| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan C. Mejuto | + 4377 word(s) | 4377 | 2021-02-23 06:58:38 | | | |
| 2 | Camila Xu | Meta information modification | 4377 | 2021-03-04 09:56:28 | | |
Video Upload Options
Cyclodextrins (CDs) are cyclic oligomers broadly used in food manufacturing as food additives for different purposes, e.g., to improve sensorial qualities, shelf life, and sequestration of components.
1. Introduction
Numerous investigations have been focused on developing relatively simple organic compounds able to catalyze organic reactions, like enzymes. Since the discovery of enzymes’ active sites, new models of synthesis of nonpeptide organic systems that could simulate the enzymatic behavior have been developed. Chemists have been developing more sophisticated molecular structures within the nanometric order. However, even though apparently the easiest way to solve it would be to assemble individual molecules directly, the most efficient alternative is to generate molecules with a complementary form capable of spontaneously self-organizing, resulting in orderly assemblies. This can be achieved, for example, with host and guest molecules [1].
Host–guest complexes are molecular aggregates stabilized via noncovalent bonds (for example, van der Waals, hydrogen bonds, and hydrophobic interactions), but never by complete covalent bonds. Host molecules are characterized by having an inner cavity where another molecule can be incorporated, this is, the guest molecule. Therefore, hosts will act as receptors and guests as substrates, inhibitors, or cofactors [2]. The resulting molecular inclusion complex can easily break under determined physiological environments [3].
Therefore, the establishment of these systems can improve physicochemical properties of the guest molecule. Different types of host molecules have been developed, but they are all characterized by acting as non-natural receptors capable of partial or total enclosure of the compounds of interest (drugs, active compounds, etc.). Likewise, numerous hosts-guest complexes have already been synthesized. Among them, crown ethers, cryptands, spherands, carcerands, and cyclodextrins (CDs) can be highlighted.
CDs are a group of relatively recent discovered compounds, which are also identified as cycloamyloses, cyclomaltoses, and Schardinger dextrins [4]. Their first reference dates from 1891. In this year, a crystalline product was isolated during a starch fermentation carried out by Bacillus amylobacter. Some authors reflected that this production was probably due to impurities in cultures and, consequently, it was supposed that CDs might be produced by cross-contamination with another species (B. macerans). Later, in 1903, two crystalline components were isolated: dextrins A and B, which were characterized by their lack of reducing power. Even so, the bacterial strain responsible of its production was unfortunately not preserved [5]. A year later, the same authors were able to isolate an organism capable of producing acetone and ethyl alcohol from plant materials that contained sugar and starch. In 1911, it was discovered that a strain of B. macerans produced large amounts of crystalline dextrins (25–30%) from starch too. These resulting products were named as crystallized dextrin α and crystallized dextrin β [4]. It was not until 1935 that the third type of dextrin (dextrin γ) was isolated. Several fractionation processes were also developed in order to produce CDs [5]. At that point, the structure of said molecules was still a mystery. It was not fully discovered until 1942, when the structure of two CDs (α and β) was established through X-ray crystallography. A few years later (1948), the same method was applied to γ-CDs. Later, in 1961, more types of CDs (9–12 residues) were discovered [6].
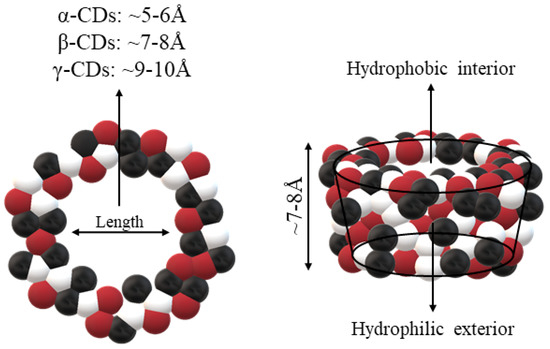
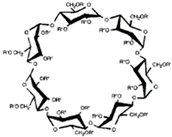
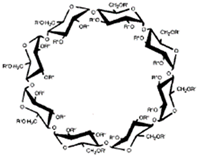
CDs are one of the most used hosts-guest complexes for organic molecules. Their structure consists of a cyclic arrangement of D (+)-glucopyranose units joined by α-(1 → 4) glycosidic links. They can be differentiated according to their number of glucoses in α-, β- and γ-CDs (6, 7, and 8 glucose units, respectively) (Table 1) [7][8]. Regarding its form, it is a truncated cone characterized as bearing primary and secondary OH groups, respectively, in the slim and wide rim. It is characterized by having cavities with hydrophobic properties. In this case, as in other complexes, Van der Waals and hydrophobic forces are responsible for keeping guest and host together with a partial or total adjustment of the cavity. Due to the establishment of an inclusion complex, guest reactivity can vary, making possible its use in a wide variety of fields [9]. Furthermore, as these receptors can improve bioavailability, they are suitable for functional delivery systems [10]. They can be found in food, pharmaceuticals, cosmetics, the textile industry, conversion and fermentation processes, and environmental and other chemical systems and applications. However, the development of hosts is still ongoing, the development of processes not only simple and reproducible, but also economically profitable is fundamental so that their widespread acceptance would be guaranteed in diverse fields such as food and drug production [11].
Table 1. Main cyclodextrins (CDs) properties [5][17].
| Properties | Unit | α-CDs | β-CDs | γ-CDs |
|---|---|---|---|---|
| Formula | C36H60O30 | C42H70O35 | C48H80O40 | |
| Structure | 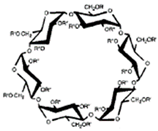 |
 |
 |
|
| Mol wt | 972.84 | 1134.98 | 1297.12 | |
| Glucopyranose units | 6 | 7 | 8 | |
| Solubility (water, 25 °C) | % w/v | 14.5 | 1.85 | 23.2 |
| Outer diameter | Å | 14.6 | 15.4 | 17.5 |
| Cavity diameter | Å | 4.7–5.3 | 6.0–6.5 | 7.5–8.3 |
| Height of torus | Å | 7.9 | 7.9 | 7.9 |
| Cavity volume | Å3 | 174 | 262 | 427 |
| Crystal form | Hexagonal plates | Monoclinic parallelograms | Quadratic prisms | |
| Others | Most accessible, lowest-priced and generally the most useful |
Regarding the importance and new advances on the field of CDs and food science, some recent reviews have been focused on the latest updates of the use of CDs in food products such as nanoparticles, nanosensors, extraction enhancers, active or smart packaging, and their most widespread use as carriers and complexes formers to protect and stabilize bioactive compounds with the aim of improving the final product [12][13]. However, future research is also focused on CDs properties and their interaction with other materials (e.g., durability, stability, or dispersion), new applications (e.g., CDs immobilization for wastewater treatment) or the development of green chemistry processes [14]. They have also been used for sequester certain molecules (i.e., cholesterol), as modulators for medicinal biomaterials, as elicitors or as synergistic agents for the production of secondary metabolites in plants [15][16].
