
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Urbano Diaz | + 1968 word(s) | 1968 | 2021-02-01 04:41:12 | | | |
| 2 | Nicole Yin | Meta information modification | 1968 | 2021-02-25 03:47:43 | | | | |
| 3 | Nicole Yin | Meta information modification | 1968 | 2021-02-25 03:48:06 | | |
Video Upload Options
Hybrid organic-inorganic catalysts have been extensively investigated by several research groups in the last decades, as they allow combining the structural robust-ness of inorganic solids with the versatility of organic chemistry. Within the field of hybrid catalysts, synthetic strategies based on silica are among the most exploitable, due to the convenience of sol-gel chemistry, to the array of silylderivative precursors that can be synthesized and to the number of post-synthetic functionalization strategies available, amongst others. Exemplificative studies involving mono- and multi-functional silica-based hybrid catalysts featuring different types of active sites (acid, base, redox) have been studied. Materials obtained through different approaches are described and their properties, as well as their catalytic performances, are compared in the literature.
1. Introduction
Since the development of M41S materials by the Mobil Oil Company in 1992, porous materials with large specific surface areas has been a field of extensive research, particularly with regard to potential applications in areas such as catalysis, adsorption, gas storage, chromatography, controlled drug-delivery and sensor technology. Like microporous crystalline zeolites, this class of materials feature very large specific surface areas, ordered pore systems and well-defined pore size distributions. However, unlike zeolites, M41S materials exhibit amorphous pore walls and their pore diameters is comprised between 2 and 10 nm. The most representative materials belonging to this class include silica solids MCM-41 (with a hexagonal arrangement of the mesopores), MCM-48 (with a cubic arrangement of the mesopores), and MCM-50 (with a laminar structure) (Figure 1)[1].
Not long after their discovery, researchers began attempting to incorporate organic components within an inorganic silica framework to achieve symbiosis of the properties of both components. The combination of the properties of organic and inorganic building blocks within a single material is highly attractive. In fact, this allows for the possibility of combining the enormous variety of functional groups, typical of organic chemistry, with the advantages of a thermally stable and robust inorganic substrate. This is particularly appealing for applications in heterogeneous catalysis. The symbiosis of organic and inorganic components can lead to materials with considerably different properties from those of their individual, isolated components.
Several synthetic methodologies allow to functionalize silica matrixes with specific organic functionalities such as C=C multiple bonds, alcohols, thiols, sulfonic and carboxylic acids, amines, as sol-gel chemistry provides versatile and effective ways of anchoring organic moieties to a porous solid silica support. As it will be shown below, these organic moieties (featuring acidic, basic, redox sites) can be catalytically active for a specific reaction and being selective toward the formation of one product, instead of another. Bounding catalytically active organic moieties to solid supports, opens up to the possibility of carrying out reactions under heterogeneous regime, allowing for recovery and reuse of these valuable catalyst over multiple cycles, easing product separation and purification[1][2].
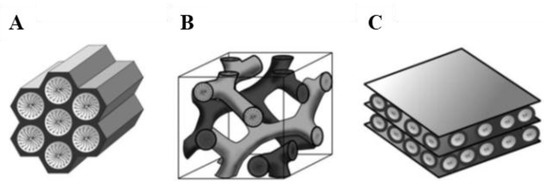
Figure 1. Structures of mesoporous M41S materials: MCM-41 (A), MCM-48 (B) and MCM-50 (C)[1]. Reprinted with permission from[1]. Copyright 2006, John Wiley and Sons.
Silica-based hybrids can be achieved by (i) post-synthetic modification of the pore surface of a purely inorganic silica material (grafting), (ii) co-condensation of a silica precursor and a silyl derivative bearing the functional group of interest (“one-pot” approach), (iii) condensation of bisilylated precursors featuring the functional group of interest in the linker between the two alkoxysilyl groups (alone or together with a minimal amount of tetra-alkoxysilyl precursor), leading to a material in which the organic functionalities are imbedded within the pore walls.
2. Post-Synthetic Modification (Grafting)
Grafting refers to the post-synthesis modification of the surfaces of mesostructured silica phases with organic groups. This process is mainly carried out by reaction of organosilanes of the type RSi(OR’)3 with the free silanol groups on the pore surfaces. Less frequently chlorosilanes ClSiR3 or silazanes HN(SiR3)2 are used (Figure 2).
In principle, by varying of the organic residue R, functionalization with different organic groups can be achieved. By implementing this approach, the mesostructure of the starting silica phase is usually retained. On the other hand, a reduction in the porosity of the hybrid, compared to the parent mesoporous silica, is usually observed, proportionally to the size of the grafted organic moieties. Furthermore, the organic silyl-derivatives might preferentially react with the surface silanol groups that they will encounter first, i.e., those located at the pore mouth or at the initial part of the mesoporous channels. This effect is modulated by several factor. Firstly, the reactivity of the alkoxy groups bounded to the silicon atom determines the ease with which hydrolysis/condensation reactions can occur.

Figure 2. Grafting of a generic silyl-derivative RSi(OR’)3 onto a mesoporous silica pore walls[1]. Reprinted with permission from[1]. Copyright 2006, John Wiley and Sons.
