| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nurul 'Izzah Ibrahim | + 4671 word(s) | 4671 | 2021-02-02 09:01:39 | | | |
| 2 | Rita Xu | -1591 word(s) | 3080 | 2021-02-26 07:49:41 | | |
Video Upload Options
Squalene (SQ), an unsaturated hydrocarbon naturally synthesized in plants and animals, could become the alternative treatment or supplementary agent for cardiovascular health.
1. Introduction
Cardiovascular diseases (CVD) have been recognized as the leading cause of mortality worldwide with approximately 17.9 million deaths per year [1]. CVD may refer to several linked pathologies that are commonly defined as coronary heart disease (CHD), cerebrovascular disease, peripheral arterial disease, rheumatic and congenital heart diseases and venous thromboembolism. CVD accounts for 31% of global mortality with the majority consisting of CHD and cerebrovascular accident [1][2]. An estimation by the World Health Organization (WHO) stated that 80% of premature CVD is preventable by controlling various risk factors to assist in reducing the increasing CVD burden on individuals and healthcare providers [3]. These risk factors include dyslipidemia, smoking, diabetes, hypertension, and family history of premature heart disease, as well as the non-independent risk variables of physical inactivity and body weight and composition [4]. In a well-recognized study, namely, the INTERHEART study, several CVD risk factors were highlighted, such as dyslipidemia, smoking, hypertension, diabetes, abdominal obesity, and it also demonstrated the protective effects of a healthy lifestyle (e.g., consumption of fruits and vegetables, and regular physical activity) [5]. Among all the risk factors, hyperlipidemia, which is defined as an abnormally elevated level of lipids or lipoproteins in the blood, has been established as the most potent risk factor [6][7]. It has been acknowledged that almost 50% of the general population have elevated cholesterol levels above the accepted normal range, corresponding with the prevalence of cardiovascular disease [8][9].
The first step to reduce cholesterol levels is lifestyle modification, including healthy diet, weight control, and physical activity, which are known to be effective. However, some individuals may find this difficult to achieve as there are limitations in most weight loss studies. For example, weight reduction might be able to reduce both cholesterol and triglyceride, but in a long-term period, almost half of the initial weight loss is regained after 1 year [10]. In a review by Makris and Foster (2011), the authors concluded that the type of diet is less significant due to the regaining of weight after the initial loss in a long-term period [11]. Due to this downside of lifestyle modification, it may be sensible to accomplish the lipid-lowering goals by preferably initiating medications more quickly rather than later. However, if lifestyle-change goals are achieved for long-term periods with no rebound, the demand for medication can be reconsidered.
In terms of pharmacotherapy, many studies have established that for most hyperlipidemia patients, statins, a class of drugs that reduce LDL-C are the preferred medical treatment [7][12][13][14]. However, despite their extensive use, statins are associated with patients’ discontinuation and nonadherence which creates a major gap in the prevention and treatment of atherosclerotic cardiovascular diseases. The main reason for the discontinuation is due to the development of statin-associated muscle symptoms [15]. It should be stated that the side effects occur more commonly at high doses and are more common in females, the elderly, patients with hypothyroidism and patients taking other drugs such as gemfibrozil and cytochrome P450 3A4 inhibitors [16]. Additionally, there is also a range of other statin-induced side effects, such as hepatotoxicity, gastrointestinal upset, as well as increased risk of getting cataracts and diabetes [15]. Due to the fear of the side effects, many people prefer complementary and alternative medicines to pharmaceutical products. This alternative is preferred as it is less expensive, requires no prescription and it is considered natural and safer. Several alternative treatments have been identified and discussed in several reviews which demonstrated efficacy in reducing plasma lipids with the use of garlic, artichoke leaf extract, fenugreek, red yeast rice, omega-3 fatty acids and plant stanols [17][18][19]. Other complementary and alternative medicines are constantly being discovered in providing alternatives to statin-intolerant patients.
