| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Miguel Angel Prieto Lage | + 1825 word(s) | 1825 | 2021-02-19 03:54:52 | | | |
| 2 | Bruce Ren | -21 word(s) | 1804 | 2021-03-01 05:15:51 | | |
Video Upload Options
Nowadays, cancer is one of the deadliest diseases in the world, which has been estimated to cause 9.9 million deaths in 2020. Conventional treatments for cancer commonly involve mono-chemotherapy or a combination of radiotherapy and mono-chemotherapy. However, the negative side effects of these approaches have been extensively reported and have prompted the search of new therapeutic drugs. In this context, scientific community started to look for innova-tive sources of anticancer compounds in natural sources, including traditional plants. Currently, numerous studies have evaluated the anticancer properties of natural compounds derived from plants, both in vitro and in vivo. In pre-clinical stages, some promising compounds could be men-tioned, such as the sulforaphane or different phenolic compounds. On the other hand, some phy-tochemicals obtained positive results in clinical stages and were further approved for cancer treatment, such as vinca alkaloids or the paclitaxel.
1. Introduction
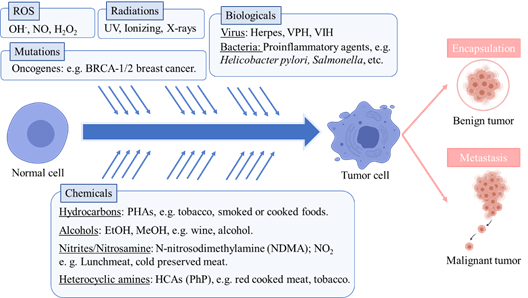
Cancer is one of the deadliest diseases globally and especially in western countries. According to the International Cancer Observatory, roughly 9.9 million people have died in 2020 as a result of developing cancer [1]. Cancer is a complex disease, generally defined as an uncontrolled proliferation and development of cells in tissues forming an amalgamation and microenvironment (tumor) that may potentially expand to a whole organ or systemically to other tissues (metastasis) [2]. This abnormal cell behavior may be the result of hereditary genetics, or an epigenetic-driven alteration of key genes (oncogenes) related to the cell cycle and regulation of cell death (apoptosis) [3]. Cancerous cells are also characterized by dysregulation of programmed apoptosis and aberrant behavior of microtubules, as they are involved in the mitotic process [3]. The World Health Organization identifies as main causes behind the development of cancer random somatic mutations, ionizing radiation, reactive oxidative species as well as several chemical and biological agents [4]. Except for random mutations, these are widely recognized exogenous carcinogens. Ionizing radiation is able to disrupt the hydrogen bonds between nucleic acids as well as altering their chemical conformation, which may yield alterations in normal DNA expression regulation[5] . Infectious diseases caused by bacteria, fungi or viruses have also been significantly correlated with developing cancer afterward in the same affected tissues. Well-established associations between infections and cancer are viruses like human papillomavirus to cervix cancer, Herpesvirus to Karposi’s sarcoma or Hepatitis B and C to liver cancer. In the same sense, bacterial infections by Helycobacter pylori are linked to gastric cancer or the genus Salmonella to colon or gallbladder cancer development post-infection [6]. Viruses that integrate their genetic material into the host may alter normal expression of genes related to cell division or even induce expression of oncogenes that could derive into oncogenesis. Some examples of these are the integration of Hepatitis B virus into telomeres, as well as genes coding for proteins X and S, which induce inflammation and neoplasia; or Human Papilomavirus integration in oncogenes E6 and E7 in the cell genome, which suppress the p53 anti-tumor gene and promote cell proliferation while simultaneously alters cell-to-cell adhesion [7][8][9]. These oncogenes have been related to increased release of inflammatory mediators like necrosis factor kappaB (Nf-κB) or Activator protein-1 (AP-1) [8]. Conversely, bacterial infections may elicit the release of toxins with cytotoxic activity and the disruption of the tissue cell matrix. Known examples are enteric toxins from S. typhi or CagA and vacuolating toxins of H. pylori, which may induce cell death, neoplasia, and also alterations in the normal cell metabolism [10][11]. Other infectious pathogens like fungi and parasitic helminths that produce direct or toxin-mediated tissular damage are also accounted as oncogenic agents [12]. Aside from specific genetic alterations, the main recognized tumor-inducing mechanism of biological agents is tissue inflammation as a result of cell damage and subsequent neoplasia which, if unchecked, can result in potential chronic inflammation of the affected tissues (e.g., hepatic cirrhosis by Hepatitis virus) [13]. Regarding reactive oxygen species, like hydroxide peroxide or hydroxyl radical, which are normal metabolic products but also arise from contact with oxygen, they are described to provoke damage and alterations of the cell membranes, lipids or DNA [14]. Indeed, reactive oxygen