2. Cyclodextrins
2.1. Definition
Industrially, CDs are enzymatically produced with cyclodextrin glucosyltransferase from modified starch. This is an economic process, whose production is estimated in 150 tons per year [7]. The resulting product is nontoxic, characterized by not being absorbed in the upper gastrointestinal tract and being entirely processed by the colon microflora [18].
Natural CDs are also characterized by poor solubility. At the beginning, this was a major inconvenience since it prevented the use of CDs as effective complexing agents. It was not until the end of the 1960s when it was revealed that, by means of chemical replacements at positions 2, 3, and 6-hydroxyl, the solubility was highly increased. In addition, depending on the grade of chemical replacement and the type of the substituents, the maximum concentration of CDs in aqueous mediums was improved. The maximum concentration that most chemically modified CDs can reach is 50% (w/v) in water by carrying out substitutions at the 2, 3, and 6 hydroxyl like (2-Hydroxypropyl)-β-CDs [8].
2.2. Mechanism
Some parameters must be considered for the study of CDs mechanism of action. The size of the cavity is essential to choose which type of CDs is more suitable to use in the interaction. The size is related to the degree of adjustment, which is a critical parameter to achieve optimal CDs incorporation. Therefore, each type of CDs will have different types of cavities. The α-CDs possess small cavities, so that they are not able to accept many molecules. The γ-CDs are larger in size compared to most molecules that can be complexed. In addition, hydrophobic CDs loads cannot efficiently interact to simplify complex formation. Therefore, it is considered that the diameter of the cavity of β-CDs is the most appropriate for molecules such as hormones, vitamins, and other compounds commonly used in tissue and cell culture applications. All these features make β-CDs the CDs of choice as complexing agent in most cases [19].
The structure of CDs allows the enclosure of hydrophobic molecules, such as vitamins and lipid soluble hormones, improving their solubility in aqueous systems. The process can be reversed by diluting the complex in a larger volume of solvent. As a result, the molecule of interest is released into the environment. The structure of the resulting complexes (Figure 1) is like a cage, with the same characteristics as those formed by cryptands, calixarenes, cyclophanes, spheres, and crown ethers. As mentioned before, all these molecules are involved in chemical reactions due to non-covalent bonds, with most of the reactions happening as the “host–guest” type [19][20].
Regarding their physical characteristics, CDs complexes can get wet; they are almost odorless and non-hygroscopic powders [18][23]. Their mechanical properties (crystallization, flow, etc.) depend on the complex formation process. The moisture content and temperature present an enormous importance in controlling the deformation or release of the CDs-guest. Other parameters to consider can be seen in Figure 2. CDs are crystallized by two main mechanisms that, depending on the type of compounds that make up the complex (CDs and guest), turn into to two key categories of crystal packing (channel or cage structures) [5]. An example of these molecules is the flavor/β-CDs complexes, which are formed after a co-crystallization, kneading, and suspension process [18].
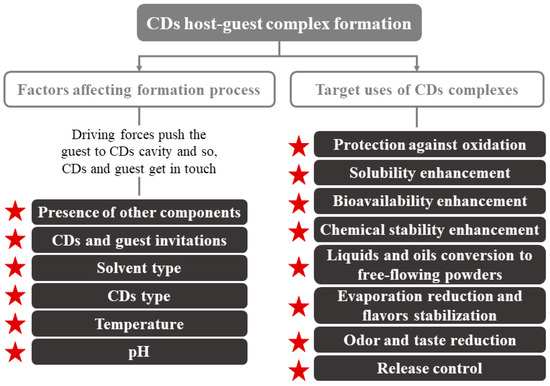
Figure 2. Factors affecting CDs inclusion complex formation and main application of CDs. Adapted from [22].
3. Applications in the Food Industry
CDs are used primarily in foods for the encapsulation of compounds of interest and the improvement of water retention, since they are hygroscopic compounds [18]. Their use can enhance several technological advantages, such as more homogeneous compositions that are easier to be standardized [18][24]. Numerous applications of CDs have been described, e.g., improving the organoleptic quality (total or partial elimination of undesired flavors/odors), increasing food shelf life, component sequestration, and pickering emulsions, among others (Figure 2).
3.1. Improving Sensorial Qualities
3.1.1. Color
Food color is the first quality parameter assessed by customers, so it is a key parameter of food quality [25]. CDs can be applied to modulate food color by increasing solubility and chemical stability of coloring compounds (natural ones and coloring components produced during food processing). They can provoke the inhibition of pro-browning polyphenol-oxidase reactions by complexing with several substrates or cofactors (e.g., chlorogenic acid, polyphenols, cinnamic acid, Cu2+) [22]. Several studies have proven the utility of CDs in food science. For instance, a study showed that the natural pigments curcumin and lycopene can form a complex with CDs, improving their solubility and reducing the degree of oxidation compared to the compounds separately [26]. Another study, conducted with chopped ginger root, showed that by adding 1–4% of CDs, the sample could be enzymatic browning stabilized for four weeks at 5 °C while being vacuum sealed. A similar inhibitory effect was observed in another study carried out with maltosyl-β-CDs in apple and pear juices. The mechanism consists of preventing ascorbic acid oxidation by an antioxidant effect, which maintains the color and the food quality [5]. α-, β-, and γ-CDs (the only CDs allowed in food industry by the U.S. Food and Drug Administration and EU) are commonly used in the elaboration of different juices to improve the color of the final product. The addition of these molecules has other effects as they can also change the concentration of individual volatile molecules as well as their chemical grouping. In the case of pear juice, adding α-CDs was recommended. By adding this molecule, the overall quality of the juice was increased since browning reactions were reduced and no significant loss of aroma quality is produced [27].