Squalene (SQ), a highly unsaturated hydrocarbon from the triterpenoid family, is synthesized in plants, animals, bacteria and fungi as a precursor for the synthesis of secondary metabolites such as sterols, hormones, or vitamins [20][21]. SQ has been discovered as a major component of shark liver oil, which was believed by the Japanese people to possess powerful healing agents [22]. High concentration of SQ in the shark liver oil has been associated with protective effects against bacterial and fungal infections, particularly in eczema and dry skin lesions [23][24]. Besides being the main source from shark liver oil, which is limited by animal protection regulations [25], SQ is also extracted from plant sources such as olive oil, soybean oil, rice, wheat germ, grape seed oil, peanut, corn, and amaranth. Among the plant sources, olive oil is the only source that provides commercial SQ, although amaranth has the highest content of SQ [26]. SQ also possesses a protective effect on the skin against UV-induced radiation damage due to its high secretion in sebum, about 10–14% of lipids on sebum [27]. In addition, SQ has demonstrated its anti-cancer properties by inhibiting Ras oncoprotein farnesylation and restricting the transformation of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) into mevalonate, which takes place in cholesterol biosynthesis pathway [28]. It has also been found that SQ exhibited a cardioprotective effect, similar to statin, via inhibition of HMG-CoA reductase in the cholesterol biosynthesis pathway. As described earlier, statin is the preferred medical treatment for reducing LDL-C; its mechanism of action is by inhibiting HMG-CoA reductase in the cholesterol biosynthesis pathway [29]. Specifically, the inhibition of HMG-CoA reductase activity occurs due to the enhanced SQ-derived cholesterol synthesis via negative feedback mechanism. In a randomized controlled trial conducted by Chan et al., (1996), it was demonstrated that SQ supplementation at the dose of 860 mg/day for 20 weeks in primary hypercholesterolemia patients had significantly decreased total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels compared to the placebo group. Additionally, this SQ supplementation had also caused a reduction in triglyceride (TG) levels by 5.3% and increased the high-density lipoprotein cholesterol (HDL-C) level by 1.8% [30]. This human trial has shown that SQ can cause hypocholesterolemic activity, which in turns may reduce the risk of cardiovascular disease.
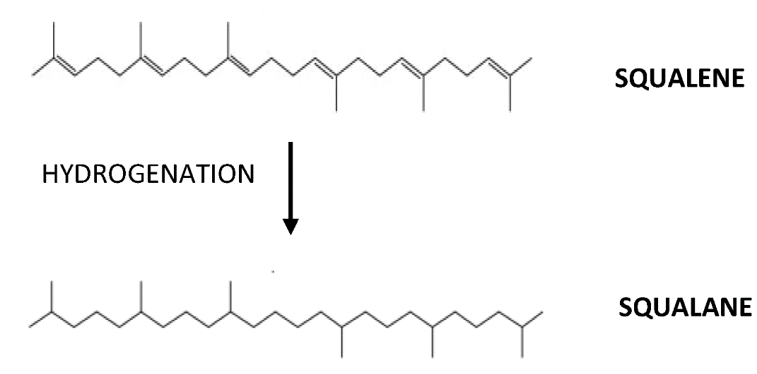
In a systematic review by Ibrahim et al., (2020) [31], on the efficacy of SQ in cardiovascular diseases, it was concluded that SQ occupies cardioprotective effects from its antioxidant property. This property is associated with the abundance of double bonds in the structure, which has enabled SQ to act as a strong antioxidant [31]. The abundance of double bonds structure contributes to the extreme reactivity of SQ in getting into the oxidized form by binding with hydrogen ions from water releasing three unbound oxygen molecules and developing into its saturated form squalane, C30H62 (Figure 1). The released oxygen may then reach the cells to intensify cellular metabolism and improve the function of certain organs in the body [32]. However, the outcome of the review was heterogeneous, with 16 studies showing positive results on SQ supplementation in animals (n = 15) and humans (n = 1), while five studies showed inconsistent or negative results, with animals (n = 3) and humans (n = 2). These discrepancies of the SQ effect on cardiovascular diseases clearly warrant further investigation [31]. Indeed, oxidative stress, which is caused by exaggerated reactive oxygen species, is closely related to inflammatory responses and is interdependently related. The exact reason for the failure or inefficiency of an agent with antioxidant property is yet to be revealed. Many studies have supported that there is an interdependent relationship between inflammation and oxidative stress [33]. In a review by Lou-Bonafonte et al., (2018), it was stated that the anti-inflammatory and antioxidant properties of SQ are responsible for its various biological actions [34]. Nonetheless, the interdependence between these properties was not elaborated. Therefore, this article elucidates the effects of SQ on antioxidant and anti-inflammatory properties for an alternative mechanism of action, besides the antioxidant property alone and HMG-CoA reductase inhibition.
Figure 1. Following hydrogenation, squalene (C30H50) will develop into its oxidized form, squalane (C30H62).
2. Antioxidant Activity of Squalene Related to Cardiovascular Health
Reactive oxygen species (ROS), which include free radicals, such as superoxide anion (O2−), lipid radicals (ROO∙), hydroxyl radical (·OH), and non-radicals, such as hydrogen peroxide (H2O2), hypochlorous acid (HClO) and peroxynitrite (ONOO−), are the by-products of numerous oxidative physiological and biochemical processes. Under physiological conditions, ROS serve as signaling molecules that involve regulation of vascular smooth muscle cell contraction, relaxation, and growth [35]. On the other hand, pathophysiological conditions may provoke an imbalance between ROS (oxidants) and antioxidants, which leads to endothelial dysfunction and subsequent cardiovascular disease conditions [35][36]. ROS can exert direct oxidizing effects on DNA, proteins and lipids contributing to cell damage, necrosis, and apoptosis [36]. In the meantime, antioxidant, which can be defined as a substance when present at concentrations lower than the oxidizable substrate, may significantly decrease or prevent the adverse effects of reactive species [37]. Antioxidants are important for the defense mechanisms associated with free radicals’ attack. Therefore, the intake of natural-derived antioxidant has been suggested for preventing degenerative diseases caused by oxidative stress, including cancer, Alzheimer’s disease and atherosclerosis [38]. Due to these, many natural products have been tested for their antioxidant property using different assays [38]. Several previous studies have demonstrated antioxidant activity of SQ ,with respect to cardiovascular health, using different methods such as lipid peroxidation, antioxidant enzymes and others, as tabulated in Table 1. In these studies, SQ has demonstrated its antioxidant effects in cardiovascular-related conditions including hyperlipidemia [39], atherosclerosis [40], myocardial infarction [41][42][43][44][45] and cardiotoxicity [46].
