| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jiujiu Yu | + 3786 word(s) | 3786 | 2021-02-14 09:37:52 | | | |
| 2 | Vicky Zhou | -11 word(s) | 3775 | 2021-02-26 06:22:42 | | |
Video Upload Options
The nucleotide-binding domain and leucine-rich repeat related (NLR) family, pyrin domain containing 3 (NLRP3) inflammasome is a multimeric protein complex that recognizes various danger or stress signals from pathogens, the host, and the environment, leading to activation of caspase-1 and inducing inflammatory responses. This pro-inflammatory protein complex plays critical roles in pathogenesis of a wide range of diseases including neurodegenerative diseases, autoinflammatory diseases, and metabolic disorders. Therefore, intensive efforts have been devoted to understanding its activation mechanisms and to searching for its specific inhibitors. Approximately forty natural compounds with anti-NLRP3 inflammasome properties have been identified.
1. Introduction
The molecular mechanisms of NLRP3 inflammasome activation have been reviewed in-depth q[1][2][3][4][5]. Here, we summarize general principles of NLRP3 inflammasome activation (Figure 1) to provide background knowledge about how natural compounds target this key pro-inflammatory protein complex.
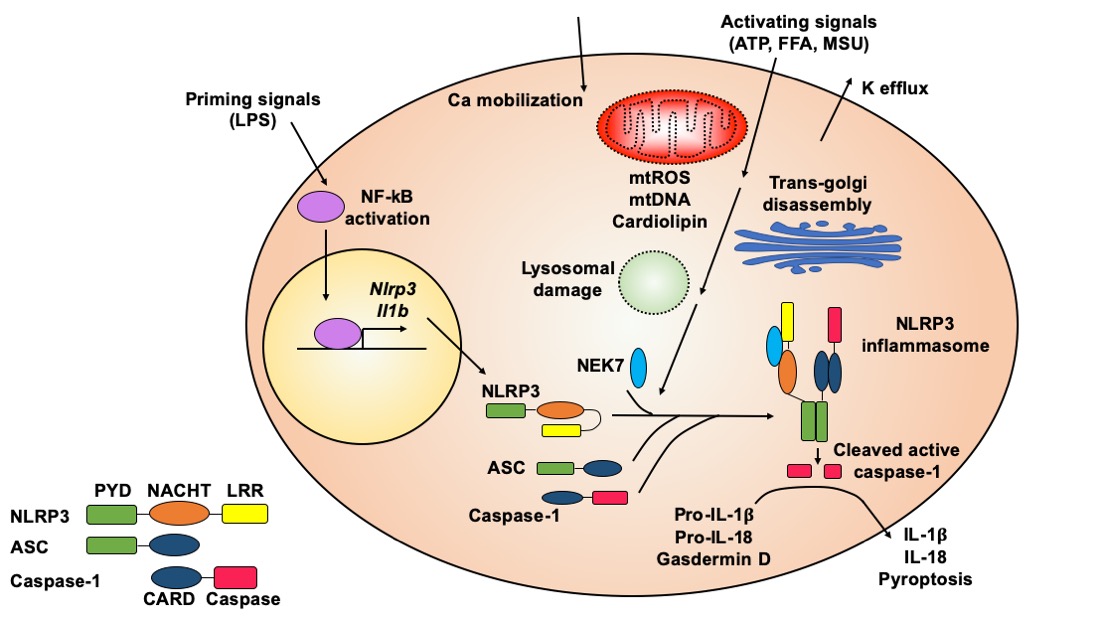
Figure 1. Molecular mechanisms of NLRP3 inflammasome activation. The NLRP3 inflammasome is activated through an initial priming step followed by an activating step. The priming signals, such as lipopolysaccharide (LPS), activate NF-κB signaling, which induces transcription of the Nlrp3 and Il1b genes. The activating signals, such as ATP, nigericin, free fatty acids (FFAs), monosodium urate (MSU) or cholesterol crystals, can trigger a series of cellular events, which typically include potassium efflux, calcium mobilization, mitochondrial (mt) damage (leading to release of reactive oxygen species (ROS), DNA, and cardiolipin), trans-Golgi disassembly, and lysosomal rupture. These upstream cellular events converge on the NLRP3 protein, causing its conformational change and leading to oligomerization of NLRP3 with the assistance of NEK7 protein. Oligomerized NLRP3 recruits ASC, which further recruits caspase-1, to form the NLRP3 inflammasome complex. Caspase-1 autocleaves itself to generate the active enzyme, which cleaves pro-IL-1β and pro-IL-18 to release mature cytokines. Active caspase-1 also cleaves gasdermin D to induce pyroptosis. Both cytokine release and pyropototic cell death trigger inflammation.