3.1.2. Flavor
Flavoring substances are historic in food, although their direct use presents a series of disadvantages like having high volatility and sensitivity to light and heat. Part of these inconveniences can be solved with the CDs based encapsulation of food flavors, which is a frequent and simple solution to maintain the stability [22][24][28]. The price of the resulting β-CDs encapsulated flavor (USD $5–6 per kg) would not be much higher than other microencapsulated flavors price. CDs encapsulation provides an effective protection of each flavor component found in a multicomponent food system to whichever process it has been subjected (freezing, thawing, and/or microwaving). This factor is very important since the substances responsible for flavor usually involve numerous compounds, so it is interesting that all these molecules become part of the complex without seeing their organoleptic properties altered [18][29][30]. This method can be also used in oils to achieve a manipulability powder that can be added to food [22][24]. The liberation of aroma in these complexes can be controlled by slow guest liberation, mask off-notes of aromatic components by affinity with CDs cavity, and increase food flavor by water dissociation of aroma due to the polar external part of CDs [22]. Due to the properties of CDs-complexes, they can be applied to enhance flavor before extrusion, being a promising alternative for applying during the process [31]. As for the evolution of aromas over time, α-, β-, and γ-CDs are the best for initial flavor retention, α being better than γ for avoiding the loss of volatiles after storage [32][33]. Some examples of the application of CDs in the flavor and taste can be seen in Table 2.
Table 2. Studies of cyclodextrins (CDs) for food flavor and taste improvement.
| CDs | Extract | Compounds | Characteristic | Effect | Ref. |
|---|---|---|---|---|---|
| A. Food Flavor Improvement | |||||
| α | Shiitake | Cyclic sulphur compounds | Shiitake mushroom | Flavor retention | [34] |
| α | European pear | Five ester types | Pear | Heat protection at 120 °C for 60 min. | [35] |
| β | Food | - | - | Encapsulation the best protection against heat and evaporation | [37][36] |
| β | Food | Several volatile compounds | - | Protection during high temperature short time extrusion cooking process | [38] |
| β | Polysaccharide solutions | ketones, hexanal, t–2-hexenal, ethyl butanoate and 1-hexanol | - | Retain some aroma compounds during thermal processes (cooking, pasteurization) | [39] |
| β | Corn starch | Eugenol | Clove | 79% odor retention during extrusion | [40] |
| β | Goat milk and its yogurt | 4-methyloctanoic acid | Goat flavor | Reduce goat flavor | [41] |
| β | Thermally processed foods | l-menthol | Menthol | Improved flavor retention | [42] |
| α, β, γ | Aqueous ethanol | l-menthol, ethyl butyrate, ethyl hexanoate, benzaldehyde, citral, and methyl anthranilate | - | Temperature dependent | [28] |
| B. Food taste improvement | |||||
| α | Soy protein | Phenylalanine, tryptophane, tyrosine, isoleucine, proline and histidine | Taste modification | Reduce bitter taste | [43] |
| β | Milk casein hydrolysate | - | Bitter | Bitter taste eliminated by adding 10% β-CDs to the protein hydrolysate | [29] |
| β | β-polymers | Limonin, naringin | Bitter | Debittering agents | [44] |
| β | Canned citrus and citrus juice | Naringin, limonin, hesperidin | Bitter, precipitation | Reduce bitter taste of naringin, limonin, and hesperidin and prevent precipitation | [22] |
| β | Fish oil | - | Taste, oxidation | Eliminate unpleasant taste, smell, and stabilization against oxidation | [45] |
| γ | Ginseng | - | Bitter | Debittering agent | [46] |
| α, β | Navel orange and grapefruit juices | Limonin, naringin | Bitter | Improve flavor | [47] |
| β, γ | Caffeine and bitter natural extracts (artichoke leaves, aloe, and gentian) | β- and γ-CDs linked to chitosan through succinyl or maleyl bridges | Bitter | Bitter-masking properties | [48] |
3.1.3. Taste
Bitterness is one of the factors that can generate the rejection of a food product. However, there are exceptions to this rule, as some products are expected to have a certain degree of bitterness, like coffee, beer, or wine [29]. By using appropriate CDs, the bitter taste of certain substances may be totally or partially eliminated since complexed compounds cannot react in the oral cavity with the taste buds. This type of taste is not perceived as only dissolved substances have flavor. This system has been applied to the bitter and astringent compounds of foods (e.g., soy), beverages (e.g., naringin in citrus juice or chlorogenic acid and polyphenols in coffee), cigarette smoke (nicotine), or oral care products or drugs [49].
The mechanism of action consists of forming complexes of enough stability with the selected CDs to make the substance, that gives the unwanted taste, insoluble in water and, therefore, in saliva, and do not cause a bad taste sensation. The effectiveness of the process will depend on the value of the complex association constant (usually 101–104 molK−1), pH (less stable complexes with ionized guest molecules), and guest/host ratio (the higher possible molar excess, the better) [49]. Finally, the complex is released throughout the digestive system. Considering this, CDs are one of the best methods for masking the unpleasant taste [22]. The most relevant publications dealing with the elimination of unwanted tastes focus on the positive effect of β-CDs, the possibilities of α-, γ-, hydroxypropyl-CDs, and maltosyl-CDs having not yet been explored. CDs can also be used in seafood and meat products, to improve texture [29]. Other cases of study of CDs for food taste improvement can be seen in Table 2.
3.2. Improving Shelf Life
CDs can protect several compounds present in foods from reactions such as oxidation, light induced reactions, heat promoted decomposition, self-decomposition, and loss through volatility or sublimation [24]. The encapsulation of CDs with lipophilic food ingredients, physically and chemically, improves the stability of flavors, vitamins, dyes, and unsaturated fats, among others. Consequently, the shelf life of the product will be increased [18]. As an example, an in vitro study demonstrated that CDs encapsulation improved the stability of rosemary bioactive compounds [24]. Different accelerated and long-term storage stability tests showed that ingredients complexed with CDs have a longer life than those traditionally formulated. Another study tested twelve different complexes with β-CDs stored for 14 years. The results showed that encapsulation resulted in a notable improvement of the stability during long-term storage. Its preserving power depends on factors that affect the host–guest union such as the structure, the polarity of the compounds, or their geometry. Different studies showed that the greatest protective effect is observed in flavors with terpenoid, phenylpropane, and alkylsulfide structures [18]. In a recent study, different nonalcoholic beverages used limonene complexes with α-, β-, and γ-CDs to improve flavor and shelf life. The study showed that although limonene content diminished in all cases, it did so to a lesser extent once β-CDs/limonene complexes were adjoined. After 10 days, which mimic nine months of storage, 40% of limonene complexed remained in the model drink [50].