species have been identified to increase in tumorous cells, enhancing their proliferation and survivability [15]. Nonetheless, the common factor besides possible genetic alterations by oxidative stress, infections and ultraviolet radiation is the associated inflammatory response [14]. On this matter, chronic inflammation is considered both cause and symptom of other ailments, but particularly of cancer, as tumorous cells secrete several pro-inflammatory molecules [16]. For example, it is well known that the pro-inflammatory mediator cyclooxygenase-2 (COX-2) is overexpressed in several types of cancer. As such, pro-inflammatory mediators are markers of cancer and could be also a possible target for anticancer therapies [17][18]. Considering chemical carcinogens aside from potential hazardous substances, the main carcinogens originate in diet. Major chemical carcinogens include polycyclic aromatic hydrocarbons (PAHs), N-nitroso compounds, heterocyclic amines (HCAs) and alcohol. PAHs like anthracene appear in combustion reactions, and are reported in grilled or smoked foods, as well as being part of urban air pollution. They are linked to lung and digestive tract cancer [19][20]. Closely related in their effects and occurrence, HCAs like 2-Amino-1-methyl-6-phenylimidazo [4,5-b]pyridine are the result of pyrolysis of proteins and amino acids in meat or fish foods [21][22]. It is worth mentioning that tobacco is reported to contain high levels of PAHs and HCAs, linking them to the pro-carcinogen effects of tobacco consumption [23]. N-nitroso compounds are additives in processed meats and include nitrites and nitrosamines like N-nitrosedimethylamine that have been correlated to gastric cancer development [24]. Ethanol as well as other alcohols present in beverages and spirits induce many metabolic and endocrine disorders along with being highly cytotoxic chemicals and attributed to cause many types of cancers [25]. Altogether, it should be considered that a variety of exogenous carcinogens from different sources can heavily prompt cancer development (Figure 1).
Figure 1. Main causes involved in the development of cancer, according to WHO.
Cancer not only displays heterogeneous cell and tissue-specific behavior, depending on the onset, inducer and individual genetic profile, but also an unpredictable and diverse evolution that hinders its treatment [26]. Conventional cancer treatment approach commonly involves chemotherapy, radiotherapy and surgery [27][28]. Regarding chemotherapeutics, their effect is cytostatic, acting through shifting the expression of cell cycle mediators, disruption of microtubules, or inducers of apoptosis. However, as radiotherapy and chemotherapy do not discriminate between normal and cancer cells, their application is paired with certain side effects, ranging from mild gastrointestinal alterations and nausea to severe gut mucosa dysfunction, cardiovascular toxicity or immunity disorders [29][30]. These side effects, which can linger for long periods after treatment, pose a major issue when selecting and applying therapeutics.
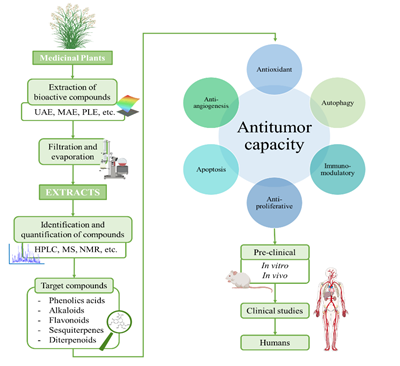
The ever-growing interest in the search of new therapeutic compounds against cancer has pushed researchers to look for innovative sources of anticancer compounds in natural sources, including plants [31]. Traditionally, plants have been used in all cultures for healing diverse diseases and improve well-being [32][33]. Further research demonstrated that traditional-used plants contain bioactive compounds, which administered in sufficient doses, have positive effects on health. These effects are attributed to the biological properties of the compounds, such as antioxidant, anti-inflammatory, antimicrobial and also anticancer. Nowadays, the potential of plants as sources of anticancer compounds is both well recorded in traditional medicine and experimental findings [34]. In several cases, phytochemical compounds have been directly employed or chemically modified to develop chemicals used in modern medicine, including anticancer drugs. According with the Food and Drug Administration (FDA), more than the 60% of the drugs employed in cancer treatment are obtained from natural resources [32]. In Figure 2, a schematic process for the development of anticancer drugs based on phytochemical compounds is presented. Briefly, this process starts with the extraction from plants and the testing of the extracts to evaluate their anticancer potential. Bioactive compounds of the extract are identified, purified and tested in pre-clinical studies, (both in vitro cell cultures and in vivo animal models) and later clinical trials in humans [35]. In these studies, some factors such efficacy, induced tumorigenic changes, possible side effects and toxicity factors must be deeply characterized [30]. Vinka alkaloids, taxanes, and camptothecin are some examples of compounds that are currently clinically employed.
