function depends on complex mechanisms involving intestinal neuromuscular circuits as well as signaling from the central nervous system. Various colonic contraction patterns have been identified [21]. The final transport path in the GI tract is the expulsion of fecal contents from the rectum. Anal continence and defecation involve anatomical factors, anorectal sensation, rectal compliance, stool consistency, anal muscle strength, mobility, and psychological factors [22]. In contrast to the intestines, the pelvic floor muscles are under conscious control. The homeostatic balance is easily disturbed by functional or structural anorectal disturbances that may coexist.
Defecation is the physiological process through which stools are eliminated from the rectum via the anal canal [23][24][25]. Defecation is initiated by urge to defecate predominantly resulting from filling of stool in the rectum. The abdominal pressure increases during defecation, the anal sphincter and puborectalis muscle relax, and the anorectal angle straightens [24][25][26]. The defecation process may be disordered, resulting in symptoms such as fecal incontinence, constipation, and pain (proctalgia) [22]. Colonic and defecatory disorders affect 25% of the population with rising incidence [22][23][27][28]. These disorders pose a major health care burden but are poorly recognized and treated [22]. For example, constipation refers to abnormally delayed or infrequent passage of usually dry hardened feces that may be associated with pain during defecation. Chronic constipation (CC) is a symptom of possibly several underlying pathophysiologic processes and affects 12–19% of Americans [29]. The etiology of CC is multifactorial. Slow colonic transit and anorectal disorders are major causes of CC. Anorectal sensitivity and contractility, stool consistency, rectal reservoir capacity and compliance, and coordination of the pelvic floor muscles play important role in the genesis of obstructed defecation. Etiology of slow colonic transit constipation includes low residual diet, female sex hormones, medication that inhibit peristalsis and coordination (such as opioids and anticholinergics), and neuromuscular disorders of the colonic smooth muscles.
There are many tests available currently to study anorectal motility disorders but it is not clear whether the tests identify the precise pathophysiologic abnormality. Commonly used tests are high-resolution anal manometry (HRAM), barium and MR defecography, balloon expulsion test (BET), and barostat testing for the rectal sensory function [22][26][30][31][32][33]. These tests have significant limitations; e.g., HRAM does not record dynamic events during real defecation, defecography does not provide information on anorectal sensation and motility, and BET does not provide physiologic information; i.e., geometry and pressure changes during the passage of simulated stool from the rectum through the anal canal. Furthermore, there is considerable disagreement between the results of various anorectal tests and not surprisingly the test results correlate poorly with symptoms and treatment outcomes [5]. Most importantly, there is no integrated diagnostic test to define the anorectal dysfunction in obstructed defecation. The need for physiologically relevant and easy-to-use diagnostic tests for identifying underlying mechanisms is substantial.
Colon is more difficult to study than anorectum due to the remote access and lack of technology. Ingestible markers visible by radiography or magnetic sensors have been used for years to study colonic transit. This is important for diagnosis of slow transit constipation but has little value for studying irritable bowel syndrome. The recent development of fiber-optic high-resolution manometry catheters with more than 72 pressure sensors has advanced the field. Recordings have been made of motor events in the entire colon over prolonged periods of time [34][35]. Various studies describe patterns of colonic motor events including high amplitude peristaltic contractions and retrograde movement of markers [21][36]. However, the abovementioned in vivo technologies have not been able to detect the shallow waves observed in colon preparations from rabbits or rodents in vitro [21]. These shallow waves are believed to play an important function for slowing colonic transit and for mixing [21].
3. Design Considerations for Fecobionics and Principle of Measurement
To be a truly bionics device for colon and anorectal transit studies, the shape and consistency of Fecobionics must be like feces and measure relevant physiological data. Indeed, the shape and properties of Fecobionics were designed to be similar to the most common form of feces in normal subjects according to the Bristol stool form scale [37]. Feces consistency changes along colon and a choice has to be made which part of colon to simulate best since feces becomes more solid during the transit. Colon and anorectal transport of feces depends on several factors that are desirable to measure. The most relevant parameters are the pressures (forces) acting on the surface of feces, the size of the intestinal conduit, friction between feces and the intestinal mucosa, and bends of the conduit. Axial pressure in the direction of the trajectory is of particular interest. Distensibility and sensitivity are other parameters of importance. All parameters must be considered in design of a bionics device for simulation of GI transport. Fecobionics is conveniently designed to quantitate the mentioned parameters. The most important variables are briefly discussed below for anorectal applications. Derived parameters are discussed in the section on detailed analysis and modeling.