3.2.1. Against Oxidation
CDs can form complexes with ingredients (flavors, unsaturated fatty acids, dyes, etc.) sensitive to oxygen or oxidizing substances, which in most cases leads to an improvement in the stability of encapsulated substrates. Several studies have shown that complexing by means of this type of compounds almost completely prevents these oxidizable substances from undergoing chemical modifications, even when warehoused in an atmosphere of 100% oxygen [18]. Another study showed that using cinnamon-CDs complexes in the manufacture of dried apple slices with cinnamon flavor not only prevented a decrease in the concentration of this compound due to evaporation, but also protected it from oxidation [51].
3.2.2. Against Light-Induced Decomposition
CDs can be also used to protect compounds of interest from deterioration factors such as light, heat, or oxidation. In addition, if the CDs cavity is filled, the entry of other molecules is prevented, so no unwanted reactions occur. Another mechanism of action is preventing reactive molecules from approaching the active sites of the host molecule. For instance, CDs have been used to protect vitamins and pharmaceutical products that contain easily oxidizable double bonds (e.g., prostaglandins). It has been demonstrated that hydroxypropyl-β-CDs protected peptides from hydrolysis and their consequent loss of ability [51].
3.2.3. Against Heat-Induced Changes
Another important problematic event is thermal degradation of natural compounds. In most cases, the application of heat causes the volatilization of less stable compounds, which might have interesting biological properties. One possible solution can be the encapsulation of bioactive compounds with CDs, resulting in a complex that would provide a barrier for preventing their loss [52]. Several studies have shown that these complexes are very useful when it comes to protecting volatile flavor compounds and essential oils against heat, generally achieving better flavor retention with CDs than with various traditional formulations [53]. For example, this system has been used in vitro for the encapsulation of vitamin A palmitate to produce enriched foods using β-CDs. As a result, there is an increase of both, in its solubility in aqueous media and in its stability against different external factors (temperature, light, and oxygen) [54]. Other examples of the application of these complexes to protect several compounds can be observed in Table 3.
Table 3. Studies of cyclodextrins (CDs) for heat protection of food ingredients.
| CDs | Subtract | Properties | Study | Effect | Info | Ref. |
|---|---|---|---|---|---|---|
| β | 2-nonanone | Aromatic, antifungal | TGA, DSC, against B. cinereal | Improve antifungal, thermal stability | complex 1:0.5 (80% growth inhibition). | [55] |
| β | cyanidin-3-O-glucoside | Several | DSC | Improve bioavailability, thermal protection | - | [56] |
| β | S. baicalensis BA | Anti-inflammatory, antioxidant, antitumor | Increase solubility, stability | 13672.67 L/mol | [57] | |
| β | S. salar EO | DSC, KFT | Thermal and oxidative stability | Complex 1:1 and 3:1 | [58] | |
| β | Benzyl isothiocyanate (papaya) | Antimicrobial | DSC, TGA | Improve stability, controlled release | 600.8 L/mol | [59] |
| β | O. basilicum EO | Aromatic, medicinal | GC-MS | Improve stability against air/oxygen and temperature | - | [60] |
| β | Methanolic extract of H. perforatum | Antioxidant | DSC | Intact at temperatures at which the free extract was oxidized | Food supplement or a novel additive to enhance the antioxidant capacity of fresh or thermally processed food | [61] |
| β | Garlic | Antimicrobial, antioxidant | TGA, DSC, SEM | Thermal and oxidative stability | Nanoencapsulation yields >60% | [62] |
| β | Garlic oil | Antimicrobial, antioxidant | DSC | Improve protection against oxidation | Complex 1:1 | [63] |
| β | Oils | Antimicrobial | DSC, against S. enterica and L. innocua | Thermal protection | Masking the sensory effect of the attributes of antimicrobial agents and potentiate their activity. | [64] |
| HP | Clove EO | Antioxidant | DPPH | Prevent degradation and loss of active compounds. prolong shelf life | Complex 1:1 | [65] |
| γ | Geraniol | Aromatic, to treat infectious diseases, preserve food | SEM analysis | High thermal stability and enhanced durability of active agents and functional food ingredients | - | [66] |
HP: hydroxypropyl beta-cyclodextrin; BA: baicalein; EO: essential oil, DSC: differential scanning calorimetry, TGA: thermogravimetric analysis, GC-MS: gas chromatography-mass spectrometry, DPPH: 2,2-diphenyl-1-picryl-hydrazyl-hydrate, SEM: scanning electron microscope, KFT: Karl Fischer titration.
3.3. Modifying Solubility
As mentioned before, CDs are capable of changing the solubility of a compound [67]. They have the ability to form stable emulsions of water in oil (e.g., mayonnaise, salad dressings), due to differences in polarity between the inside and outside of the molecule [18]. In addition, CDs can also increase the solubility of certain compounds in water, by forming dynamic, noncovalent, water-soluble inclusion complexes [68]. However, in many cases, the solubility of the complex is not appropriate, so it is necessary to modify the external surface of CDs. Neutral (hydroxypropyl) or ionic groups (hydroxy, carboxymethyl, tertiary amine, or quaternary amine) can be used to increase the solubility up to 60%. On the other hand, to improve solubility in organic solvents, the modification is carried out with aliphatic groups or smaller groups (hexyl, acetyl). Thus, complexation with CDs is a mechanism to increase or decrease the solubility of a guest component. A common example of its application is to reduce the bitterness in citrus juices, by creating naringin-β-CDs complex. In fact, the rate of transformation of naringin to naringenin in inclusion complexes or free can reach 98.7% and 56.2%, respectively. It might be concluded that β-CDs can improve the aqueous solubility, which also means that the rate of enzymatic hydrolysis of naringin will be increased [69]. The increase in solubility will also affect aromatic compounds, such as vanillin, used at the vanillin/β-CDs inclusion complex. This complex increased solubility in water with respect to the free compound. Moreover, the formation of this complex protects vanillin inside the CDs cavity, which avoids damage from several factors such as oxidation according to the results obtained in differential scanning calorimetry (DSC) studies. Therefore, the vanillin/β-CDs complex can be used as a food additive for its higher antioxidant activity [70]. CDs may be also applied to improve the solubility of vitamins (Figure 3). The union of β-CDs with the essential oil from guava leaves also increases solubility and stability. The resulting complex presented antioxidant (against light) and antibacterial (Staphylococcus aureus and Escherichia coli) activities [71]. Similar effects were achieved by encapsulation of black pepper essential oil or yarrow essential oil [72][73].