In general, plant-anticancer compounds have been considered a possible option to develop new chemotherapeutics and also to enhance the effectivity of the conventional ones [36][37][38]. Nevertheless, these compounds present many drawbacks, such as low stability or solubility, difficulty to be extracted from natural sources and even negative side effects [39]. Thus, the application of these compounds still has to face several challenges and further research is necessary. In this review, current information about phytochemical compounds currently employed clinically in cancer treatment and also promising compounds in pre-clinical and clinical level will be addressed. In addition, the main challenges lying facing the use of these compounds as therapeutic agents and possible strategies to solve them will be described.
Figure 2. Schematic process for the development of anticancer drugs based on plant-derived compounds.
2. Phytochemicals Currently Used in Cancer Therapy
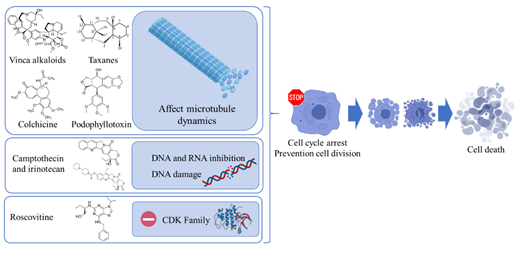
Along the last decade, many works have compiled ethnomedicinal and ethnopharmacological uses of very different plant species. Numerous experimental works based on the evaluation through in vitro and in vivo assays have confirmed the therapeutic application of many natural compounds, which have been later included as part of approved treatments, including in anticancer agents (Figure 3) [40].
Figure 3. Main mechanisms of phytochemical compounds employed in cancer therapy.
This section summarizes current available information regarding the clinical status of the main plant compounds proposed for cancer treatment. Vinka alkaloids, taxanes, camptothecin derivatives, podophyllotoxin and derivatives and roscovitine are the most used in clinical studies. Table 1 collects an overview of the data supporting the development of plant compounds as anticancer agents, including clinical trials and clinical uses.
Table 1. Anticancer compounds employed in clinical treatment of cancer [41][42][43][44].
|
Compounds |
Source-Extraction |
Mechanism Action |
Clinical Development |
Commercial Name |
|
Vinca alkaloids |
Catharanthus roseu (Leaves) Isolated by semi-synthetic routes |
Inhibit the tubulin polymerization of tumor cells and also cause mitotic spindle destruction |
In clinical use; combination trials |
Vinorelbine, Vincristine, Vinblastine, Vindesine, Vinflunine, Vincamine, Vintafolide |
|
Paclitaxel, docetaxel |
Taxus spp.(Bark) Synthesis, semi-synthesis, and plant cell culture |
Stabilization of microtubules and inhibition of depolymerization into tubulin, which stops the cell cycle in the G2/M phase leading to cell death |
In clinical use; Phase I-III clinical trials; early treatment settings; non-small lung cancer, breast cancer, ovarian cancer, Kaposi sarcoma. Research and development in alternative drug administration using nanoparticles, naocochealtes and nanoliposomes. |
Taxol®, Taxotere®, Abraxane®, Jevtana®, Taxoprexin®, Xytotax® |
|
Camptotecin, irinotecan |
Camptotheca acuminata (leaves) Water extraction |
Binding to the TOP1 cleavage complex, leading to an accumulation of DNA strand breaks upon replication, causing apoptosis during the S phase of the cell cycle |
Ovarian, lung, colorectal and pediatric cancer |
Topotecan, irinotecan, belotecan |
|
Podophyllotoxin and analogues |
Podophyllum spp. (rhizome, roots) Alcohol extraction |
Blockage of cell division metaphase of mitosis |
Lymphomas and testicular cancer trials |
No rentable |
|
Roscovitine |
Raphanus sativus(Radish) Chloroform extraction |
Inhibition of cyclin dependent kinases; reduction of cell cycle progression |
Phase II clinical trials in Europe |
Roscovitine, seliciclib |
References
- International Agency for Research on Cancer Global Cancer Observatory 2020.