Although the precise molecular mechanisms of NLRP3 inflammasome activation are not fully understood, it is generally accepted that NLRP3 inflammasome activation requires an initial priming step followed by an activation step (Figure 1) [1][2]. A priming signal, such as lipopolysaccharide (LPS), activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, leading to increased expression of the Il1b and Nlrp3 genes. The priming signal also induces the deubiquitination of NLRP3 protein, which is a prerequisite for optimal activation of the NLRP3 inflammasome [6][7][8]. Activating signals for the NLRP3 inflammasome include a variety of molecules with diverse and un-related chemical structures. For example, ATP, nigericin, alum, free fatty acids (FFAs), cholesterol crystals, or monosodium urate (MSU) crystals can serve as an activating signal to promote the assembly and formation of the NLRP3 inflammasome [1][2].
The exact signaling cascade from activating signals or NLRP3 agonists to inflammasome assembly is still not clear. Some upstream cellular events that have been implicated in NLRP3 inflammasome formation include potassium efflux, calcium mobilization, production of mitochondrial reactive oxygen species (ROS), release of mitochondrial DNA, cytosolic exposure of mitochondrial inner member lipid cardiolipin, cellular translocation of inflammasome subunits, lysosomal destabilization, and trans-Golgi disassembly (Figure 1) [9][1][3]. However, some of these upstream cellular events are agonist specific. For example, lysosomal destabilization is often observed when crystal agonists or protein aggregates are used to activate the NLRP3 inflammasome [10][11][12]. In general, it seems that all NLRP3 agonists trigger cellular stress, which could be sensed by the NLRP3 protein, leading to NLRP3 inflammasome assembly and activation.
The NLRP3 protein contains an N-terminal pyrin domain (PYD), a central nucleotide-binding and oligomerization domain (NACHT), and a C-terminal leucine-rich repeat domain (LRR) (Figure 1) [13]. In the absence of activating signals, LRR internally interacts with NACHT, which causes the NLRP3 protein to form a closed structure. The activating signal triggers a conformational transition of NLRP3 proteins, leading to the oligomerization of NLRP3 through their NACHT domains. Recently, it has been shown that adjacent NLRP3 proteins are bridged through never in mitosis gene a (NIMA)-related kinase 7 (NEK7) to facilitate oligomerization [14]. Oligomerized NLRP3 forms a platform to recruit ASC through PYD–PYD interaction. ASC contains an N-terminal PYD and a C-terminal caspase activation and recruitment domain (CARD). After PYD of ASC interacts with PYD of NLRP3, the CARD of ASC further recruits caspase-1 through CARD–CARD interaction, since caspase-1 contains an N-terminal CARD and a C-terminal caspase domain. The recruitment of caspase-1 in the inflammasome complex triggers its autocleavage to generate the active enzyme [15][9]. Because both PYD and CARD form filaments, the resulting NLRP3 inflammasome is a highly polymerized and filamentous protein complex with high molecular mass [16].
While proper innate immune responses protect against insults, aberrant activation of the NLRP3 inflammasome causes or contributes to the onset and progress of various diseases, including Cryopyrin-associated periodic syndromes, gout, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, obesity, atherosclerosis and type 2 diabetes.
2. Anti-NLRP3 Inflammasome Natural Compounds
2.1. Oridonin
Oridonin is an ent-kaurane diterpenoid extracted from Rabdosia rubescens, a traditional medicinal herb that has been commonly used to treat inflammatory diseases in China for centuries [17]. Oridonin strongly suppressed NLRP3 inflammasome activation in primary macrophages by blocking formation of the inflammasome complex [18]. This natural compound had no effects on the protein levels of NLRP3, ASC, caspase-1, NEK7, or pro-IL-1β. It also had no impact on potassium efflux or mitochondrial ROS production, when the NLRP3 inflammasome was activated using LPS and nigericin. The inhibitory effects of oridonin on the NLRP3 inflammasome were specific, considering it had no impact on activation of other inflammasomes, including the Absent in melanoma 2 (AIM2) inflammasome and NLR family CARD domain-containing protein 4 (NLRC4) inflammasome. Further investigation revealed that oridonin directly formed a covalent bond with cysteine 279 on the NACHT domain of the NLRP3 protein through a carbon–carbon double-bond. Binding of oridonin to the NLRP3 protein impeded the interaction between NEK7 and NLRP3 and therefore blocked further assembly and formation of the NLRP3 inflammasome complex. In MSU crystal-induced peritonitis and gouty arthritis in mice, oridonin attenuated the NLRP3 inflammasome-dependent acute inflammatory responses. After the mice were fed with a high-fat diet (HFD) for 12 weeks to induce obesity and chronic inflammation, daily intraperitoneal administration of oridonin prevented further body weight gain and improved glucose tolerance and insulin sensitivity [18].