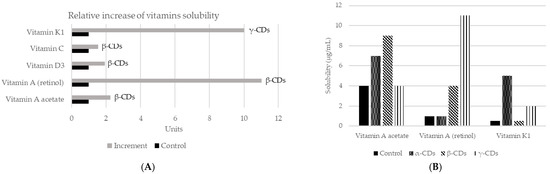
Figure 3. Changes in solubility in water of different vitamins according to the use of different CDs. (A) Relative increase of the solubility of vitamins using different CDs complexes. Adapted from [74][75]. (B) Vitamins solubility (µg/mL) when the three main types of CDs applied in a concentration of 5%, using saline solution as control and measured by mass spectrometry [74]. More recently, new studies have assessed the solubility of vitamin E + large ring cyclodextrins [76] and vitamin A together with β-CDs [77].
3.4. Sequestration of Selected Components
Among the most recent CDs applications, reduction of unwanted compounds (flavor, trans-fats, allergens, toxins) is included [24]. Allergens can be avoided with β-CDs. It has been demonstrated that they form host–guest systems with allergenic aroma molecules (eugenol, isoeugenol, benzyl alcohol, or anisyl alcohol) and proteins [78][79]. An example of this possible application is the preparation of soybean milk of low allergenicity. They are also able of forming complexes with mycotoxins like ochratoxin A from cereals, coffee, beer, wine, and cocoa [80]. CDs have been demonstrated to sequestrate other mycotoxins, such as aflatoxin, ochratoxin, patulin, zearalenone, zearalenol, and citrinin [79]. A study used 1% β-CDs during apple juice processing to reduce mycotoxins (patulin) and inhibit enzymatic browning by 70% and 75%, respectively [81].
Other application of CDs is the sequestration of cholesterol from food products [24][82]. They can be applied in food with high content of these fatty acids to make it healthier. This is the case of milk, butter, and egg yolks [83]. In fact, this property is widely used in the industry to produce products without cholesterol since this compound is retained in the β-CDs cavity. CDs did also sequester reducing sugars capable of reacting with proteins (Maillard reaction) and that could give an undesirable color and adverse effects in the nervous system and fertility, being a possible carcinogen. The mechanism is based on the complexation of proteins with CDs, which protect them from reaction. They can also be applied to decrease acrylamide content in food products and food intermediates [84].
3.5. Pickering Emulsions
Pickering emulsions are strictly defined as emulsions that are stabilized by an adsorbed layer of solid particles at the emulsion drop surface [85]. Their properties are usually determined by particle size, particle wettability, particle concentration, oil/water ratio, pH, salt concentration, and solvent type [86]. CDs can form CDs-oil complexes, however, in most cases, high concentration of CDs is needed. To solve this inconvenience, modified CDs can be used such as soft colloidal CDs polymer (CDs nanogel) [87]. Thermal stability in water of this type of emulsions with different oils has been investigated, observing activity at room temperature and the dissolution/fusion of inclusion complexes with high melting temperatures (near to or higher than 100 °C) [86]. Another study has considered the union between β-CDs and octadecenylsuccinic anhydride (ODS) under alkaline conditions. ODS-β-CDs particles exhibited a higher emulsifying capacity compared to β-CDs. The resulting pickering emulsions formed by ODS-β-CDs particles were more stable during storage [88].
3.6. Other Food Applications
Apart from food processing, CDs also have other technological advantages such as improving nutritional properties and for developing nutraceutical products [18]. Several studies have been conducted in this aspect. α-CDs complex can be used to keep certain products (cereal, snacks) crunchy after storage and also as soluble dietary fiber in beverages and foods [30]. Other CDs complexes can be used in the preservation of food. They can be used indirectly in the prevention of microbial growth, being added in plastic packaging films. In this way, they preserve food during storage and also prevent loss of aroma [18]. Finally, CDs have been demonstrated to be useful to remove contaminants from food products, including herbicides, insecticides, fungicides, repellents, pheromones, and growth regulators [5].
References
- Wenz, G. An overview of host-guest chemistry and its application to nonsteroidal anti-inflammatory drugs. Clin. Drug Investig. 2000, 19, 21–25, doi:10.2165/00044011-200019002-00003.
- Cram, D.J.; Cram, J.M. Host-Guest Chemistry. Science 1974, 183, 803–809, doi:10.1097/EDE.0b013e3181.
- Lehn, J.M. Supramolecular chemistry: Receptors, catalysts, and carriers. Science 1985, 227, 849–856, doi:10.1126/science.227.4689.849.
- Eastburn, S.D.; Tao, B.Y. Applications of modified cyclodextrins. Biotechnol. Adv. 1994, 12, 325–339, doi:10.1016/0734-9750(94)90015-9.
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046, doi:10.1016/S0032-9592(03)00258-9.
- Buschmann, H.J.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191.
- Shibatani, T. Industrial Application of Immobilized Biocatalysts in Japan. In Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 11, pp. 585–591.
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Supramolecular cyclodextrin complex: Diversity, safety, and applications in ocular therapeutics. Exp. Eye Res. 2019, 189, 107829, doi:10.1016/j.exer.2019.107829.
- Bortolus, P.; Grabner, G.; Köhler, G.; Monti, S. Photochemistry of cyclodextrin host-guest complexes. Coord. Chem. Rev. 1993, 125, 261–268, doi:10.1016/0010-8545(93)85023-W.
- Da Hu, Q.; Tang, G.P.; Chu, P.K. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025, doi:10.1021/ar500055s.
- Wenz, G. Cyclodextrins as Building Blocks for Supramolecular Structures and Functional Units. Angew. Chem. Int. Ed. Engl. 1994, 33, 803–822, doi:10.1002/anie.199408031.
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143, doi:10.1016/j.tifs.2020.08.009.
- Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Latest developments in the application of cyclodextrin host-guest complexes in beverage technology processes. Food Hydrocoll. 2020, 106, 105882, doi:10.1016/j.foodhyd.2020.105882.
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603, doi:10.1002/pi.5992.
- Braga, S.S. Cyclodextrins: Emerging medicines of the new millennium. Biomolecules 2019, 9, 803–822, doi:10.1002/anie.199408031.