- Valent, P.; Bonnet, D.; De Maria, R.; Lapidot, T.; Copland, M.; Melo, J. V.; Chomienne, C.; Ishikawa, F.; Schuringa, J.J.; Stassi, G.; et al. Cancer stem cell definitions and terminology: The devil is in the details. Nat. Rev. Cancer 2012, 12, 767–775, doi:10.1038/nrc3368.
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633, doi:10.1016/j.tcb.2013.07.006.
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; WHO Press, World Health Organization, 2014; ISBN 978-92-832-0443-5.
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986, doi:10.1111/j.1365-4632.2010.04474.x.
- International Agency for Research on Cancer Biological Agents, Volume 100B: A Review on Human Carcinogens; IARC: Lyon, 2012; Vol. 105; ISBN 978 92 832 1319 2.
- Zhao, L.H.; Liu, X.; Yan, H.X.; Li, W.Y.; Zeng, X.; Yang, Y.; Zhao, J.; Liu, S.P.; Zhuang, X.H.; Lin, C.; et al. Genomic and oncogenic preference of HBV integration in hepatocellular carcinoma. Nat. Commun. 2016, 7, 1–10, doi:10.1038/ncomms12992.
- Martin, D.; Gutkind, J.S. Human tumor-associated viruses and new insights into the molecular mechanisms of cancer. Oncogene 2008, 27, S31–S42, doi:10.1038/onc.2009.351.
- Hansen, A.; Henderson, S.; Lagos, D.; Nikitenko, L.; Coulter, E.; Roberts, S.; Gratrix, F.; Plaisance, K.; Renne, R.; Bower, M.; et al. KSHV-encoded miRNAs target MAF to induce endothelial cell reprogramming. Genes Dev. 2010, 24, 195–205, doi:10.1101/gad.553410.
- Wen, S.; Moss, S.F. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Lett. 2009, 282, 1–8, doi:10.1016/j.canlet.2008.11.016.
- Scanu, T.; Spaapen, R.M.; Bakker, J.M.; Pratap, C.B.; Wu, L. en; Hofland, I.; Broeks, A.; Shukla, V.K.; Kumar, M.; Janssen, H.; et al. Salmonella Manipulation of Host Signaling Pathways Provokes Cellular Transformation Associated with Gallbladder Carcinoma. Cell Host Microbe 2015, 17, 763–774, doi:10.1016/j.chom.2015.05.002.
- Elsland, D.; Neefjes, J. Bacterial infections and cancer. EMBO Rep. 2018, 19, 1–11, doi:10.15252/embr.201846632.
- Mesri, E.A.; Cesarman, E.; Boshoff, C. Kaposi’s sarcoma and its associated herpesvirus. Nat. Rev. Cancer 2010, 10, 707–719, doi:10.1038/nrc2888.
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616, doi:10.1016/j.freeradbiomed.2010.09.006.
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64, doi:10.1016/j.semcdb.2017.05.023.
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596, doi:10.1038/nrclinonc.2015.105.
- Masferrer, J.L.; Leahy, K.M.; Koki, A.T.; Zweifel, B.S.; Settle, S.L.; Woerner, B.M.; Edwards, D.A.; Flickinger, A.G.; Moore, R.J.; Seibert, K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000, 60, 1306–1311.
- Qu, X.; Tang, Y.; Hua, S. Immunological approaches towards cancer and inflammation: A cross talk. Front. Immunol. 2018, 9, doi:10.3389/fimmu.2018.00563.
- Shen, H.; Tao, S.; Liu, J.; Huang, Y.; Chen, H.; Li, W.; Zhang, Y.; Chen, Y.; Su, S.; Lin, N.; et al. Global lung cancer risk from PAH exposure highly depends on emission sources and individual susceptibility. Sci. Rep. 2014, 4, 1–8, doi:10.1038/srep06561.
- Bansal, V.; Kim, K.H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38, doi:10.1016/j.envint.2015.06.016.
- Sugimura, T.; Wakabayashi, K.; Nakagama, H.; Nagao, M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Gann Monogr. Cancer Res. 2004, 52, 71–96.
- Puangsombat, K.; Gadgil, P.; Houser, T.A.; Hunt, M.C.; Smith, J.S. Occurrence of heterocyclic amines in cooked meat products. Meat Sci. 2012, 90, 739–746, doi:10.1016/j.meatsci.2011.11.005.