Table 1. Antioxidant effects of squalene in cardiovascular-related conditions.
|
Assays for Determining Antioxidant Activity |
Cardiovascular-Related Conditions |
Study Type |
Experimental Model |
Findings |
Reference |
|
Paraoxonase |
Hyperlipidemia |
Animal |
Wild-type, ApoA1- and ApoE-deficient C57BL/6J mice |
Reduction in reactive oxygen species (ROS) level and plasma malondialdehyde in lipoprotein fractions independently of the animal background. |
[39] |
|
Paraoxonase |
Atherosclerosis |
Animal |
Female and male ApoE knockout mice |
No significant changes in paraoxonase activity in both sexes. |
[40] |
|
8-isoprostaglandin F2α |
Decreased level of plasma 8-isoprostaglandin F2α in both sexes. |
||||
|
Catalase (CAT) and superoxide dismutase (SOD) |
Myocardial infarction (MI) |
Animal |
Isopreterenol MI-induced Wistar male rats |
Increased CAT and SOD activities. Increased GPX and GST activities. |
|
|
Glutathione peroxidase (GPX) and Glutathione-S-Transferase (GST) |
|||||
|
Glutathione (GSH) |
Increased GSH. |
||||
|
Thiobarbituric Acid (TBARS) |
Decreased lipid peroxidation in plasma and heart tissue. |
||||
|
GPx and GSH |
Cardiotoxicity |
Animal |
Cyclophosphomide- induced cardiotoxicity in male Wistar rats |
Increased GSH and decreased GPx. |
[46] |
Gabas-Rivera et al., (2014), have measured the levels of ROS in isolated lipoprotein fractions including very low density lipoprotein (VLDL), low density lipoprotein (LDL) and high density lipoprotein (HDL). In the study, mice of the C57BL/6J strain have been used due to the higher predisposition to atherosclerosis development [39]. Three models of mice from this strain have been used, namely, wild-type, ApoE-deficient and ApoA1-deficient. ApoE and ApoA1 are examples of apolipoprotein, an important component of lipoprotein particles that facilitates the transport of cholesterol, TG and phospholipids between plasma and cells [47]. Therefore, mice that lacked both apolipoproteins have impaired elimination of lipoproteins, providing a possibility to explore changes in lipids [48]. In this study, the supplementation of 1 g/kg SQ for 11 weeks significantly increased plasma HDL-C level for all animal backgrounds, indicating that SQ exhibits an atheroprotective effect. It was also shown that SQ supplementation had significantly reduced the ROS level in LDL and HDL fractions for both wild-type and ApoA1-deficient mice. Meanwhile, SQ had demonstrated a significant reduction in ROS level for isolated VLDL and HDL in ApoE-deficient mice. This study has shown that SQ can reduce ROS levels in normal, ApoA1- and ApoE-deficient mice. In addition, plasma malondialdehyde (MDA), which is one of the most frequently used lipid peroxidation indicators, also showed significant reduction for all animal backgrounds following SQ supplementation [39]. This study indicates that SQ supplementation can produce antioxidant activity by reducing the oxidative stress level in lipoprotein fractions.
The SQ effect on antioxidant defenses was also studied by Guillén et al., (2008) via paraoxonase activity and plasma 8-isoprostaglandin F2α level. In this study, they categorized ApoE-knockout mice into two groups; male or female to investigate the relation of SQ administration modulation accordingly to sex-dependent manner. In terms of the atherosclerotic lesion, male mice receiving SQ showed a significant decrease in lesion area, while no change was observed in female mice indicating that SQ modulates lesion in a sex-specific manner. However, it was shown that SQ administration for 10 weeks via beverages did not induce significant changes in the activity of the paraoxonase in either sex [40]. Paraoxonase is an enzyme with anti-atherosclerotic property that generally inhibits the accumulation of lipoperoxides and inhibits the lipid oxidation of LDL [49]. In contrast, SQ has a significantly low lipid peroxidation in both sexes as observed via the measurement of prostaglandin namely 8-isoprostaglandin F2α [40]. The measurement of this prostaglandin, which was enhanced in cardiovascular risk factors, is a reliable method for identifying subjects with enhanced rates of lipid peroxidation [50].