- Almagro, L.; Pedreño, M.Á. Use of cyclodextrins to improve the production of plant bioactive compounds. Phytochem. Rev. 2020, 19, 1061–1080, doi:10.1007/s11101-020-09704-6.
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754, doi:10.1021/cr970022c.
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142, doi:10.1016/j.tifs.2003.09.019.
- de Menezes, P.P.; de Andrade, T.A.; Frank, L.A.; de Souza, E.P.B.S.S.; das Trindade, G.G.G.; Trindade, I.A.S.; Serafini, M.R.; Guterres, S.S.; de Araújo, A.A.S. Advances of nanosystems containing cyclodextrins and their applications in pharmaceuti-cals. Int. J. Pharm. 2019, 559, 312–328, doi:10.1016/j.ijpharm.2019.01.041.
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180, doi:10.1016/j.ijpharm.2012.06.055.
- Bruns, C.J. Exploring and exploiting the symmetry-breaking effect of cyclodextrins in mechanomolecules. Symmetry 2019, 11, 1249, doi:10.3390/sym11101249.
- Linde, G.A.; Laverde, A.; Colauto, N.B. Changes to Taste Perception in the Food Industry: Use of Cyclodextrins. In Handbook of Behavior, Food and Nutrition; Preedy, V.R., Watson, R.R., Martin, C.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 99–118.
- Radu, C.-D.; Parteni, O.; Ochiuz, L. Applications of cyclodextrins in medical textiles. J. Control. Release 2016, 224, 146–157, doi:10.1016/J.JCONREL.2015.12.046.
- dos Santos, C.; Buera, P.; Mazzobre, F. Novel trends in cyclodextrins encapsulation. Applications in food science. Curr. Opin. Food Sci. 2017, 16, 106–113, doi:10.1016/j.cofs.2017.09.002.
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Modern extraction techniques optimized to extract betacyanins from Gomphrena globosa L. Ind. Crops Prod. 2017, 105, 29–40, doi:10.1016/j.indcrop.2017.05.008.
- Blanch, G.P.; Ruiz del Castillo, M.L.; del Mar Caja, M.; Pérez-Méndez, M.; Sánchez-Cortés, S. Stabilization of all-trans-lycopene from tomato by encapsulation using cyclodextrins. Food Chem. 2007, 105, 1335–1341, doi:10.1016/j.foodchem.2007.04.060.
- Andreu-Sevilla, A.J.; López-Nicolás, J.M.; Carbonell-Barrachina, Á.A.; García-Carmona, F. Comparative Effect of the Addi-tion of α-, β-, or γ-Cyclodextrin on Main Sensory and Physico-Chemical Parameters. J. Food Sci. 2011, 76, S347–S353, doi:10.1111/j.1750-3841.2011.02190.x.
- Reineccius, T.A.; Reineccius, G.A.; Peppard, T.L. Flavor release from cyclodextrin complexes: Comparison of alpha, beta, and gamma types. J. Food Sci. 2003, 68, 1234–1239, doi:10.1111/j.1365-2621.2003.tb09631.x.
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640, doi:10.1016/j.foodhyd.2009.01.001.
- Cravotto, G.; Binello, A.; Baranelli, E.; Carraro, P.; Trotta, F. Cyclodextrins as food additives and in food processing. Curr. Nutr. Sci. 2006, 2, 343–350.
- Yuliani, S.; Bhandari, B.; Rutgers, R.; D’Arcy, B. Application of microencapsulated flavor to extrusion product. Food Rev. Int. 2004, 20, 163–185, doi:10.1081/FRI-120037159.
- Reineccius, T.A.; Reineccius, G.A.; Peppard, T.L. Encapsulation of flavors using cyclodextrins: Comparison of flavor reten-tion in alpha, beta, and gamma types. J. Food Sci. 2002, 67, 3271–3279, doi:10.1111/j.1365-2621.2002.tb09577.x.
- Astray, G.; Mejuto, J.C.; Morales, J.; Rial-Otero, R.; Simal-Gándara, J. Factors controlling flavors binding constants to cy-clodextrins and their applications in foods. Food Res. Int. 2010, 43, 1212–1218, doi:10.1016/j.foodres.2010.02.017.
- Yoshii, H.; Yasuda, M.; Furuta, T.; Kuwahara, H.; Ohkawara, M.; Linko, P. Retention of cyclodextrin complexed shiitake (Lentinus edodes) flavors with spray drying. Dry. Technol. 2005, 23, 1205–1215, doi:10.1081/DRT-200059343.
- Tobitsuka, K.; Miura, M.; Kobayashi, S. Retention of a European pear aroma model mixture using different types of saccha-rides. J. Agric. Food Chem. 2006, 54, 5069–5076, doi:10.1021/jf060309n.
- Pagington, J.S. β-cyclodextrin and its uses in the flavour industry. In Developments in Food Flavours; Birch, G.G., Lindley, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1986.
- Hedges, A.R.; Shieh, W.J.; Sikorski, C.T. Use of Cyclodextrins for Encapsulation in the Use and Treatment of Food Products. In Encapsulation and Controlled Release of Food Ingredients; ACS Publications: Washington, DC, USA, 1995; Volume 590, pp. 60–71.
- Bhandari, B.; D’Arcy, B.; Young, G. Flavour retention during high temperature short time extrusion cooking process: A review. Int. J. Food Sci. Technol. 2001, 36, 453–461, doi:10.1046/j.1365-2621.2001.00495.x.
- Jouquand, C.; Ducruet, V.; Giampaoli, P. Partition coefficients of aroma compounds in polysaccharide solutions by the phase ratio variation method. Food Chem. 2004, 85, 467–474, doi:10.1016/J.FOODCHEM.2003.07.023.
- Kollengode, A.N.R.; Hanna, M.A. Cyclodextrin complexed flavors retention in extruded starches. J. Food Sci. 1997, 62, 1057–1060, doi:10.1111/j.1365-2621.1997.tb15037.x.
- Young, O.A.; Gupta, R.B.; Sadooghy-Saraby, S. Effect of cyclodextrins on the flavour of goat milk and its yoghurt. J. Food Sci. 2012, 77, 122–127.
- Reineccius, T.A.; Reineccius, G.A.; Peppard, T.L. Utilization of β-Cyclodextrin for Improved Flavor Retention in Thermally Processed Foods. J. Food Sci. 2004, 69, FCT58–FCT62, doi:10.1111/j.1365-2621.2004.tb17856.x.