In a recent study [19], oridonin was reported to suppress NLRP3 inflammasome activation in the murine macrophage-like RAW 264.7 cell line. In an LPS-induced acute lung injury mouse model, intraperitoneal administration of oridonin reduced the protein levels of NLRP3, caspase-1, ASC, and IL-1β in lung tissues. Histologically, inflammatory cell infiltration and alveolar hemorrhage of lung were blunted by oridonin treatment. Yan et al. [20] reported that the NLRP3 inflammasome was activated in the pericontusional cerebral cortex upon induction of traumatic brain injury in mice using the weight-drop method. Oridonin reduced both mRNA and protein levels of the Nlrp3, Pycard, and Casp1 genes. The levels of IL-1β and IL-18, downstream products of NLRP3 inflammasome activation, were also decreased in the cerebral cortex. Meanwhile, oridonin treatment attenuated the neurological deficits associated with traumatic brain injury.
2.2. Isoliquiritigenin
Isoliquiritigenin (ILG, 2′,4′,4-trihydroxychalcone), a flavonoid with a chalcone structure (Figure 2 and Table 1), is the main active compound of roots and rhizomes of Chinese licorice (Glycyrrhiza uralensis), which has been used as a natural sweeter and medicinal food worldwide [21]. ILG exhibits various bioactivities, such as antioxidant and anti-inflammatory functions [22]. In 2014, ILG was reported, for the first time, to be a potent inhibitor of the NLRP3 inflammasome [23]. The inhibitory effect of ILG on the NLRP3 inflammasome was considered specific since ILG had no inhibitory effects on the AIM2 inflammasome. It had no impact on mitochondrial ROS production but inhibited ASC oligomerization during NLRP3 inflammasome activation. However, the exact molecular targets of ILG were not further investigated. In the same study, ILG supplement (0.5% w/w) in the HFD reduced body weight gain (despite similar food intake), improved glucose tolerance and insulin sensitivity, inhibited hepatic steatosis, and suppressed inflammation in epididymal white adipose tissue (eWAT). Expression of the Nlrp3, Casp1, Pycard, and Il1b genes was also reduced in the eWAT of ILG-treated mice. Correspondingly, the release of IL-1β and caspase-1 was diminished from ex vivo cultured eWAT of ILG-treated mice [23]. A subsequent study from Lee et al. [24] showed a HFD supplemented with 0.02% (w/w) ILG did not affect body weight gain but slightly decreased food intake. The discrepancy between these two studies could be attributed to differences in HFD composition and ILG dose. Nevertheless, Lee et al. still found that supplementation of ILG inhibited hepatic steatosis, improved glucose tolerance and insulin sensitivity, and suppressed systemic chronic inflammation [24].
In a rat disease model of intracerebral hemorrhage (ICH), intraperitoneal administration of ILG decreased brain injury and neurological deficits, accompanied by decreased mRNA and protein levels of the Nlrp3, Pycard, and Casp1 genes in the ipsilateral hemisphere, as well as reduced levels of mature IL-1β and IL-18 in the brain and blood [25]. In this study, ILG was found to simultaneously dampen NF-κB signaling and boost transcription of the Nrf2 gene in the damaged brain. NF-κB signaling is involved in the priming step of NLRP3 inflammasome activation [1]. The Nrf2 gene expresses the transcription factor erythroid-2 related factor 2 (Nrf2), which is a key mediator that controls the expression of many antioxidant and detoxification enzymes and has been implicated in alleviating NLRP3 inflammasome activity [26]. Therefore, the actions of ILG on the priming step and Nrf2 regulation may contribute to its anti-inflammasome function in vivo.
In an LPS-induced acute lung injury mouse model, ILG alleviated the severity of lung injury, reduced the production of IL-1β, and lowered protein levels of NLRP3, ASC, cleaved caspase-1, and pro-IL-1β [27]. In carrageenan-induced pleurisy and lung injury in mice, pre-treatment with ILG reduced lung injury, decreased the levels of cytokines and neutrophil infiltration in the pleural exudate, as well as protein levels of NLRP3, cleaved caspase-1, and mature IL-1β in the lung tissues [28].
2.3. Silibinin
Silibinin (SB), also called Silybin, is a natural polyphenolic flavonoid from milk thistle (Silybum marianum), which has been used as an anti-inflammatory and hepatoprotective herb [29]. Incubation of SB with differentiated THP-1 cells (a human monocytic leukemia cell line) for two hours decreased caspase-1 autocleavage when the NLRP3 inflammasome was activated using LPS and ATP [30]. SB had no impact on the protein levels of NLRP3, ASC, and caspase-1 but resulted in decreased ROS production and inflammasome complex formation. Incubation of SB with RAW264.7 cells for three hours inhibited NF-κB signaling. The anti-NLRP3 inflammasome and anti-NF-κB properties of SB may confer its protective effects on LPS-induced acute lung injury in mice [30]. In another study, SB significantly reduced IL-1β release in THP-1 cells when the NLRP3 inflammasome was activated using LPS and MSU [31].
When mice were fed with a HFD to induce liver steatosis, the protein levels of NLRP3 and cleaved caspase-1 in the liver were increased but were largely blocked by the oral administration of SB [32]. In vitro, SB suppressed IL-1β release from primary hepatocytes and HepG2 cells that had been incubated with 100 μM of palmitic acid.