Farvin and colleagues have performed several antioxidant experiments using SQ at 2% concentration, that was embedded in the animals’ standard diet for 45 days. The rats were injected for two days with isoproterenol to induce myocardial infarction (MI). The prior treatment of SQ had significantly reduced diagnostic marker enzymes [44], which demonstrates the cardioprotective effect of SQ. SQ supplementation has shown significantly increased activities of antioxidant enzymes (GPx and GST), as well as anti-peroxidative enzymes (CAT and SOD) in the MI-induced rats [45]. Subsequently, SQ has exhibited the ability to counteract lipid peroxidation in plasma and heart tissue [42][43][44]. The measurement of lipid peroxidation was conducted by means of malondialdehyde (MDA) via thiobarbituric acid (TBA) assay as the oxidative stress biomarker [42]. MDA is a commonly used oxidative stress biomarker in various health problems, including cardiovascular diseases, cancer, psychiatry and chronic obstructive pulmonary disease [51]. Farvin et al. had also shown that SQ had maintained glutathione (GSH) levels in the heart tissue at near-normal levels in isoproterenol-induced MI rats [43][44]. GSH is an antioxidant that prevents damage to cellular components against exogenous and endogenous toxins including reactive oxygen (ROS) and nitrogen (RNS) species [52]. The level of endogenous antioxidants, such as ascorbic acid and alpha tocopherol in the heart tissue, has also been measured, whereby the SQ administration has significantly reduced the isoproterenol-induced decline in these antioxidants level. Impairment of α-tocopherol status and inadequacy intake may cause damage to the cardiac muscles [53].
Dhandapani et al., (2007), conducted a study to determine the antioxidant status and lipid peroxidation of SQ and polyunsaturated fatty acids (PUFA) on isoproterenol MI-induced rats. In terms of cardiovascular-related results, it was found that SQ supplementation had significantly decreased the diagnostic marker enzymes such as alanine aminotransferase (ALT), alanine aminotransferase(AST), lactate dehydrogenase (LDH) and creatine phosphokinase (CPK). In terms of antioxidant and lipid peroxidation, the SQ-given group showed significantly reduced lipid peroxides and significantly elevated GSH and antioxidant enzymes including GPx, GST, CAT and SOD in the heart tissue compared to the negative control group. These results may indicate that SQ has exerted cardioprotection against isoproterenol-MI-induced changes.
Cardiovascular toxicity has been defined by the National Cancer Institute (NCI) as toxicity that affects the heart, including angina, acute arrhythmia, and myocardial infarction [54][55]. Most commonly, this type of toxicity is related to patients receiving chemotherapy (doxorubicin, anthracycline, cyclophosphamide) or targeted therapy (trastuzumab, bevacizumab and tyrosine kinase inhibitors) [55]. Although survival rates of cancer may have been improved due to advancement in chemotherapy and targeted therapies, the patients may however suffer from cardiac side effects. To overcome the cardiac side effects caused by certain drug administration, SQ has also been suggested. A study by Motawi et al., (2010), where SQ at 35 mg/kg body weight was orally supplemented to cyclophosphamide-induced rats has demonstrated a significant reduction in all cardiac markers, including CPK, LDH and AST. For antioxidant activity, the SQ treatment caused a significant decrease in GPx, while an increase in GSH level when compared to the cyclophosphamide-control group [46]. This study may indicate the protective effect of SQ via antioxidant capacity to attenuate the cardiotoxicity effects due to cyclophosphamide exposure.
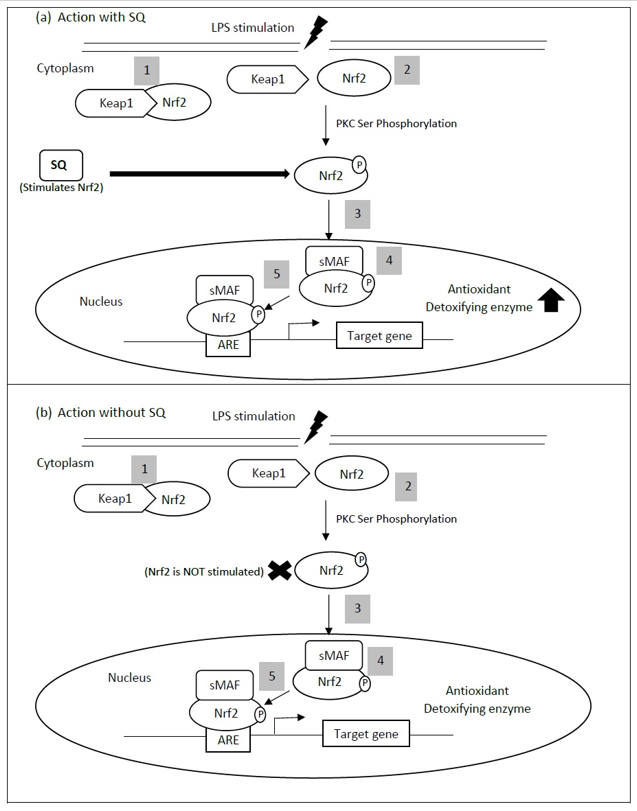