- Linde, G.A.; Junior, A.L.; Faria, E.V. de; Colauto, N.B.; de Moraes, F.F.; Zanin, G.M. Taste modification of amino acids and protein hydrolysate by α-cyclodextrin. Food Res. Int. 2009, 42, 814–818, doi:10.1016/j.foodres.2009.03.016.
- Binello, A.; Robaldo, B.; Barge, A.; Cavalli, R.; Cravotto, G. Synthesis of cyclodextrin-based polymers and their use as debit-tering agents. J. Appl. Polym. Sci. 2008, 107, 2549–2557, doi:10.1002/app.27249.
- Choi, M.J.; Ruktanonchai, U.; Soottitantawat, A.; Min, S.G. Morphological characterization of encapsulated fish oil with β-cyclodextrin and polycaprolactone. Food Res. Int. 2009, 42, 989–997, doi:10.1016/j.foodres.2009.04.019.
- Lee, S.H.; Yu, H.J.; Cho, N.S.; Park, J.H.; Kim, T.H.; Kim, K.H.; Lee, S.K. A method for preparing the inclusion complex of ginseng extract with gamma-cyclodextrin, and the composition comprising the same. In International Patent Classification IPC A23L; IPC Publication: Geneva, Switzerland; 2006; pp. 1–212.
- Shaw, P.E.; Tatum, J.H.; Wilson, C.W. Improved Flavor of Navel Orange and Grapefruit Juices by Removal of Bitter Com-ponents with β-Cyclodextrin Polymer. J. Agric. Food Chem. 1984, 32, 832–836, doi:10.1021/jf00124a034.
- Binello, A.; Cravotto, G.; Nano, G.M.; Spagliardi, P. Synthesis of chitosan-cyclodextrin adducts and evaluation of their bit-ter-masking properties. Flavour Fragr. J. 2004, 19, 394–400, doi:10.1002/ffj.1434.
- Szejtli, J.; Szente, L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 2005, 61, 115–125, doi:10.1016/j.ejpb.2005.05.006.
- Saldanha do Carmo, C.; Pais, R.; Simplício, A.L.; Mateus, M.; Duarte, C.M.M. Improvement of Aroma and Shelf-Life of Non-alcoholic Beverages Through Cyclodextrins-Limonene Inclusion Complexes. Food Bioprocess Technol. 2017, 10, 1297–1309, doi:10.1007/s11947-017-1897-0.
- Hedges, A.R. Cyclodextrins: Properties and Applications. In Starch; Academic Press: Cambridge, MA, USA, 2009; pp. 833–851; ISBN 9780127462752.
- Capelezzo, A.P.; Mohr, L.C.; Dalcanton, F.; de Mello, J.M.M.; Fiori, M.A. β-Cyclodextrins as Encapsulating Agents of Essen-tial Oils. In Cyclodextrin—A Versatile Ingredient; IntechOpen: London, UK, 2018.
- Furuta, T.; Yoshii, H.; Fujimoto, T.; Yasunishi, A.; Linko, Y.-Y.; Linko, P. A Short-Cut Method for Estimating l-Menthol Re-tention Included in Cyclodextrin During Drying a Single Drop. In Proceedings of the Eighth International Symposium on Cy-clodextrins; Springer: Berlin/Heidelberg, Germany, 1996; pp. 583–586.
- Vilanova, N.; Solans, C. Vitamin A Palmitate-β-cyclodextrin inclusion complexes: Characterization, protection and emulsi-fication properties. Food Chem. 2015, 175, 529–535, doi:10.1016/j.foodchem.2014.12.015.
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975, doi:10.1016/j.foodchem.2015.10.023.
- Fernandes, A.; Rocha, M.A.A.; Santos, L.M.N.B.F.; Brás, J.; Oliveira, J.; Mateus, N.; de Freitas, V. Blackberry anthocyanins: β-Cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. 2018, 245, 426–431, doi:10.1016/j.foodchem.2017.10.109.
- Zhou, Q.; Wei, X.; Dou, W.; Chou, G.; Wang, Z. Preparation and characterization of inclusion complexes formed between baicalein and cyclodextrins. Carbohydr. Polym. 2013, 95, 733–739, doi:10.1016/j.carbpol.2013.02.038.
- Hǎdǎruga, D.I.; Ünlüsayin, M.; Gruia, A.T.; Birǎu, C.; Rusu, G.; Hǎdǎruga, N.G. Thermal and oxidative stability of Atlantic salmon oil (Salmo salar L.) and complexation with β-cyclodextrin. Beilstein J. Org. Chem. 2016, 12, 179–191, doi:10.3762/bjoc.12.20.
- Li, W.; Liu, X.; Yang, Q.; Zhang, N.; Du, Y.; Zhu, H. Preparation and characterization of inclusion complex of benzyl isothi-ocyanate extracted from papaya seed with β-cyclodextrin. Food Chem. 2015, 184, 99–104, doi:10.1016/j.foodchem.2015.03.091.
- Hădărugă, D.; Hădărugă, N.; Costescu, C.; David, I.; Gruia, A. Thermal and oxidative stability of the Ocimum basilicum L. essential oil/β-cyclodextrin supramolecular system. Beilstein J. Org. Chem. 2014, 10, 2809–2820.
- Kalogeropoulos, N.; Yannakopoulou, K.; Gioxari, A.; Chiou, A.; Makris, D.P. Polyphenol characterization and encapsulation in β-cyclodextrin of a flavonoid-rich Hypericum perforatum (St John’s wort) extract. LWT—Food Sci. Technol. 2010, 43, 882–889, doi:10.1016/j.lwt.2010.01.016.
- Hadaruga, D.I.; Hadaruga, N.G.; Rivis, A.; Gruia, A.; Pinzaru, I.A. Thermal and oxidative stability of the Allium sativum L. bioactive compounds/α- and β-cyclodextrin nanoparticles. Rev. Chim. 2007, 58, 1009–1015.
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and release characterisation of garlic oil-β- cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685, doi:10.1016/j.foodchem.2011.02.036.
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT—Food Sci. Technol. 2013, 51, 86–93, doi:10.1016/j.lwt.2012.11.011.
- Cetin Babaoglu, H.; Bayrak, A.; Ozdemir, N.; Ozgun, N. Encapsulation of clove essential oil in hydroxypropyl be-ta-cyclodextrin for characterization, controlled release, and antioxidant activity. J. Food Process. Preserv. 2017, 41, e13202, doi:10.1111/jfpp.13202.