4. Fecobionics Assessment of Functional Data
Anorectal Fecobionics data have been published on normal human subjects as well as in a small number of patients with fecal incontinence and constipation [7][8][9]. Most attention has until now been given to pressure analysis. Typically, the front, rear, and bag pressures, as well as the difference between front and rear pressures are plotted as function of time (Figure 1). Endpoints such as the resting and maximum pressures can be derived from the plots. Based on pressure-time plots, it was possible to divide defecations into five phases based on distinct pressure patterns [7]. Rear-front pressure (preload-afterload) plots are another informative way to express pressure data. The preload-afterload concept is known from cardiology, where left ventricle pressure-volume measurements provide substantial insights into heart contractility, preload (heart filling), and afterload (vascular resistance) [24][38][39]]. This type of analysis is beneficial for understanding anorectal function.
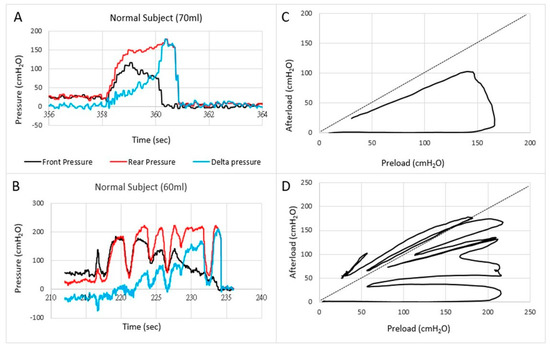
Figure 1. Examples of pressures as a function of time (A,B) and the front pressure plotted as a function of the rear pressure in two normal subjects (C,D). The first contraction usually follows the line of pressure unity where after the front pressure progressively decreases due to anal sphincter relaxation. In some subjects, this happened in one contraction (C) whereas others use several contractions (D).
Translation of the learnings from cardiology to gastroenterology for Fecobionics testing, the filling of the bag inside the rectum until the subject feels the urge, corresponds to the preload. At this point, the subject initiates abdominal contractions to generate the propulsive force needed to expel the device. The driving force is picked up by the rear pressure sensor whereas the front pressure sensor records the afterload. The diagram allows evaluation of pressure cycles without the time element. When the front pressure exceeds rear pressure, data are above the unity line (defecation cannot happen against a pressure gradient). Fecobionics (and feces) will be expelled when the recto-anal pressure gradient is large enough to overcome the friction between the surface and mucosa. Measurement of axial pressures at front and rear, and the bag pressure is essential in this regard. Repeated contractions shift the tracings downwards where a cut-off is reached, i.e., the anal pressure drops quickly followed by device expulsion [7][8][9]. Afterload is the resistance that the propulsive force must work against to evacuate feces. The resistance depends on several factors including anal diameter, anal pressure, anorectal angle, and friction. The diagrams show clockwise contraction cycles, for normal subjects usually 2–5 cycles, reflecting the number of abdominal muscle contractions that are needed to defecate. Fecobionics is uniquely designed to quantify these preload-afterload properties. Figure 1 shows representative defecations from two normal subjects. For comparison, fecal incontinence patients are often below the unity line and defecate instantly. In contrast, constipation patients are often above the line of unity for an extended time and use multiple contraction attempts.
The preload–afterload diagrams are very intuitive but must be quantified. Therefore, defecation indices are computed as the areas under the front pressure curve (reflecting anal resistance) and rear pressure curve (reflecting propulsive force). The defecation indices can be normalized with respect to duration, maximum pressure amplitude, as well as other factors. The general learnings from yet unpublished studies is that fecal incontinence patients have a low index whereas constipation patients have high index. Data points to that subtypes exist both for fecal incontinence and constipation patients. The afterload seems especially important since obstructed (dyssynergic) defecation [40][41][42] and anal stricture will be associated with increased afterload. The preload and afterload may be clinically important for differentiating subtypes of patients. For example, the current dyssynergia classification [40][41][42] uses a 2 × 2 diagram where two subtypes show abnormal expulsion pressures and two subtypes are associated with anal sphincter function. The classification is being criticized for being too simple and dyssynergic abnormality has been found in 90% of healthy subjects with HRAM [43]. Preliminary data with Fecobionics point to that more than four subtypes exist. This needs further investigation.