All studies mentioned above have demonstrated that SQ exerts a cardioprotective effect via its antioxidant activity. A transcription factor known as nuclear factor E2-related factor 2 (Nrf2) is accepted as a master regulator of antioxidant responses to cellular stress [56]. The SQ antioxidant activity might be related to this transcription factor, as SQ has been shown to significantly increase total and phosphorylated nuclear factor E2-related factor 2 (Nrf2) protein expression in lipopolysaccharide-treated cells (Figure 2) [57]. Nrf2 responds to oxidative stress by binding to antioxidant response element (ARE) in the promoter of genes coding for antioxidant enzymes [58]. Nrf2-regulated gene expression is mainly controlled by Kelch-like ECH-associated protein 1 (KEAP1) that mediates its protein ubiquitination and degradation. KEAP1, which acts as an adapter molecule for CUL-E3 ligase, will undergo cysteine modification upon exposure to oxidative stress triggering dissociation of KEAP1 from CUL-E3 ligase [59]. In addition, Nrf2 serine (Ser) 40 could be phosphorylated by protein kinase C (PKC) and dissociated from KEAP1 [58]. The altered KEAP1 structure causes the release of Nrf2, which is then translocated into the nucleus [60]. Upon entry into the nucleus, Nrf2 molecules will dimerize with other transcription factors, including small Maf (sMaf) forming a heterodimer, and bind to the ARE to induce gene transcription [61]. Nrf2 is involved in the induction of genes encoding many cytoprotective enzymes including heme oxygenase-1 (HO-1), glutamate cysteine ligase (GCL), NAD(P)H: quinone oxidoreductase-1 (NQO1), superoxide dismutase (SOD), glutathione S-transferase (GST), glutathione peroxidase (GPx) catalase (CAT), and thioredoxin [60][62]. Increased oxidative stress in the affected myocardium is a well-established phenomenon. Concerning heart failure, ROS causes impairment of cardiac function and increases arrhythmia risk by a direct toxic effect of increased cell necrosis and apoptosis [63][64]. Several Nrf2 downstream target genes, such as HO-1, SOD and GPx, have demonstrated protection against abnormal myocardial remodeling, pathological myocardial hypertrophy and heart failure [65][66][67]. Additionally, an overexpression of Nrf2 genes in the transverse aortic constriction mouse model of pressure overload has attenuated ROS production and hypertrophic growth in cardiomyocytes, and cardiac fibroblasts [68]. These previous studies may have indicated the protective effect of Nrf2 in cardiovascular health via reduction in oxidative stress.
Figure 2. Keap1-Nrf2-ARE pathway. A proposed mechanism for the antioxidant property of squalene (SQ), where SQ stimulates the total and phosphorylated Nrf2. The stimulation of Nrf2,therefore, activates the transcription of antioxidant or detoxifying enzyme, which then causes a reduction in myocardial injury. (1) During normal conditions in the cytoplasm, Nrf2 resides as an inactive complex, with its repressor, Keap1. (2) Upon activation by oxidative stress (LPS stimulation), an oxidation of Keap1 cysteine residues or phosphorylation of Nrf2 serine (Ser) 40 may occur with respect to protein kinase C (PKC), which subsequently causes the release of Nrf2. (3) Nrf2 is then translocated into the nucleus and (4) dimerized with a small transcription factor, sMaf, forming a heterodimer. It then (5) binds to the antioxidant response element (ARE) genes. ARE is responsible for the regulation of antioxidant or detoxifying enzyme transcription. This figure is modified from Vomhof-DeKrey and Picklo, 2012 [58].
References
- World Health Organization. Cardiovascular Disease. Available online: https://www.who.int/health-topics/cardiovascular-diseases/ (accessed on 5 November 2020).
- Stewart, J.; Manmathan, G.; Wilkinson, P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc. Dis. 2017, 6, 2048004016687211, doi:10.1177/2048004016687211.
- World Health Organization. The Challenge of Cardiovascular Disease—Quick Statistics. Available online: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/cardiovascular-diseases/data-and-statistics (accessed on 16 November 2020).
- Schenck-Gustafsson, K. Traditional Cardiovascular Disease Risk Factors. In ESC CardioMed (3 edn); Camm, T.F.L.A.J., Maurer, G., Serruys, P.W., Eds.; 73, United Kingdom: 2020.
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952, doi:10.1016/s0140-6736(04)17018-9.
- Sudhakaran, S.; Bottiglieri, T.; Tecson, K.M.; Kluger, A.Y.; McCullough, R.O.P.A. Alteration of lipid metabolism in chronic kidney disease, the role of novel antihyperlipidemic agents, and future directions. Cardiovasc. Med. 2018, 19, 77–88, doi:10.31083/j.rcm.2018.03.908.
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Care 2013, 40, 195–211, doi:10.1016/j.pop.2012.11.003.
- Yao, Y.S.; Di Li, T.; Zeng, Z.H. Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids Health Dis. 2020, 19, 23, doi:10.1186/s12944-019-1171-8.
