| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Festo Damian | + 1135 word(s) | 1135 | 2021-02-17 06:31:22 | | | |
| 2 | Karina Chen | Meta information modification | 1135 | 2021-03-04 09:03:29 | | | | |
| 3 | Karina Chen | Meta information modification | 1135 | 2021-03-04 09:04:51 | | |
Video Upload Options
The development of soft gelatin capsules (SGCs) dosage forms has attracted a great deal of interest in the oral delivery of poorly water-soluble drugs. This is attributed to the increased number of poorly soluble drugs in the pipeline, and hence the challenges of finding innovative ways of developing bioavailable and stable dosage forms. Encapsulation of these drugs into SGCs is one of the approaches that is utilized to deliver the active ingredients to the systemic circulation to overcome certain formulation hurdles. Once formulated, encapsulated drugs in the form of SGCs require suitable in vitro dissolution test methods to ensure drug product quality and performance.
1. Advantages and Disadvantages of SGCs
SGCs offer several advantages when compared to traditional oral solid dosage forms, and their popularity as a dosage form is increasing for several reasons, that include:
- Consumer preference: SGCs dosage forms were developed to conceal the unpleasant taste and odor of drugs. Compared to tablets, SGCs are more comfortable to swallow when used with water because the soft gelatin capsule is self-lubricating [1][2]. SGCs look more appealing and enjoyable to consumers as they can easily be produced in various shapes, sizes, and colors (Figure 1), and different drug delivery system like chewable softgels [3]and meltable softgels [4].
Figure 1. A selection of SGCs with various sizes and shapes.
- Technical advantages: SGCs have high dosage accuracy and uniformity [1][2]as well as higher consistent manufacturing requirements and product stability. It is possible to deliver an active pharmaceutical ingredient with a higher degree of accuracy and greater consistency between different manufacturing lots due to more accurate compounding, blending, and dispensing of liquid fill materials. SGC products tend to have higher stability as the entire encapsulation process can be done under inert conditions to protect drugs against oxidation and degradation. This is especially important for drugs that are subject to hydrolytic and oxidative degradation.
- Safety aspects: The tight sealing of the gelatin shell protects the fill material from air and environmental contaminations. The shell can be formulated to block ultraviolet (UV) as well as visible light. Also, SGC formulation helps to avoid dust handling contaminations and enhances operator safety [2].
- Bioavailability advantages: SGCs can increase the bioavailability of poorly soluble drugs by improving solubility and enhancing absorption within the GIT [5][6]. Water-insoluble drugs are formulated in form of SGCs using lipophilic vehicles as a portion of fill material, greatly enhancing uptake of such drugs within the GIT [7].
Despite these advantages, SGCs are not a first-line oral dosage form of choice for most pharmaceutical companies due to the following reasons:
- SGC technology is believed to be relatively expensive to produce, and this can increase prices to consumers. Many pharmaceutical companies do not have the specialized equipment necessary to fill SGCs, and most of them rely on contract laboratories/manufacturers for their supplies.
- Unlike solid dosage forms, SGCs can also be affected by humidity and microbial contamination. This can cause product stability issues if the medications are not kept in sealed containers or a cool and dry place.
- Depending on the nature of the drug that is dissolved within the lipophilic vehicle, there is a chance that the drug could migrate into the shell of the capsule. This migration can cause issues during absorption within the body, as the release rate of the drug would be altered. Likewise, SGCs are not generally capable of holding water-based liquids, as there is a possibility that the medication could diffuse out of the soft-gelatin capsule [1][2].
- Another issue concerning the use of soft gelatin drug products is the fact that some groups have dietary restrictions that prevent them from consuming animal products found in SGCs. Gelatin is primarily made from bones, skins, and other parts of animals such as pigs and cows. Because capsule shells are made from animal parts, many vegetarians also opt not to use them. Due to this, there is emerging technologies on animal-free substitute gelatin capsules made from seaweed extract or other sources, but they are generally more expensive and harder to find [8].
- Gelatin is extremely water-soluble, which helps it dissolve in the body. The downside of this property is that SGCs are sensitive to heat and humidity. In hot or humid climates, capsules may stick together or even break open before consumers have a chance to use them [9].
- Alkaline or acidic solutions are not good candidates for soft gelatin fill because they can cause hydrolysis and leakage of the gelatin shell unless their pH is adjusted to neutral [10].
