
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Subroto Babul Chatterjee | + 1039 word(s) | 1039 | 2021-02-24 04:44:04 | | | |
| 2 | Vicky Zhou | + 74 word(s) | 1113 | 2021-03-02 07:31:58 | | |
Video Upload Options
Lactosylceramide (LacCer), also known as CD17/CDw17, is a member of a large family of small molecular weight compounds known as glycosphingolipids. It plays a pivotal role in the biosynthesis of glycosphingolipids, primarily by way of serving as a precursor to the majority of its higher homolog sub-families such as gangliosides, sulfatides, fucosylated-glycosphingolipids and complex neutral glycosphingolipids—some of which confer “second-messenger” and receptor functions. LacCer is an integral component of the “lipid rafts,” serving as a conduit to transduce external stimuli into multiple phenotypes, which may contribute to mortality and morbidity in man and in mouse models of human disease.
1. Introduction
Glycosphingolipids (GSLs) are a family of small molecular weight molecules composed of fatty acids, sugars, and an amino acid. The biosynthesis of sphingolipids is initiated upon the condensation of its two fundamental constituents, palmitoyl-coenzyme A (palmityl-CoA) and L-serine, to form sphinganine. Next, the addition of another fatty acid leads to the formation of ceramide (Figure 1). Subsequently, sugars such as glucose or galactose are sequentially added via specific glycosyltransferases to ceramide giving rise to glucosylceramide, galactosylceramide, and about 300 