2.4. Cardamonin
Cardamonin (2′,4′-dihydroxy-6′-methoxychalcone, CDN) is the major flavonoid extracted from the seeds of Alpinia Katsumadai, a traditional Chinese medicinal herb of the ginger family that has been used for the treatment of multiple inflammatory disorders [33]. This natural compound was found to suppress caspase-1 autocleavage and IL-1β release upon NLRP3 inflammasome activation in immortalized mouse bone marrow-derived macrophages (BMDMs), primary BMDMs, human THP-1 cell line-differentiated macrophages, and human PBMCs [34]. It is interesting to note that CDN was incubated with cells for one hour after the LPS priming step but before the addition of various NLRP3 activators, suggesting that the action of CDN on NLRP3 inflammasome activity was direct and prompt. The inhibitory effects of CDN on the NLRP3 inflammasome were specific because it did not inhibit activation of the NLRC4 or AIM2 inflammasome. One-hour incubation of CDN did not affect the protein levels of NLRP3, ASC, or caspase-1 but impeded the assembly of the NLRP3 inflammasome. When CDN was intraperitoneally injected one hour prior to LPS injection, CDN protected mice from death and significantly reduced serum IL-1β levels. The in vivo efficacy of CDN was comparable to MCC950 [34], which is a well-established potent chemical inhibitor of the NLRP3 inflammasome [35].
In another study [36], similar doses of CDN were incubated with THP-1 cells or BMDMs for 21 h, followed by NLRP3 inflammasome activation. The lengthy incubation of cells with CDN decreased the NLRP3 protein level and expression of the Il1b gene, accompanied by decreased levels of cleaved caspase-1 and mature IL-1β. Further investigation indicated that the aryl hydrocarbon receptor (AhR) and its downstream axis Nrf2/NADPH:quinon acceptor oxidoreuctase 1 (NQO1) mediated the inhibitory effects of CDN on NLRP3 inflammasome activity [36], although the study did not further elucidate whether AhR/Nrf2/NQO1 targeted the priming step or activating step during NLRP3 inflammasome activation. The same study showed that oral or rectal administration of CDN significantly alleviated disease severity and reduced inflammation in dextran sulfate sodium (DSS)- or 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice, suggesting the promising therapeutic potential of CDN in the management of inflammatory bowel disease.
2.5. Caffeic Acid Phenethyl Ester
Caffeic Acid Phenethyl Ester (CAPE), a natural polyphenolic compound in honeybee propolis, has been reported to possess antioxidant and anti-inflammatory properties [37]. Remarkably, CAPE suppressed caspase-1 autocleavage, IL-1β release, and ASC oligomerization when LPS-primed BMDMs were treated with MSU to activate the NLRP3 inflammasome [38]. In MSU-induced gouty arthritis in mice, orally administered CAPE significantly attenuated foot swelling and neutrophil infiltration in foot tissues, accompanied by decreased release of IL-1β and IL-18 and reduced levels of cleaved caspase-1 in foot tissues. Further mechanistic studies using reconstitution experiments, pull down assay, and surface plasmon resonance (SPR) analysis showed that CAPE directly bound to ASC. CAPE specifically bound to PYD of ASC, but not to PYD of NLRP3 or CARD of ASC. Molecular modeling analysis suggested that CAPE seemed to interact with Glu13 and Lys24 of ASC through hydrogen bonds and with Lys21 and Leu45 through lipophilic interactions. The association of CAPE with ASC prevented the interaction of ASC and NLRP3 in BMDMs upon inflammasome activation. Because CAPE targeted ASC, the AIM2 inflammasome, which is composed of the sensor AIM2, the adaptor ASC, and the effector caspase-1, was unsurprisingly inhibited by CAPE treatment [38].
A recent study [39] showed that CAPE suppressed caspase-1 autocleavage, IL-1β release, and inflammasome formation in both THP-1 cells and BMDMs when the NLRP3 inflammasome was activated using LPS and ATP. The level of NLRP3 protein was decreased in a dose-dependent manner by CAPE treatment. Interestingly, CAPE did not affect the mRNA level of the Nlrp3 gene. Instead, during NLRP3 inflammasome activation, CAPE suppressed deubiquitination of the NLRP3 protein, which is a prerequisite for the assembly of the NLRP3 inflammasome complex [40]. CAPE was found to promote interaction of NLRP3 with the ubiquitin-conjugating enzyme Cullin1 but to decrease interaction of NLRP3 with the deubiquitinating enzyme COP9 signalosome subunit 5 (CSN5). This study suggests another mechanism by which CAPE suppresses activation of the NLRP3 inflammasome. CAPE was also shown to suppress NLRP3 inflammasome activity in human aortic valve interstitial cells (AVICs) cultured in osteogenic induction medium [41].