Fecobionics has recently been applied to the colon in a canine study [12]. From in vitro experiments in rodents and rabbits, it was well known that shallow contractions (ripples) exist [21]. These were recorded as subtle diameter changes detected by video capture. However, such waves are not detectable with manometry. Fecobionics was able to pick up shallow antegrade and retrograde contractions in the canine colon, as well as antegrade peristaltic contractions. The shallow contractions are believed to serve an important function for slowing down colonic transit and facilitate mixing of fecal content. Figure 2 shows an example of shallow contractions in the transverse colon. The color contour topography plot, originally developed by Clouse for high-resolution manometry, is employed [44].
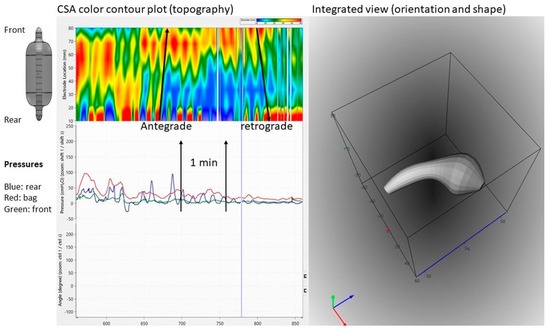
Figure 2. Graphical user interface showing data recordings from the canine colon. Upper left: Color contour plot generated from multiple cross-sectional area (CSA) recordings demonstrating antegrade and retrograde shallow waves that are not recorded by manometry. Bottom left: Pressure recordings from the front, bag, and rear. Right: 3D plot of the orientation, shape, and bending of Fecobionics (at the time point indicated by the vertical marker in the left diagrams.
References
- Haase, A.M.; Gregersen, T.; Schlageter, V.; Scott, M.S.; Demierre, M.; Kucera, P.; Dahlerup, J.F.; Krogh, K. Pilot study trialling a new ambulatory method for the clinical assessment of regional gastrointestinal transit using multiple electromagnetic capsules. Neurogastroenterol. Motil. 2014, 26, 1783–1791.
- Mark, E.B.; Poulsen, J.L.; Haase, A.M.; Espersen, M.; Gregersen, T.; Schlageter, V.; Scott, S.M.; Krogh, K.; Drewes, A.M. Ambulatory assessment of colonic motility using the electromagnetic capsule tracking system. Neurogastroenterol. Motil. 2019, 31, e13451.
- Mark, E.B.; Klinge, M.W.; Grønlund, D.; Poulsen, J.L.; Schlageter, V.; Scott, S.M.; Krogh, K.; Drewes, A.M. Ambulatory assessment of colonic motility using the electromagnetic capsule tracking system: Effect of opioids. Neurogastroenterol. Motil. 2020, 32, e13753.
- Mark, E.B.; Bødker, M.B.; Grønlund, D.; Østergaard, L.R.; Frøkjaer, J.B.; Drewes, A.M. MRI analysis of fecal volume and dryness: Validation study using an experimental oxycodone-induced constipation model. J. Magn. Res. Imaging 2019, 1, 1–13.
- Palit, S.; Thin, N.; Knowles, C.H.; Lunniss, P.J.; Bharucha, A.E.; Scott, S.M. Diagnostic disagreement between tests of evacuatory function: A prospective study of 100 constipated patients. Neurogastroenterol. Motil. 2016, 28, 1589–1598.
- Sun, D.; Huang, Z.; Zhuang, Z.; Ma, Z.; Man, L.K.; Liao, D.; Gregersen, H. Fecobionics: A novel bionics device for studying defecation. Ann. Biomed. Eng. 2019, 47, 576–589.