complex GSLs [1]. The large body of literature gathered over the last three decades amply describe the important roles of sphingosine, sphingosine-1-phosphate (S1P) [2], and ceramide (Cer) [3] in regulating critical phenotypes such as cell proliferation, angiogenesis, and apoptosis. In fact, ceramide has become synonymous to apoptosis. Nevertheless, there are some critical or beneficial functions of ceramide such as serving as a barrier in the skin, which covers nearly the entire mammalian body. This is an area of ceramide biology that requires discussion. Thus, this review will attempt to bring this beneficial function of ceramide to the attention of readers. The next step in GSL synthesis involves the transfer of galactose from uridine diphosphate galactose (UDP-galactose) to GlcCer to form lactosylceramide (LacCer, Figure 1). LacCer can then be used to synthesize more complex GSLs, such as globosides, sulfatides, and gangliosides, in the Golgi apparatus [4].
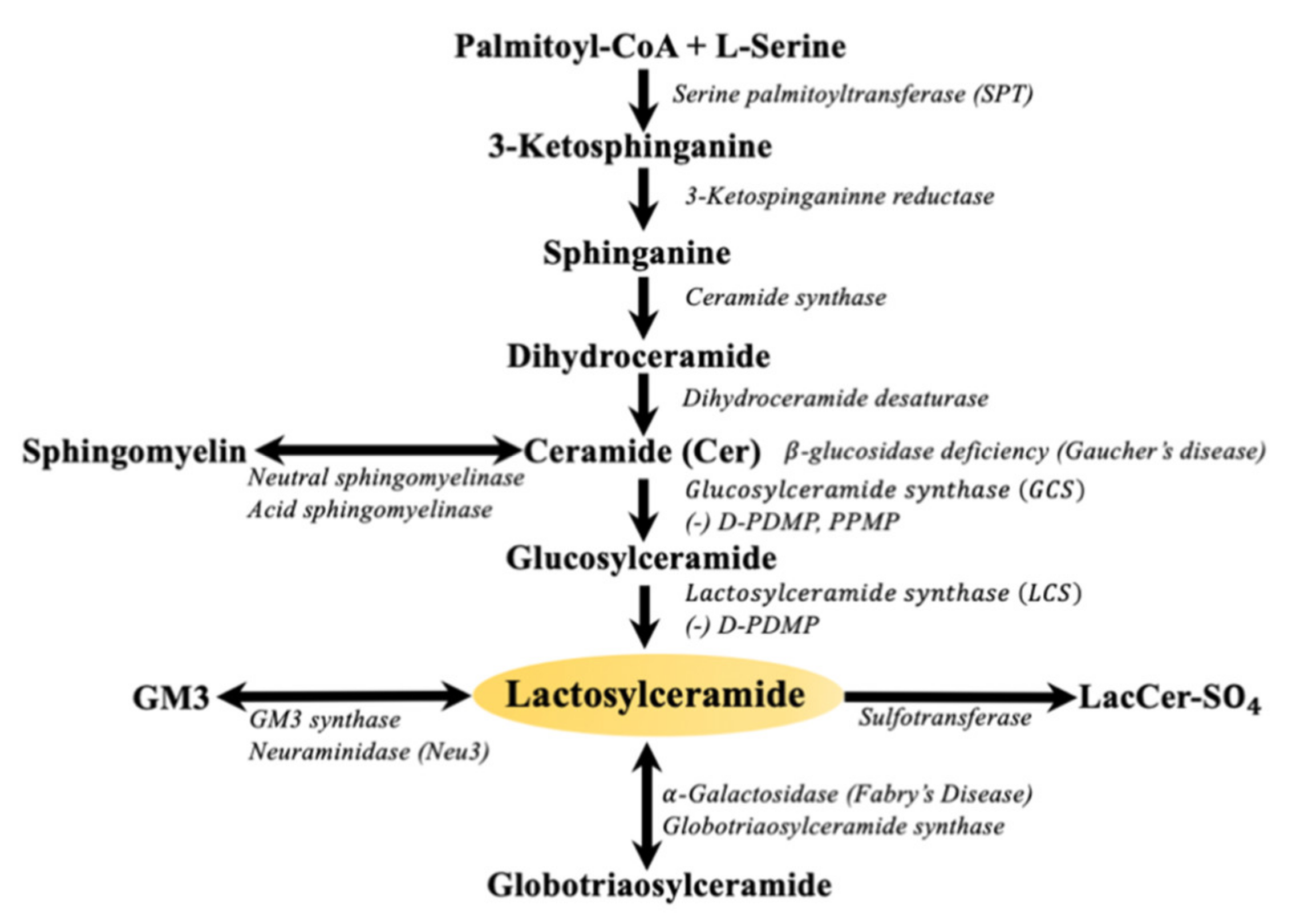
In contrast to lipids used for structure and energy, bioactive lipids, which include sphingolipids, ceramide, sphingosine, and sphingosine-1-phosphate, are lipids that respond to specific stimuli and are parts of signaling pathways [3]. There is evidence that GlcCer and LacCer may be considered bioactive lipids. For instance, exogenous LacCer treatment is associated with increased cell adhesion, angiogenesis, reactive oxygen species, and inflammation independent of the above bioactive lipids [5].