2.6. Ginsenosides
Ginsenosides are steroid glycosides and triterpene saponins (Figure 2 and Table 2) that are specifically enriched in the ginseng, the root of plants in the genus Panax. Their herb medicines have been used in Asia for thousands of years to improve stamina and treat a variety of diseases, such as gastric ulcer, diabetes, and cancer [42]. More than thirty ginsenosides have been identified, among which Rb1, compound K (CK), Rg3, and 20S-protopanaxatriol (PPT) have been indicated in inhibiting NLRP3 inflammasome activity [43].
Rb1 is the most abundant ginsenoside in Korean ginseng (Panax ginseng), and CK is a metabolite of Rb1 generated through hydrolysis by intestinal bacteria [44]. Chen et al. [45] observed when eWAT of mice were cultured ex vivo with high glucose (33 mM) for 24 h, the level of NLRP3 protein and cleaved caspase-1 in the eWAT tissue and IL-1β release in the medium were increased, but all were blunted by pretreatment with Rb1 or CK. In diabetes spontaneous mutation (Leprdb) mice (often called db/db mice), oral administration of CK improved glucose tolerance, insulin sensitivity, cognitive function, and memory [46]. Remarkably, the levels of mature IL-1β, NLRP3, cleaved caspase-1, and ASC were decreased in the hippocampus of db/db mice treated with CK. Although these animal studies pointed to the possible regulatory effects of Rb1 and CK on the NLRP3 inflammasome, the experimental design was not able to determine whether the decreased IL-1β release and cleaved caspase-1 in the tissues were caused by decreased levels of the NLRP3 inflammasome subunits or due to diminished activation of the NLRP3 inflammasome. The direct effects of Rb1 or CK on NLRP3 inflammasome activation need to be further investigated.
In a recent study [47], ginsenoside Rg3 was shown to specifically inhibit activation of the NLRP3 inflammasome, but not the NLRC4 or AIM2 inflammasome. In LPS-primed THP-1 cells, Rg3 reduced caspase-1 autocleavage and the secretion of IL-1β and IL18, but it had no impact on the release of IL-6 or TNFα when the NLRP3 inflammasome was stimulated by nigericin, ATP, or alum. Rg3 treatment did not affect upstream events of NLRP3 inflammasome activation, such as mitochondrial ROS production or potassium efflux. Instead, Rg3 abrogated the interaction of NEK7 and NLRP3 and therefore disrupted NLRP3 inflammasome assembly. In LPS-induced endotoxic shock in mice, pretreatment with Rg3 via intraperitoneal injection suppressed the levels of IL-1β in both the peritoneal cavity and circulation and improved the survival of mice [47]. Rg3 was metabolized into six metabolites after its oral administration in rats [48]. Therefore, it is important to determine whether Rg3 could reach innate immune cells in vivo and/or whether any Rg3 metabolites retain inhibitory effects on the NLRP3 inflammasome.
Ginsenoside PPT is one of the terminal metabolites commonly detected in human circulation after ingestion of ginseng [49]. Jiang et al. reported that PPT suppressed activation of the NLRP3 inflammasome, but not AIM2 or NLRC4 inflammasome, in LPS-primed peritoneal macrophages [50]. PPT blocked ASC oligomerization and subsequent caspase-1 autocleavage and IL-1β release upon NLRP3 inflammasome activation by nigericin, ATP, or silica, but it had no impact on the protein levels of NLRP3, ASC, or caspase-1. In LPS-induced endotoxic shock in mice, PPT decreased serum IL-1β levels. In the mouse model of MSU-induced acute peritonitis, PPT attenuated the level of IL-1β and neutrophil infiltration in peritoneal lavage fluids [50]. In another recent study, PPT was shown to ameliorate thioacetamide-induced hepatic fibrosis and decrease protein levels of NLRP3, cleaved caspaspe-1, and mature IL-1β in fibrotic livers [51].
3. Conclusion
Tozser et al. in 2016 [52] summarized nine natural compounds with anti-NLRP3 inflammasome function. Most of these natural compounds were included in a more recent review by Jahan et al. in 2017 [53], which overviewed 34 natural compounds possessing inhibitory actions on the NLRP3 inflammasome. In the last three years, five more new natural compounds have been identified with anti-NLRP3 inflammasome properties; these are summarized in this review. Although ILG was included in Jahan’s review, only one report was available at that time. Therefore, we added ILG to the list of most recently identified natural compounds and included an overview of ILG’s actions based on four more recent research articles.
The study of natural compounds with anti-NLRP3 inflammasome function is still at an early stage. Many outstanding questions remain unanswered in this new field. Although some elegant studies have shown that oridonin or CAPE inhibits activation of the NLRP3 inflammasome through unique mechanisms or by targeting multiple targets, more investigation is warranted to elucidate the detailed molecular mechanisms that enable each natural compound to inhibit the NLRP3 inflammasome. It also would be important to study the structural basis of anti-inflammasome properties of these natural compounds and their derivatives, since such studies will facilitate modification and optimization during drug development. Future studies on the bioavailability, efficacy, and safety of these natural compounds in vivo are needed, as such information is critical to determine their suitability for treatment of specific diseases. In addition, because natural compounds could be converted into a variety of metabolites after oral administration, it would be critical to assess what types of metabolites reach innate immune cells and whether these metabolites retain anti-NLRP3 inflammasome activities. An ultimate question in the field of anti-NLRP3 inflammasome natural compounds is how to develop efficient and rapid strategies to fractionate and separate the crude natural extracts and identify new active natural components. The whole natural compound field is facing the same challenge, requiring multidisciplinary efforts and expertise from chemistry, molecular and cell biology, physiology, and pharmacology.