- Zárate, A.; Manuel-Apolinar, L.; Saucedo, R.; Hernández-Valencia, M.; Basurto, L. Hypercholesterolemia as a Risk Factor for Cardiovascular Disease: Current Controversial Therapeutic Management. Med. Res. 2016, 47, 491–495, doi:10.1016/j.arcmed.2016.11.009.
- Cheng, V.Y.; Berman, D.S.; Rozanski, A.; Dunning, A.M.; Achenbach, S.; Al-Mallah, M.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; Chang, H-J.; et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: Results from the multinational coronary CT angiography evaluation for clinical outcomes: An international multicenter registry (CONFIRM). Circulation 2011, 124, 2423–2432.
- Makris, A.; Foster, G.D. Dietary Approaches to the Treatment of Obesity. Clin. N. Am. 2011, 34, 813–827, doi:10.1016/j.psc.2011.08.004.
- Zodda, D.; Giammona, R.; Schifilliti, S. Treatment Strategy for Dyslipidemia in Cardiovascular Disease Prevention: Focus on Old and New Drugs. Pharmacy 2018, 6, 10, doi:10.3390/pharmacy6010010.
- Last, A.R.; Ference, J.D.; Menzel, E.R. Hyperlipidemia: Drugs for Cardiovascular Risk Reduction in Adults. Fam. Physician 2017, 95, 78–87.
- Safeer, R.S.; LaCivita, C.L. Choosing drug therapy for patients with hyperlipidemia. Fam. Physician 2000, 61, 3371–3382.
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Res. 2019, 124, 328–350, doi:10.1161/circresaha.118.312782.
- Iii, W.H.S.; Khan, B.V.; Sperling, L.S. Management of the statin-intolerant patient. Treat. Options Cardiovasc. Med. 2009, 11, 263–271.
- Bouknight, P.; Mackler, L.; Heffington, M. FPIN’s clinical inquiries. Best alternatives to statins for treating hyperlipidemia. Fam. Physician 2007, 76, 1027–1029.
- Wider, B.; Pittler, M.H.; Thompson-Coon, J.; Ernst, E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst. Rev. 2013, 2013, CD003335, doi:10.1002/14651858.cd003335.pub4.
- Nies, L.K.; Cymbala, A.A.; Kasten, S.L.; Lamprecht, D.G.; Olson, K.L. Complementary and Alternative Therapies for the Management of Dyslipidemia. Pharmacother. 2006, 40, 1984–1992.
- Ghimire, G.P.; Thuan, N.H.; Koirala, N.; Sohng, J.K. Advances in Biochemistry and Microbial Production of Squalene and Its Derivatives. Microbiol. Biotechnol. 2016, 26, 441–451, doi:10.4014/jmb.1510.10039.
- Rohmer, M.; Seemann, M.; Horbach, S.; Bringer-Meyer, S.; Sahm, H. Glyceraldehyde 3-Phosphate and Pyruvate as Precursors of Isoprenic Units in an Alternative Non-Mevalonate Pathway for Terpenoid Biosynthesis. Am. Chem. Soc. 1996, 118, 2564–2566, doi:10.1021/ja9538344.
- Wołosik, K.; Knaś, M.; Zalewska, A.; Niczyporuk, M.; Przystupa, A.W. The importance and perspective of plant-based squalene in cosmetology. Cosmet. Sci. 2013, 64, 59–66.
- Nowicki, R.; Barańska-Rybak, W. Shark liver oil as a supporting therapy in atopic dermatitis. Polski Merkur. Lek. 2007, 22, 312–313.
- Okada, K.; Matsumoto, K. Effect of Skin Care with an Emollient Containing a High Water Content on Mild Uremic Pruritus. Apher. Dial. 2004, 8, 419–422, doi:10.1111/j.1526-0968.2004.00175.x.
- Turchini, G.M.; Ng, W.-K.; Tocher, D.R. Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; CRC Press: Boca Raton, FL, USA, 2010.
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant Sources, Extraction Methods, and Uses of Squalene. J. Agron. 2018, 2018, 1829160, doi:10.1155/2018/1829160.
- Pappas, A. Epidermal surface lipids. Dermato-Endocrinol. 2009, 1, 72–76, doi:10.4161/derm.1.2.7811.
- Gunes, F. Medical use of squalene as a natural antioxidant. J. Marmara Univ. Inst. Health Sci. 2013, 3, 221–229.
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. Cell. Mol. Med. 2001, 5, 378–387, doi:10.1111/j.1582-4934.2001.tb00172.x.
- Chan, P.; Tomlinson, B.; Lee, C.B.; Lee, Y.S. Effectiveness and safety of low-dose pravastatin and squalene, alone and in combination, in elderly patients with hypercholesterolemia. Clin. Pharmacol. 1996, 36, 422–427.
- Ibrahim, N.; Fairus, S.; Zulfarina, M.S.; Mohamed, I.N. The Efficacy of Squalene in Cardiovascular Disease Risk-A Systematic Review. Nutrient 2020, 12, 414, doi:10.3390/nu12020414.