2. Convergence: Lactosylceramide-Centric Signaling Pathways Induce Inflammation, Oxidative Stress, and Other Phenotypic Outcomes
The past few decades have witnessed a marked advancement in the development of mass spectrometry technology to quantify lipids, the availability of mouse models of human disease and pathology, and several molecular tools to dissect signaling pathways. These developments have led to numerous pre-clinical studies that help us better understand the interplay of GSLs in vascular biology and in the pathophysiology of diseases in order to develop novel drug targets and biomarkers of diseases. Thus, the major conclusions drawn in the field of LacCer metabolism are the following (Figure 2):
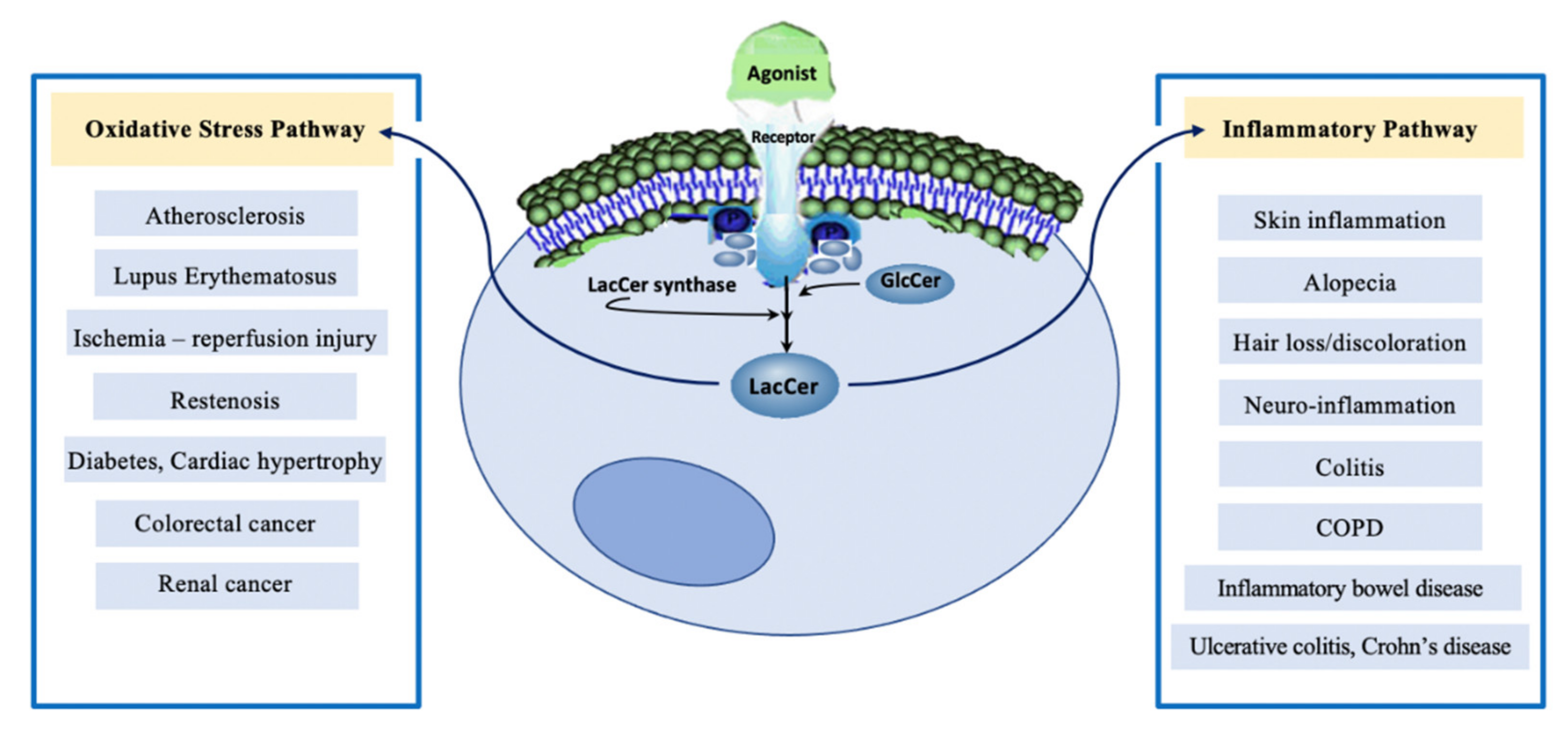
- The LacCer-centric inflammatory pathway can begin to explain the pathophysiology of skin inflammation, neuro-inflammation, and hair greying/loss due to aging as well as other inflammatory diseases, e.g., COPD and inflammatory bowel disease.
- A western diet, which induced skin inflammation, hair loss, and hair discoloration, implicates the LacCer biosynthetic pathway involving neutrophil infiltration and TSG-6 expression. This was reversed by blocking LacCer synthesis and raising skin ceramide levels.