Successful development of a drug from bench to bedside requires tremendous financial and time investment. Generally, it takes 10–15 years and USD 500 million–USD 2 billion to develop a single drug [54][55]. Promising drug leads undergo comprehensive examination in preclinical studies of absorption, distribution, metabolism, and excretion (ADME), efficacy, and toxicity, followed by modification and production optimization. Afterward, the candidates selected from preclinical studies enter the clinical trials (typically four phases) and are evaluated for their efficacy and safety in humans. Unfortunately, the whole process is associated with high failure frequency often due to low efficacy in humans [56]. For example, only 5% of oncology drugs entering Phase I clinical trials are eventually approved by the US Food and Drug Administration (FDA) [57]. Compared to synthetic chemicals, development of natural product-sourced drugs is more time consuming and associated with higher cost, due to the extra efforts needed to identify the active components in crude extracts and to modify (often extensively) the base molecules [58]. Therefore, there is a long way to go for natural products with anti-NLRP3 inflammasome functions to be eventually developed into drugs.
When these natural compound-based drugs become available in clinic, their cost-effectiveness would facilitate their routine use in treatment of chronic diseases such as autoinflammatory disease CAPS. Very often, such natural compound-derived drugs can be taken orally and thus avoid the side effects associated with frequent injection. However, one complication of using NLRP3 inflammasome inhibitors in treating chronic inflammation lies in potential inherent adverse effects. Proper activation of the NLRP3 inflammasome is part of the host’s protective inflammatory response against pathogens and other detrimental insults [9][59]. Long-term and continuous inhibition of the NLRP3 inflammasome may compromise the host’s normal immune defense function and therefore increase the risk of infections. One possible solution is to use a highly specific drug against the NLRP3 inflammasome and thus leave other innate immune responses intact. Intermittent administration of these inhibitor-based drugs could be another option. Furthermore, selection of different natural compounds with different molecular targets or tissue distribution could help to reach maximal clinical outcomes while limiting undesirable side effects.
Finally, it is worthwhile mentioning that many natural compounds could function through highly selective and totally unexpected new pathways. Therefore, investigating the molecular mechanisms underlying their anti-NLRP3 inflammasome functions could possibly reveal new insights and pathways of NLRP3 inflammasome activation, thus contributing to a better understanding of the complex mechanisms of NLRP3 inflammasome activation.
References
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9, 857–865.
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286.
- Sharif, H.; Wang, L.; Wang, W.L.; Magupalli, V.G.; Andreeva, L.; Qiao, Q.; Hauenstein, A.V.; Wu, Z.; Nunez, G.; Mao, Y.; et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 2019, 570, 338–343.
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832.
- Lu, A.; Magupalli, V.G.; Ruan, J.; Yin, Q.; Atianand, M.K.; Vos, M.R.; Schroder, G.F.; Fitzgerald, K.A.; Wu, H.; Egelman, E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014, 156, 1193–1206.
- Ding, Y.; Ding, C.; Ye, N.; Liu, Z.; Wold, E.A.; Chen, H.; Wild, C.; Shen, Q.; Zhou, J. Discovery and development of natural product oridonin-inspired anticancer agents. Eur. J. Med. Chem. 2016, 122, 102–117.
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W.; et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 2018, 9, 2550.
- Yang, H.; Lv, H.; Li, H.; Ci, X.; Peng, L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-kappaB pathways. Cell Commun. Signal. 2019, 17, 62.
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161.
- Yan, C.; Yan, H.; Mao, J.; Liu, Y.; Xu, L.; Zhao, H.; Shen, J.; Cao, Y.; Gao, Y.; Li, K.; et al. Neuroprotective effect of oridonin on traumatic brain injury via inhibiting NLRP3 inflammasome in experimental mice. Front. Neurosci. 2020, 14, 557170.
- Lee, Y.; Kwon, E.Y.; Choi, M.S. Dietary isoliquiritigenin at a low dose ameliorates insulin resistance and NAFLD in diet-induced obesity in C57BL/6J mice. Int. J. Mol. Sci. 2018, 19, 3281.
- Liu, Q.; Lv, H.; Wen, Z.; Ci, X.; Peng, L. Isoliquiritigenin activates nuclear factor erythroid-2 related factor 2 to suppress the NOD-like receptor protein 3 inflammasome and inhibits the NF-kappaB pathway in macrophages and in acute lung injury. Front. Immunol. 2017, 8, 1518.