- Kelly, G.S. Squalene and its potential clinical uses. Med. Rev. 1999, 4, 29–36.
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931, doi:10.1155/2016/5698931.
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current Insights into the Biological Action of Squalene. Nutr. Food Res. 2018, 62, e1800136, doi:10.1002/mnfr.201800136.
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Chim. Acta 2019, 493, 36–51, doi:10.1016/j.cca.2019.02.022.
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Signal. 2012, 24, 981–990, doi:10.1016/j.cellsig.2012.01.008.
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. Agric. Food Chem. 2005, 53, 1841–1856, doi:10.1021/jf030723c.
- Moharram, H.; Youssef, M. Methods for Determining the Antioxidant Activity: A Review. J. Food Sci. Technol. 2014, 11, 31–42.
- Gabás-Rivera, C.; Barranquero, C.; Martínez-Beamonte, R.; Navarro, M.A.; Surra, J.C.; Osada, J. Dietary Squalene Increases High Density Lipoprotein-Cholesterol and Paraoxonase 1 and Decreases Oxidative Stress in Mice. PLoS ONE 2014, 9, e104224, doi:10.1371/journal.pone.0104224.
- Guillén, N.; Acín, S.; Navarro, M.A.; Perona, J.S.; Arbonés-Mainar, J.M.; Arnal, C.; Sarría, A.J.; Surra, J.C.; Carnicer, R.; Orman, I.; et al. Squalene in a sex-dependent manner modulates atherosclerotic lesion which correlates with hepatic fat content in apoE-knockout male mice. Atherosclerosis 2008, 197, 72–83, doi:10.1016/j.atherosclerosis.2007.08.008.
- Dhandapani, N.; Ganesan, B.; Anandan, R.; Jeyakumar, R.; Rajaprabhu, D.; Ezhilan, R.A. Synergistic effects of squalene and polyunsaturated fatty acid concentrate on lipid peroxidation and antioxidant status in isoprenaline-induced myocardial infarction in rats. J. Biotechnol. 2007, 6, 6, ISSN 1684-5315.
- Farvin, K.S.; Anandan, R.; Kumar, S.H.S.; Shiny, K.; Mathew, S.; Sankar, T.; Nair, P.V. Cardioprotective Effect of Squalene on Lipid Profile in Isoprenaline-Induced Myocardial Infarction in Rats. Med. Food 2006, 9, 531–536, doi:10.1089/jmf.2006.9.531.
- Farvin, K.S.; Kumar, S.H.S.; Anandan, R.; Mathew, S.; Sankar, T.; Nair, P.V. Supplementation of squalene attenuates experimentally induced myocardial infarction in rats. Food Chem. 2007, 105, 1390–1395, doi:10.1016/j.foodchem.2007.05.034.
- Farvin, K.H.S.; Anandan, R.; Sankar, T.V.; Nair, P.G.V. Protective Effect of Squalene against Isoproterenol-Induced Myocardial Infarction in Rats. Clin. Biochem. Nutr. 2005, 37, 55–60.
- Farvin, K.S.; Anandan, R.; Kumar, S.H.S.; Shiny, K.S.; Sankar, T.V.; Thankappan, T.K. Effect of squalene on tissue defence system in isoproterenol-induced myocardial infarction in rats. Res. 2004, 50, 231–236.
- Motawi, T.M.; Sadik, N.A.E.-H.; Refaat, A. Cytoprotective effects of DL-alpha-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: An experimental study on rat myocardium, testicles and urinary bladder. Food Chem. Toxicol. 2010, 48, 2326–2336, doi:10.1016/j.fct.2010.05.067.
- Yao, X.; Gordon, E.M.; Figueroa, D.M.; Barochia, A.V.; Levine, S.J. Emerging Roles of Apolipoprotein E and Apolipoprotein A-I in the Pathogenesis and Treatment of Lung Disease. J. Respir. Cell Mol. Biol. 2016, 55, 159–169, doi:10.1165/rcmb.2016-0060tr.
- Boskou, D.; Clodoveo, M. Squalene: A Trove of Metabolic Actions. In Products from Olive Tree; Clodoveo, M., Boskou, D., Eds.; IntechOpen, London, UK: 2016.
- Mehdi, M.M.; Rizvi, S.I. Human Plasma Paraoxonase 1 (PON1) Arylesterase Activity During Aging: Correlation with Susceptibility of LDL Oxidation. Med. Res. 2012, 43, 438–443, doi:10.1016/j.arcmed.2012.08.012.
- Kim, J.-Y.; Lee, J.-W.; Youn, Y.-J.; Ahn, M.-S.; Ahn, S.G.; Yoo, B.-S.; Lee, S.-H.; Yoon, J.; Choe, K.-H. Urinary Levels of 8-Iso-Prostaglandin F2α and 8-Hydroxydeoxyguanine as Markers of Oxidative Stress in Patients with Coronary Artery Disease. Korean Circ. J. 2012, 42, 614–617, doi:10.4070/kcj.2012.42.9.614.