2. Manufacture of SGCs
Before discussing shell and fill formulation of SGCs, an overview of the industrial SGC filling process is briefly highlighted in this section. These steps are critical as they can influence the dissolution characteristics of SGCs. For details of the manufacturing process of SGCs, readers are referred to Gullapalli [11]. The main steps during the SGC manufacturing process are as follows:
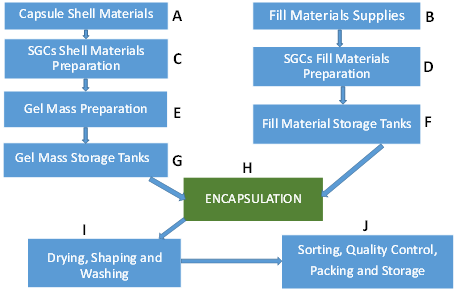
- Raw materials such as raw gelatin, plasticizers, and purified water are mixed under vacuum conditions at a temperature of about 70 °C depending on gel formulation (Figure 2C, D). Any undissolved gel is removed by filtration.
- All compounds required for a specific formulation are added and mixed under appropriate vacuum and nitrogen blanket conditions. During the mixing process, samples are taken from a different area of the vessel to ensure homogeneity of the mixture and for viscosity control. The vessel size depends on the development of the commercial stage of a drug product; for example, gelatin melting tanks can range from small scale (e.g., 100 L) to large scale (e.g., 1200 L), while gelatin service tanks can range from 50 L (lab scale) to 300 L (large scale) [12]. These vessels are connected to a mill to reduce particle size to below 180 µm for the encapsulation process.
- The mixture is stored in the receiver containers (Figure 2F, G).
- When fill material and gel are ready, the encapsulation with soft gelatin will start (Figure 2H). Gel and fill material are connected to the encapsulation machine through heated tubing and under the nitrogen atmosphere. After encapsulation, SGCs enter a tumbler for the initial drying process. Then, they are stored in a drying tunnel for several days, depending on formulation and capsule size, to dry further. Most of the moisture is removed during this stage, and capsules’ moisture content and hardness is measured at the end of this step.
- After capsules are dry to the desired level, they are sorted and graded (Figure 2I and 2J)—grading is especially critical and important for blister production.
- Capsules are polished with a cloth soaked with isopropanol for a defined period and then with a dry cloth for the same amount of time. In the end, capsules are printed and packaged (Figure 2J).
Figure 2. Schematic representation of (A–J) different steps in the manufacturing of SGCs.
References
- Augsburger, L.L. Hard and Soft Shell Capsules. In Modern Pharmaceutics; Banker, G.S., Rhodes, C.T., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 512–575.
- Reich, G. Formulation and physical properties of soft capsules. In Pharmaceutical Capsules; Podczeck, F., Jones, B.E., Eds.; Pharmaceutical Press: London, UK, 2004; pp. 201–212.
- Hassan, E.M.; Kindt, W.W.; Gordon, R. Chewable Soft Capsule; Patheon Softgels Inc.: High Point, NC, USA, 2012.
- Schwab, E. Hot Melt-Filled Soft Capsules; Swiss Caps Rechte und Lizenzen AG: Kirchberg, Switzerland, 2010; p. 15.
- Bekerman, T.; Golenser, J.; Domb, A. Cyclosporin nanoparticulate lipospheres for oral administration. J. Pharm. Sci. 2004, 93, 1264–1270.
- Lissy, M.; Scallion, R.; Stiff, D.D.; Moore, K. Pharmacokinetic comparison of an oral diclofenac potassium liquid-filled soft gelatin capsule with a diclofenac potassium tablet. Expert Opin. Pharmacother. 2010, 11, 701–708.
- Bende, G.; Biswal, S.; Bhad, P.; Chen, Y.; Salunke, A.; Winter, S.; Wagner, R.; Sunkara, G. Relative bioavailability of diclofenac potassium from softgel capsule versus powder for oral solution and immediate-release tablet formulation. Clin. Pharm. Drug Dev. 2016, 5, 76–82.
- Fonkwe, L.G.; Archibald, D.A.; Gennadios, A. Non-Gelatin Capsule Shell Formulation in Greensboro, NC; Patheon Softgels Inc.: High Point, NC, USA, 2005.
- Jones, B.E.; Podczeck, F.; Lukas, P. Capsule shell manufacture. In Pharmaceutical Dosage Forms: Capsules; Augsburger, L.L., Hoag, S.W., Eds.; Taylor & Francis Group: London, UK, 2018; pp. 75–110.
- Bekerman, T.; Golenser, J.; Domb, A. Cyclosporin nanoparticulate lipospheres for oral administration. J. Pharm. Sci. 2004, 93, 1264–1270.
- Gullapalli, R. Soft gelatin capsules (Softgels). J. Pharm. Sci. 2010, 99, 4107–4148.
- Joysun Pharm. Softgel Making Machine. Available online: https://www.jspharma.net/category/softgel-capsule-auxiliary-equipment/