- TNF-α, a major pro-inflammatory cytokine, recruits players in the LacCer-centric inflammatory pathway, such as cPLA2 enzyme to generate eicosanoids/prostaglandins, leading to inflammation, neutrophil migration/infiltration, and expression of neutrophil/monocyte cell adhesion molecule, Mac-1 (CD11b). This LacCer-centric inflammatory pathway is shared in neuro-inflammation, ulcerative colitis, autophagy in emphysema due to COPD and cigarette smoking.
- The LacCer-centric oxidative stress pathway may increase our understanding of atherosclerosis and lupus erythematosus via downstream generation of ROS to: (a) oxidize LDL in a cyclical fashion, raising the blood levels of oxidized LDL, (b) induce cell proliferation via activating phosphokinases Akt-1 and mTOR-C1 signaling cascade, (c) regulate cell–cell adhesion involving cell adhesion molecules, ICAM-1, PECAM-1, and Mac-1 (CD11b), (d) cause shear stress induced mechano-transduction and ischemia reperfusion injury, and (e) activate the cPLA2 enzyme and PECAM-1 gene expression to induce angiogenesis required for atherosclerotic plaque survival and cancer metastasis and tumor growth in colorectal cancer and possibly other types of cancer.
- LacCer synthase is recruited and activated by VEGF and β-FGF to induce angiogenesis in vitro and in vivo via increased expression of ICAM-1 and PECAM-1 followed by monocyte and neutrophil TEM contributing to inflammation.
- LacCer plays an important role in mitochondrial function by downregulating respiration and Ca2+ retention in diabetic rats. Both in mitochondria of diabetic mice and in N-SMase-deficient cells, ceramide generation may not be sufficient to induce apoptosis and necrosis; its glycosylation to LacCer is required.
In sum, LacCer synthase is a target for the convergence of diverse agonists that activate this enzyme to generate LacCer. In turn, LacCer activates NADPH oxidase to present an “oxidative stress” environment leading to several phenotypes in vivo and in vitro. LacCer can also activate the inflammatory pathway by activating cPLA2 and a cascade of other molecules. Both these pathways can be reversed or controlled by blocking LacCer synthase with the use of gene manipulation, glycosyltransferase inhibitors, and/or other therapies.
3. Perspectives
- We are only beginning to understand the role of LacCer synthase in other inflammatory diseases such as Lupus Erythematosus. As Lupus predominantly afflicts women in their early reproductive years, this commands greater and urgent attention.
- In diabetes, the mitochondria—the powerhouse of energy production and Ca2+ metabolism—becomes dysfunctional presumably due to increased LacCer levels. Further studies, including the targeting of drugs to improve mitochondrial health, are needed in this area. Such studies, in turn, will improve cardiac health.
- Since knowledge of GlcCer and LacCer levels has increased the predictive value of atherosclerosis and diabetes disease onset and progression and cardiac function, these measurements could be prescribed as routine tests in clinical settings.
- The pre-clinical studies above have provided a wealth of information on the role of LacCer synthase in several inflammatory diseases, cancer and atherosclerosis to set the stage for future human trials using various therapeutic modalities.
References
- Basu, S.; Kaufman, B.; Roseman, S. Enzymatic synthesis of ceramide-glucose and ceramide lactose by glycosyltransferase from embryonic chick brain. J. Biol. Chem. 1968, 243, 5802–5804.
- Spiegel, S. Sphingosine-1-phosphate: From insipid lipid to a key regulator. J. Biol. Chem. 2020, 295, 3371–3384.
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell. Biol. 2018, 19, 175–191.
- Schömel, N.; Geisslinger, G.; Wegner, M.S. Influence of glycosphingolipids on cancer cell energy metabolism. Prog. Lipid Res. 2020, 79, 101050. [PubMed]
- Chatterjee, S. Sphingolipids in atherosclerosis and vascular biology. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1523–1533.