- Gregersen, H.; Krogh, K.; Liao, D. Fecobionics: Integrating anorectal function measurements. Clin. Gastroenterol. Hepatol. 2018, 16, 981–983.
- Gregersen, H. Fecobionics: A novel bionic test of anorectal function and defecation. Gastroenterology 2017, 152, S317.
- Gregersen, H.; Chen, S.C.; Leung, W.W.; Wong, C.; Mak, T.; Ng, S.; Futaba, K. Novel Fecobionics Defecatory Function Testing. Clin. Transl. Gastroenterol. 2019, 10, e00108.
- Liao, D.; Chen, A.S.; Lo, K.M.; Zhao, J.; Futaba, K.; Gregersen, H. Theoretical tools to analyze anorectal mechanophysiological data generated by the Fecobionics device. J. Biomech. Eng. 2019.
- Sun, D.; Liao, D.; Chen, S.S.; Wong, C.; Leung, W.W.; Futaba, K.; Mak, T.; Ng, S.; Gregersen, H. Mechanophysiological analysis of anorectal function using simulated feces in human subjects. J. Adv. Res. 2020.
- Gregersen, H.; Wang, Y.; Guo, X.; Field, F.; Nelsen, M.; Combs, W.; Wang, M.; Kassab, G.S. Simulated colonic feces reveals novel contraction patterns. Gastroenterology 2020.
- Liao, D.; Zhao, J.; Gregersen, H. Gastrointestinal tract modelling in health and disease. World J. Gastroenterol. 2009, 15, 169–176.
- Cheng, L.K.; Du, P.; O’Grady, G. Mapping and modeling gastrointestinal bioelectricity: From engineering bench to bedside. Physiology 2013, 28, 310–317.
- Cheng, L.K.; Komuro, R.; Austin, T.M.; Buist, M.L.; Pullan, A.J. Anatomically realistic multiscale models of normal and abnormal gastrointestinal electrical activity. World J. Gastroenterol. 2007, 13, 1378–1383.
- Cheng, L.K.; O’Grady, G.; Du, P.; Egbuji, J.U.; Windsor, J.A.; Pullan, A.J. Gastrointestinal system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 65–79.
- Liao, D.; Gregersen, H.; Hausken, T.; Gilja, O.H.; Mundt, M.; Kassab, G. Analysis of surface geometry of the human stomach using real-time 3-D ultrasonography in vivo. Neurogastroenterol. Motil. 2004, 16, 315–324.
- Liao, D.; Zhao, J.; Gregersen, H. Biomechanical functional and sensory modelling of the gastrointestinal tract. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2008, 9, 3281–3299.
- Noakes, K.F.; Bissett, I.P.; Pullan, A.J.; Cheng, L.K. Anatomically realistic three-dimensional meshes of the pelvic floor & anal canal for finite element analysis. Ann. Biomed. Eng. 2008, 36, 1060–1071.
- Du, P.; Paskaranandavadivel, N.; Angeli, T.R.; Cheng, L.K.; O’Grady, G. The virtual intestine: In silico modeling of small intestinal electrophysiology and motility and the applications. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 69–85.
- Corsetti, M.; Costa, M.; Bassotti, G.; Bharucha, A.E.; Borrelli, O.; Dinning, P.; Di Lorenzo, C.; Huizinga, J.D.; Jimenez, M.; Rao, S.; et al. First translational consensus on terminology and definitions of colonic motility in animals and humans studied by manometric and other techniques. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 559–579.
- Rao, S.S.; Bharucha, A.E.; Chiarioni, G.; Felt-Bersma, R.; Knowles, C.; Malcolm, A.; Wald, A. Functional Anorectal Disorders. Gastroenterology 2016, 150, 1430–1442.
- Barleben, A.; Mills, S. Anorectal anatomy and physiology. Surg. Clin. 2010, 90, 1–15.
- Gregersen, H.; Christensen, J. Clinical Biomechanics in the Gut. An Introduction; Bentham Science: Sharjah, UAE, 2016; ISBN 978-1-68108-119-9.
- Gibbons, C.P. The mechanics of the anal sphincter complex. J. Biomech. 1988, 21, 601–604.