- Juliana, C.; Fernandes-Alnemri, T.; Kang, S.; Farias, A.; Qin, F.; Alnemri, E.S. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 2012, 287, 36617–36622.
- Gao, Y.; Lv, X.; Yang, H.; Peng, L.; Ci, X. Isoliquiritigenin exerts antioxidative and anti-inflammatory effects via activating the KEAP-1/Nrf2 pathway and inhibiting the NF-kappaB and NLRP3 pathways in carrageenan-induced pleurisy. Food Funct. 2020, 11, 2522–2534.
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489.
- Zhang, B.; Wang, B.; Cao, S.; Wang, Y.; Wu, D. Silybin attenuates LPS-induced lung injury in mice by inhibiting NF-kappaB signaling and NLRP3 activation. Int. J. Mol. Med. 2017, 39, 1111–1118.
- Ding, Y.; Ding, C.; Ye, N.; Liu, Z.; Wold, E.A.; Chen, H.; Wild, C.; Shen, Q.; Zhou, J. Discovery and development of natural product oridonin-inspired anticancer agents. Eur. J. Med. Chem. 2016, 122, 102–117.
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W.; et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 2018, 9, 2550.
- Yang, H.; Lv, H.; Li, H.; Ci, X.; Peng, L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-kappaB pathways. Cell Commun. Signal. 2019, 17, 62.
- Yan, C.; Yan, H.; Mao, J.; Liu, Y.; Xu, L.; Zhao, H.; Shen, J.; Cao, Y.; Gao, Y.; Li, K.; et al. Neuroprotective effect of oridonin on traumatic brain injury via inhibiting NLRP3 inflammasome in experimental mice. Front. Neurosci. 2020, 14, 557170.
- Honda, H.; Nagai, Y.; Matsunaga, T.; Okamoto, N.; Watanabe, Y.; Tsuneyama, K.; Hayashi, H.; Fujii, I.; Ikutani, M.; Hirai, Y.; et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J. Leukoc. Biol. 2014, 96, 1087–1100.
- Lee, Y.; Kwon, E.Y.; Choi, M.S. Dietary isoliquiritigenin at a low dose ameliorates insulin resistance and NAFLD in diet-induced obesity in C57BL/6J mice. Int. J. Mol. Sci. 2018, 19, 3281.
- Zeng, J.; Chen, Y.; Ding, R.; Feng, L.; Fu, Z.; Yang, S.; Deng, X.; Xie, Z.; Zheng, S. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-kappaB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J. Neuroinflamm. 2017, 14, 119.
- Liu, Q.; Lv, H.; Wen, Z.; Ci, X.; Peng, L. Isoliquiritigenin activates nuclear factor erythroid-2 related factor 2 to suppress the NOD-like receptor protein 3 inflammasome and inhibits the NF-kappaB pathway in macrophages and in acute lung injury. Front. Immunol. 2017, 8, 1518.
- Gao, Y.; Lv, X.; Yang, H.; Peng, L.; Ci, X. Isoliquiritigenin exerts antioxidative and anti-inflammatory effects via activating the KEAP-1/Nrf2 pathway and inhibiting the NF-kappaB and NLRP3 pathways in carrageenan-induced pleurisy. Food Funct. 2020, 11, 2522–2534.
- Zhang, B.; Wang, B.; Cao, S.; Wang, Y.; Wu, D. Silybin attenuates LPS-induced lung injury in mice by inhibiting NF-kappaB signaling and NLRP3 activation. Int. J. Mol. Med. 2017, 39, 1111–1118.
- Matias, M.L.; Gomes, V.J.; Romao-Veiga, M.; Ribeiro, V.R.; Nunes, P.R.; Romagnoli, G.G.; Peracoli, J.C.; Peracoli, M.T.S. Silibinin downregulates the NF-kappaB pathway and NLRP1/NLRP3 inflammasomes in monocytes from pregnant women with preeclampsia. Molecules 2019, 24, 1548.
- Zhang, B.; Xu, D.; She, L.; Wang, Z.; Yang, N.; Sun, R.; Zhang, Y.; Yan, C.; Wei, Q.; Aa, J.; et al. Silybin inhibits NLRP3 inflammasome assembly through the NAD(+)/SIRT2 pathway in mice with nonalcoholic fatty liver disease. FASEB J. 2018, 32, 757–767.
- Shibata, S. A drug over the millennia: Pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 2000, 120, 849–862.
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A review: The pharmacology of isoliquiritigenin. Phytother. Res. 2015, 29, 969–977.
- Jhang, J.J.; Yen, G.C. The role of Nrf2 in NLRP3 inflammasome activation. Cell. Mol. Immunol. 2017, 14, 1011–1012.
- Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 2014, 21, 415–422.
- Chen, C.; Shenoy, A.K.; Padia, R.; Fang, D.; Jing, Q.; Yang, P.; Su, S.B.; Huang, S. Suppression of lung cancer progression by isoliquiritigenin through its metabolite 2, 4, 2’, 4’-Tetrahydroxychalcone. J. Exp. Clin. Cancer Res. 2018, 37, 243.