- Kayaci, F.; Sen, H.S.; Durgun, E.; Uyar, T. Functional electrospun polymeric nanofibers incorporating geraniol-cyclodextrin inclusion complexes: High thermal stability and enhanced durability of geraniol. Food Res. Int. 2014, 62, 424–431, doi:10.1016/j.foodres.2014.03.033.
- Rakmai, J.; Cheirsilp, B.; Cid, A.; Torrado-Agrasar, A.; Mejuto, J.C.; Simal-Gandara, J. Encapsulation of Essential Oils by Cyclodextrins: Characterization and Evaluation. In Cyclodextrin—A Versatile Ingredient; IntechOpen: London, UK, 2018.
- Sharma, N.; Baldi, A. Exploring versatile applications of cyclodextrins: An overview. Drug Deliv. 2016, 23, 739–757, doi:10.3109/10717544.2014.938839.
- Cui, L.; Zhang, Z.H.; Sun, E.; Jia, X. Bin Effect of β-cyclodextrin complexation on solubility and enzymatic conversion of naringin. Int. J. Mol. Sci. 2012, 13, 14251–14261, doi:10.3390/ijms131114251.
- Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658, doi:10.1016/j.foodchem.2006.01.053.
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Simal-Gándara, J.; Torrado-Agrasar, A. Antioxidant and antimicrobial properties of encapsulated guava leaf oil in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2018, 111, 219–225, doi:10.1016/j.indcrop.2017.10.027.
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Torrado-Agrasar, A.; Simal-Gándara, J. Physico-chemical characterization and evalua-tion of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Food Hydrocoll. 2017, 65, 157–164, doi:10.1016/j.foodhyd.2016.11.014.
- Rakmai, J.; Cheirsilp, B.; Torrado-Agrasar, A.; Simal-Gándara, J.; Mejuto, J.C. Encapsulation of yarrow essential oil in hy-droxypropyl-beta-cyclodextrin: Physiochemical characterization and evaluation of bio-efficacies. CyTA—J. Food 2017, 15, 409–417, doi:10.1080/19476337.2017.1286523.
- Alpha-Cyclodextrin as a Novel Food. Available online: https://www.foodstandards.gov.au/code/applications/documents/ A494_Alpha_cylclodextrin_FAR_FINAL.pdf (accessed on 26 January 2021).
- Braithwaite, M.C.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Tomar, L.K.; Tyagi, C.; Pillay, V. A novel multi-tiered experi-mental approach unfolding the mechanisms behind cyclodextrin-vitamin inclusion complexes for enhanced vitamin solu-bility and stability. Int. J. Pharm. 2017, 532, 90–104, doi:10.1016/j.ijpharm.2017.08.109.
- Kuttiyawong, K.; Saehu, S.; Ito, K.; Pongsawasdi, P. Synthesis of large-ring cyclodextrin from tapioca starch by amylomalt-ase and complex formation with Vitamin E acetate for solubility enhancement. Process Biochem. 2015, 50, 2168–2176, doi:10.1016/j.procbio.2015.10.014.
- Xu, X.; Peng, S.; Bao, G.; Zhang, H.; Yin, C. β-cyclodextrin inclusion complexes with vitamin A and its esters: A comparative experimental and molecular modeling study. J. Mol. Struct. 2021, 1223, 129001, doi:10.1016/j.molstruc.2020.129001.
- Decock, G.; Fourmentin, S.; Surpateanu, G.G.; Landy, D.; Decock, P.; Surpateanu, G. Experimental and theoretical study on the inclusion compounds of aroma components with β-cyclodextrins. Supramol. Chem. 2006, 18, 477–482, doi:10.1080/10610270600665749.
- Fenyvesi, E.; Vikmon, M.; Szente, L. Cyclodextrins in Food Technology and Human Nutrition: Benefits and Limitations. Crit. Rev. Food Sci. Nutr. 2016, 56, 1981–2004, doi:10.1080/10408398.2013.809513.
- Verrone, R.; Catucci, L.; Cosma, P.; Fini, P.; Agostiano, A.; Lippolis, V.; Pascale, M. Effect of β-cyclodextrin on spectroscopic properties of ochratoxin a in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 475–479, doi:10.1007/s10847-006-9237-4.
- Essa, H.A.; Ayesh, A.M. Mycotoxins reduction and inhibition of enzymatic browning during apple juice processing. Availa-ble online: https://www.kau.edu.sa/Files/857/Researches/58183_28330.pdf (accessed on 26 January 2021).
- dos Santos, C.; Buera, M.P.; Mazzobre, M.F. Phase solubility studies and stability of cholesterol/β-cyclodextrin inclusion complexes. J. Sci. Food Agric. 2011, 91, 2551–2557, doi:10.1002/jsfa.4425.
- Smith, D.M.; Awad, A.C.; Bennink, M.R.; Gill, J.L. Cholesterol Reduction in Liquid Egg Yolk using β-Cyclodextrin. J. Food Sci. 1995, 60, 691–694, doi:10.1111/j.1365-2621.1995.tb06207.x.
- Plank, D.W.; Novak, D.J. Method for Reducing Acrylamide Levels in Food Products and Food Products Produced Thereby. U.S. Patent No. 7,264,838, 4 September 2007.
- Murray, B.S. Pickering emulsions for food and drinks. Curr. Opin. Food Sci. 2019, 27, 57–63, doi:10.1016/j.cofs.2019.05.004.
- Diaz-Salmeron, R.; Chaab, I.; Carn, F.; Djabourov, M.; Bouchemal, K. Pickering emulsions with α-cyclodextrin inclusions: Structure and thermal stability. J. Colloid Interface Sci. 2016, 482, 48–57, doi:10.1016/j.jcis.2016.07.033.
- Kawano, S.; Kida, T.; Akashi, M.; Sato, H.; Shizuma, M.; Ono, D. Preparation of Pickering emulsions through interfacial adsorption by soft cyclodextrin nanogels. Beilstein J. Org. Chem. 2015, 11, 2355–2364, doi:10.3762/bjoc.11.257.
- Xi, Y.; Luo, Z.; Lu, X.; Peng, X. Modulation of Cyclodextrin Particle Amphiphilic Properties to Stabilize Pickering Emulsion. J. Agric. Food Chem. 2018, 66, 228–237, doi:10.1021/acs.jafc.7b03940.