- World Health Organization WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco; 3rd ed.; Geneva, World Health Organization, 2011; ISBN 978 92 4 068781 3.
- Song, P.; Wu, L.; Guan, W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: A me-ta-analysis. Nutrients 2015, 7, 9872–9895, doi:10.3390/nu7125505.
- Praud, D.; Rota, M.; Rehm, J.; Shield, K.; Zatoński, W.; Hashibe, M.; La Vecchia, C.; Boffetta, P. Cancer incidence and mor-tality attributable to alcohol consumption. Int. J. Cancer 2016, 138, 1380–1387, doi:10.1002/ijc.29890.
- Litzenburger, U.M.; Buenrostro, J.D.; Wu, B.; Shen, Y.; Sheffield, N.C.; Kathiria, A.; Greenleaf, W.J.; Chang, H.Y. Sin-gle-cell epigenomic variability reveals functional cancer heterogeneity. Genome Biol. 2017, 18, 1–12, doi:10.1186/s13059-016-1133-7.
- Qin, S.Y.; Cheng, Y.J.; Lei, Q.; Zhang, A.Q.; Zhang, X.Z. Combinational strategy for high-performance cancer chemother-apy. Biomaterials 2018, 171, 178–197, doi:10.1016/j.biomaterials.2018.04.027.
- Gautam, L.; Jain, A.; Shrivastava, P.; Vyas, S.; Vyas, S.P. Emergence of novel targeting systems and conventional therapies for effective cancer treatment. In Nano Drug Delivery Strategies for the Treatment of Cancers; INC, 2021; pp. 1–35 ISBN 9780128197936.
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 1–3, doi:10.3389/fphar.2018.00245.
- Glass, C.K.; Mitchell, R.N. Winning the battle, but losing the war: mechanisms and morphology of can-cer-therapy-associated cardiovascular toxicity. Cardiovasc. Pathol. 2017, 30, 55–63, doi:10.1016/j.carpath.2017.06.009.
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533, doi:10.3390/ijms19113533.
- babaei, G.; Aliarab, A.; Abroon, S.; Rasmi, Y.; Aziz, S.G.G. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018, 106, 239–246, doi:10.1016/j.biopha.2018.06.131.
- Garcia-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Scientific basis for the industrialization of traditionally used plants of the Rosaceae family. Food Chem. 2020, 330, 127197, doi:10.1016/j.foodchem.2020.127197.
- Lopes, C.M.; Dourado, A.; Oliveira, R. Phytotherapy and Nutritional Supplements on Breast Cancer. Biomed Res. Int. 2017, 2017, 1–42, doi:10.1155/2017/7207983.
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From pre-clinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1–17, doi:10.3389/fphar.2019.01614.
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150, doi:https://doi.org/10.1016/j.apjtb.2017.10.016.
- Mao, Q.Q.; Xu, X.Y.; Shang, A.; Gan, R.Y.; Wu, D.T.; Atanasov, A.G.; Li, H. Bin Phytochemicals for the prevention and treatment of gastric cancer: Effects and mechanisms. Int. J. Mol. Sci. 2020, 21, doi:10.3390/ijms21020570.
- Redondo-Blanco, S.; Fernández, J.; Gutiérrez-del-Río, I.; Villar, C.J.; Lombó, F. New insights toward colorectal cancer chemotherapy using natural bioactive compounds. Front. Pharmacol. 2017, 8, 1–22, doi:10.3389/fphar.2017.00109.
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837, doi:10.1038/nature03194.
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661, doi:10.1021/acs.jnatprod.5b01055.
- Desai, A.; Qazi, G.; Ganju, R.; El-Tamer, M.; Singh, J.; Saxena, A.; Bedi, Y.; Taneja, S.; Bhat, H. Medicinal Plants and Cancer Chemoprevention. Curr. Drug Metab. 2008, 9, 581–591, doi:10.2174/138920008785821657.
- Moraes, D.F.C.; de Mesquita, L.S.S.; do Amaral, F.M.M.; de Sousa Ribeiro, M.N.; Malik, S. Anticancer drugs from plants. In Biotechnology and Production of Anti-Cancer Compounds; Springer International Publishing, 2017; pp. 121–142 ISBN 9783319538808.
- Greenwell, M.; Rahman, P.K.S.. Medicinal Plants : Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112, doi:10.13040/IJPSR.0975-8232.6(10).4103-12.Medicinal.
- FDA Food and Drud Administration Approved Drug Products - Orange Book. Orange B. 2020.