- Abenavoli, L.; Izzo, A.A.; Milic, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213.
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Munoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255.
- Vue, B.; Zhang, S.; Zhang, X.; Parisis, K.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.H. Silibinin derivatives as anti-prostate cancer agents: Synthesis and cell-based evaluations. Eur. J. Med. Chem. 2016, 109, 36–46.
- Wang, J.; Qiu, R.; Yuan, L.; Meng, F.; Tang, Q. Analysis on the Alpinia katsumadai components of Zingiberaceae plants and their functions on myeloma resistance. Pak. J. Pharm. Sci. 2015, 28, 1065–1068.
- Break, M.K.B.; Hossan, M.S.; Khoo, Y.; Qazzaz, M.E.; Al-Hayali, M.Z.K.; Chow, S.C.; Wiart, C.; Bradshaw, T.D.; Collins, H.; Khoo, T.J. Discovery of a highly active anticancer analogue of cardamonin that acts as an inducer of caspase-dependent apoptosis and modulator of the mTOR pathway. Fitoterapia 2018, 125, 161–173.
- Dong, F.; Wang, S.; Yang, A.; Li, Q.; Wang, Y.; Dai, L.; Tao, Y.; Wei, X.; Zhang, J. Systematic screening and characterization of cardamonin metabolites using UHPLC-Q-Exactive Orbitrap MS after oral administration to rats. Arab. J. Chem. 2020, 13, 8768–8782.
- Elliott, E.I.; Sutterwala, F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015, 265, 35–52.
- Murtaza, G.; Karim, S.; Akram, M.R.; Khan, S.A.; Azhar, S.; Mumtaz, A.; Bin Asad, M.H. Caffeic acid phenethyl ester and therapeutic potentials. Biomed. Res. Int. 2014, 2014, 145342.
- Hofseth, L.J.; Wargovich, M.J. Inflammation, cancer, and targets of ginseng. J. Nutr. 2007, 137, 183S–185S.
- Yi, Y.S. Roles of ginsenosides in inflammasome activation. J. Ginseng. Res. 2019, 43, 172–178.
- Chen, W.; Wang, J.; Luo, Y.; Wang, T.; Li, X.; Li, A.; Li, J.; Liu, K.; Liu, B. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J. Ginseng Res. 2016, 40, 351–358.
- Li, C.W.; Deng, M.Z.; Gao, Z.J.; Dang, Y.Y.; Zheng, G.D.; Yang, X.J.; Chao, Y.X.; Cai, Y.F.; Wu, X.L. Effects of compound K, a metabolite of ginsenosides, on memory and cognitive dysfunction in db/db mice involve the inhibition of ER stress and the NLRP3 inflammasome pathway. Food Funct. 2020, 11, 4416–4427.
- Shi, Y.; Wang, H.; Zheng, M.; Xu, W.; Yang, Y.; Shi, F. Ginsenoside Rg3 suppresses the NLRP3 inflammasome activation through inhibition of its assembly. FASEB J. 2020, 34, 208–221.
- Jiang, J.; Sun, X.; Akther, M.; Lian, M.L.; Quan, L.H.; Koppula, S.; Han, J.H.; Kopalli, S.R.; Kang, T.B.; Lee, K.H. Ginsenoside metabolite 20(S)-protopanaxatriol from Panax ginseng attenuates inflammation-mediated NLRP3 inflammasome activation. J. Ethnopharmacol. 2020, 251, 112564.
- Song, J.; Cui, Z.Y.; Lian, L.H.; Han, X.; Hou, L.S.; Wang, G.; Gao, L.; Zhu, Y.; Jiang, Y.C.; Dou, J.Y.; et al. 20S-Protopanaxatriol Ameliorates Hepatic Fibrosis, Potentially Involving FXR-Mediated Inflammatory Signaling Cascades. J. Agric. Food Chem. 2020, 68, 8195–8204.
- Akao, T.; Kida, H.; Kanaoka, M.; Hattori, M.; Kobashi, K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 1998, 50, 1155–1160.
- Qian, T.; Cai, Z.; Wong, R.N.; Mak, N.K.; Jiang, Z.H. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 816, 223–232.
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693.
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361.
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856.
- Adams, C.P.; Brantner, V.V. Estimating the cost of new drug development: Is it really 802 million dollars? Health Aff. (Millwood) 2006, 25, 420–428.
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The price of innovation: New estimates of drug development costs. J. Health Econ. 2003, 22, 151–185.
- Prasad, S.; Gupta, S.C.; Aggarwal, B.B. Serendipity in cancer drug discovery: Rational or coincidence? Trends Pharmacol. Sci. 2016, 37, 435–450.
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–715.
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695.
- Tartey, S.; Kanneganti, T.D. Differential role of the NLRP3 inflammasome in infection and tumorigenesis. Immunology 2019, 156, 329–338.