- Bharucha, A.E. Update on tests of colon and rectal structure and function. J. Clin. Gastroenterol. 2006, 40, 96–103.
- Higgins, P.D.; Johanson, J.F. Epidemiology of constipation in North America: A systematic review. Am. J. Gastroenterol. 2004, 99, 750–759.
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.
- Sonnenberg, A.; Koch, T.R. Epidemiology of constipation in the United States. Dis. Colon. Rect. 1989, 32, 1–8.
- Tirumanisett, P.; Prichard, D.; Fletcher, J.G.; Chakraborty, S.; Zinsmeister, A.R.; Bharucha, A.E. Normal values of assessment of anal sphincter morphology, anorectal motion, and pelvic organ prolapse with MRI in healthy women. Neurogastroenterol. Motil. 2018, 30, e13314.
- Van Koughnett, J.A.M.; da Silva, G. Anorectal physiology and testing. Gastroenterol. Clin. N. Am. 2013, 42, 713–728.
- Chiarioni, G.; Kim, S.M.; Vantini, I.; Whitehead, W.E. Validation of the balloon evacuation test: Reproducibility and agreement with findings from anorectal manometry and electromyography. Clin. Gastroenterol. Hepatol. 2014, 12, 2049–2054.
- Carrington, E.V.; Heinrich, H.; Knowles, C.H.; Fox, M.; Rao, S.; Altomare, D.F.; Bharucha, A.E.; Burgell, R.; Chey, W.D.; Chiarioni, G.; et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol. Motil. 2019, e13679.
- Dinning, P.G. A new understanding of the physiology and pathophysiology of colonic motility? Neurogastroenterol. Motil. 2018, 30, e13395.
- Dinning, P.G.; Di Lorenzo, C. Colonic dysmotility in constipation. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 89–101.
- Corsetti, M.; Pagliaro, G.; Demedts, I.; Deloose, E.; Gevers, A.; Scheerens, C.; Rommel, N.; Tack, J. Pan-Colonic Pressurizations Associated With Relaxation of the Anal Sphincter in Health and Disease: A New Colonic Motor Pattern Identified Using High-Resolution Manometry. Am. J. Gastroenterol. 2017, 112, 479–489.
- Heaton, K.W.; Radvan, J.; Cripps, H.; Mountford, R.A.; Braddon, F.E.; Hughes, A.O. Defecation frequency and timing, and stool form in the general population: A prospective study. Gut 1992, 33, 818–824.
- Gregersen, H. Biomechanics of the Gastrointestinal Tract; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 1852335203.
- Takeuchi, M.; Odake, M.; Takeoka, H.; Hayashi, Y.; Hata, K.; Yokoyama, M. Comparison between preload recruitable stroke work and he end-systolic pressure volume relationship in man. Eur. Heart J. 2003, 13, 80–84.
- Rao, S.S.C.; Patcharatrakul, T. Diagnosis and treatment of dyssynergic defecation. J. Neurogastroenterol. Motil. 2016, 22, 423–435.
- Rao, S.S.; Tuteja, A.K.; Vellema, T.; Kempf, J.; Stessman, M. Dyssynergic defecation: Demographics, symptoms, stool patterns, and quality of life. J. Clin. Gastroenterol. 2014, 38, 680–685.
- Heinrich, H.; Sauter, M.; Fox, M.; Weishaupt, D.; Halama, M.; Misselwitz, B.; Buetikofer, S.; Reiner, C.; Fried, M.; Schwizer, W.; et al. Assessment of obstructive defecation by high-resolution anorectal manometry compared with magnetic resonance defecography. Clin. Gastroenterol. Hepatol. 2015, 13, 1310–1317.
- Grossi, U.; Carrington, E.V.; Bharucha, A.E.; Horrocks, E.J.; Scott, S.M.; Knowles, C.H. Diagnostic accuracy study of anorectal manometry for diagnosis of dysynergic defecation. Gut 2016, 65, 447–455.
- Clouse, R.E.; Staiano, A.; Alrakawi, A.; Haroian, L. Application of topographical methods to clinical esophageal manometry. Am. J. Gastroenterol. 2000, 95, 2720–2730.