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts 2015, 5, 123–127, doi:10.15171/bi.2015.20.
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Pharmacol. 2014, 5, 196, doi:10.3389/fphar.2014.00196.
- Górnicka, M.; Ciecierska, A.; Hamulka, J.; Drywień, M.E.; Frackiewicz, J.; Górnicki, K.; Wawrzyniak, A. α-Tocopherol Protects the Heart, Muscles, and Testes from Lipid Peroxidation in Growing Male Rats Subjected to Physical Efforts. Oxidative Med. Cell. Longev. 2019, 2019, 8431057, doi:10.1155/2019/8431057.
- Sishi, B.J.N. Chapter 10—Autophagy Upregulation Reduces Doxorubicin-Induced Cardiotoxicity, in Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging; Hayat, M.A., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 157–173.
- Chang, H.M.; Moudgil, R.; Scarabelli, T.; Okwuosa, T.M.; Yeh, E.T. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 1. Am. Coll. Cardiol. 2017, 70, 2536–2551.
- Zhu, Y.-P.; Zheng, Z.; Hu, S.; Ru, X.; Fan, Z.; Qiu, L.; Zhang, Y. Unification of Opposites between Two Antioxidant Transcription Factors Nrf1 and Nrf2 in Mediating Distinct Cellular Responses to the Endoplasmic Reticulum Stressor Tunicamycin. Antioxidants 2019, 9, 4, doi:10.3390/antiox9010004.
- Cárdeno, A.; Aparicio‐Soto, M.; La Paz, S.M.-D.; Bermudez, B.; Muriana, F.J.G.; Alarcón-De-La-Lastra, C. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. Funct. Foods 2015, 14, 779–790, doi:10.1016/j.jff.2015.03.009.
- Vomhof-DeKrey, E.E.; Picklo, M.J. The Nrf2-antioxidant response element pathway: A target for regulating energy metabolism. Nutr. Biochem. 2012, 23, 1201–1206, doi:10.1016/j.jnutbio.2012.03.005.
- Rachakonda, G.; Xiong, Y.; Sekhar, K.R.; Stamer, S.L.; Liebler, D.C.; Freeman, M.L. Covalent Modification at Cys151 Dissociates the Electrophile Sensor Keap1 from the Ubiquitin Ligase CUL3. Chem. Res. Toxicol. 2008, 21, 705–710.
- Surh, Y.-J.; Kundu, J.K.; Na, H.-K. Nrf2 as a Master Redox Switch in Turning on the Cellular Signaling Involved in the Induction of Cytoprotective Genes by Some Chemopreventive Phytochemicals. Planta Med. 2008, 74, 1526–1539.
- Zhang, H.; Forman, H.J. Reexamination of the electrophile response element sequences and context reveals a lack of consensus in gene function. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2010, 1799, 496–501.
- Eggler, A.L.; Gay, K.A.; Mesecar, A.D. Molecular mechanisms of natural products in chemoprevention: Induction of cytoprotective enzymes by Nrf2. Mol. Nutr. Food Res. 2008, 52 (Suppl. 1), S84–S94.
- Howden, R. Nrf2 and Cardiovascular Defense. Oxidative Med. Cell. Longev. 2013, 2013, 104308.
- Chesley, A.; Lundberg, M.S.; Asai, T.; Xiao, R.P.; Ohtani, S.; Lakatta, E.G.; Crow, M.T. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3’-kinase. Circ. Res. 2000, 87, 1172–1179.
- Wiesel, P.; Patel, A.P.; Carvajal, I.M.; Wang, Z.Y.; Pellacani, A.; Maemura, K.; Difonzo, N.; Rennke, H.G.; Layne, M.D.; Yet, S.-F.; et al. Exacerbation of chronic renovascular hypertension and acute renal failure in heme oxygenase-1-deficient mice. Circ. Res. 2001, 88, 1088–1094.
- Lu, Z.; Xu, X.; Hu, X.; Zhu, G.; Zhang, P.; Van Deel, E.; French, J.; Fassett, J.; Oury, T.; Bache, R.; et al. Extracellular Superoxide Dismutase Deficiency Exacerbates Pressure Overload–Induced Left Ventricular Hypertrophy and Dysfunction. Hypertension 2008, 51, 19–25.
- Matsushima, S.; Kinugawa, S.; Ide, T.; Matsusaka, H.; Inoue, N.; Ohta, Y.; Yokota, T.; Sunagawa, K.; Tsutsui, H. Overexpression of glutathione peroxidase attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2237–H2245.
- Li, J.; Ichikawa, T.; Villacorta, L.; Janicki, J.S.; Brower, G.L.; Yamamoto, M.; Cui, T. Nrf2 Protects against Maladaptive Cardiac Responses to Hemodynamic Stress. Arter. Thromb. Vasc. Biol. 2009, 29, 1843–1850.