The diffusion of the silyl-derivative compounds through the mesopores also play a crucial role and is primarily determined by its size, relative to the pores diameter, and by the chemical affinity between the organic moieties and surface silanol groups. Lastly, while in an initial stage of the grafting, silyl-derivatives are allowed to diffuse through the pore channels before the catalyst (acid or base) is added into the mixture, molecules baring acidic or base functionalities can catalyze their own hydrolysis and condensation before reaching optimal diffusion, leading to a worse distribution of organic moieties throughout the porous framework. A key advantage, unique to this method, is the fact that the templating agent used for generating the mesopores is entirely removed by calcination, prior to the grafting procedure. The grafting approach allows for introducing organic functionalities onto a pre-existing mesoporous silica framework and for that, in these hybrids, the organic moieties are expected to be pending from the pore walls, partially occupying the mesoporous channels. Grafting procedures are also employed to modify the polarity of the support’s surface. In fact, silanol-capping hydrophobic moieties, e.g., trimethyl-silyl species, can make the pore walls more hydrophobic, with beneficial effect on the properties of the hybrid materials, for certain applications.
3. Co-condensation Method (“one-pot”)
An alternative method to synthesize ordered hybrid organic-inorganic mesoporous silica phases is the co-condensation method. It is possible to prepare mesostructured silica phases by the co-condensation of tetra-alkoxysilanes, typically tetraethyl ortosilicate (TEOS) or tetramethyl ortosilicate (TMOS), together with trialkoxyorganosilanes of the type RSi(OR’)3 in the presence of structure-directing agents, obtaining materials with organic residues pending covalently from the pore walls (Figure 3).
By using structure-directing agents that are typically implemented in the synthesis of pure mesoporous silica phases (e.g., MCM or SBA silica phases), organo-functionalized silica hybrids can be prepared in such a way that the organic linkers, connecting the functional group of interest to the inorganic support, are pending directly from the pore walls, with the silicon atoms deriving from hydrolysis/condensation of the alkoxy-silyl groups being imbedded into the silica pore walls. Since the organic functionalities are direct components of the silica matrix, pore blocking is not a problem in the co-condensation method, although a slight decrease in the pore diameter can be detected. Moreover, the organic units are generally more homogeneously distributed than in hybrid mesoporous materials obtained by grafting process.
Nevertheless, the co-condensation method presents also a number of disadvantages: the degree of mesoscopic order of the resulting materials tend to decreases with increasing concentration of RSi(OR’)3 in the synthesis mixture. Consequently, the relative amount of organic moieties in the modified silica phases does not normally exceed 40 mol%. Furthermore, the yield with which the organic groups can be incorporated into the pore-wall network is generally not 100% and it decreases proportionally to the amount of organic silyl-derivative in the synthesis mixture. These observations can be explained by the fact that an increasing proportion of RSi(OR’)3 in the reaction mixture promotes homocondensation reactions, favoring the formation of oligomers. These phenomena are caused by the different hydrolysis and condensation rates of the different silyl-type precursors. It is a potential problem whenever this synthetic strategy is implemented, as the homogeneous distribution of the organic functionalities in the framework cannot be guaranteed. Moreover, exceeding in the loading of organic groups can lead to a reduction in the pore diameter, pore volume, and specific surface areas. From a purely methodological point of view, a higher degree of care needs to be taken in the removal of the surfactant, used to generate the mesopores. Calcination cannot be implemented as it would compromise the integrity of the organic groups Si-R. Consequently, template removal is commonly done by continuous extraction, carried out using a soxhlet apparatus. Although fairly effective, an incomplete removal of the template is usually observed.
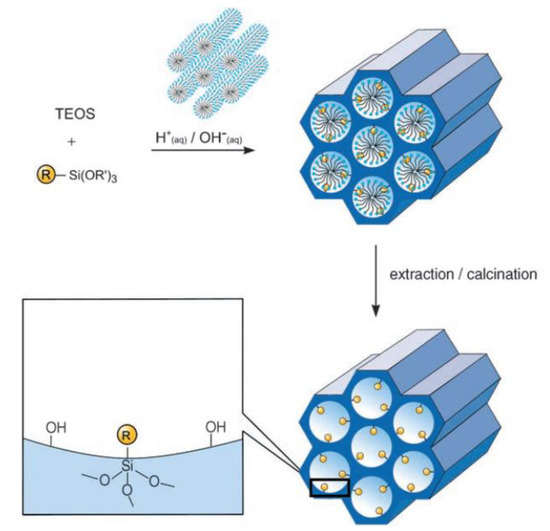
Figure 3. Co-condensation between tetraethyl ortosilicate (TEOS) and a generic silyl-derivative compound, RSi(OR’)3, followed by calcination/extraction to achieve hybrid organic-inorganic mesoporous silica[1]. Reprinted with permission from[1]. Copyright 2006, John Wiley and Sons.
An additional tool to incorporate specific organic functionalities within silica-based materials are tethering procedures. For that, firstly, mono-silanes RSi(OR`)3 are incorporated into a silica support (either by co-condensation or grafting). Afterwards, in a post-synthetic step, an organocatalyst featuring the active site of interest and a specific functional group R* is reacted with the hybrid material. R and R* need to be chosen properly, so that they can react and form a covalent bond that can guarantee the anchoring of the organocatalyst. Example of such R/R* pairs include primary amine and isocyanate, reacting to form urea group; thiol and terminal alkene, reacting in presence of a radical initiator to form a thioether; a halide (Br or I) functioning as good leaving group in a SN2 reaction and a strong nucleophile.
4. Periodic Mesoporous Organosilicas (PMOs)
PMOs are a class of silica-based organic–inorganic hybrid materials obtained by hydrolysis and condensation reactions of bridged silsesquioxane precursors of general formula (R’O)3Si-R-Si(OR’)3, used as the only silicon source[3][4]. In contrast with the two methods previously reported (grafting and direct synthesis), in this case, the organic building blocks bear two alkoxy-silyl groups that can largely improve their incorporation in the three-dimensional network as well as their homogeneous distribution throughout the solid (Figure 4A). Moreover, a major difference between PMOs and silica-based hybrids obtained by co-condensation and grafting is that in the former, active sites are imbedded into the pore walls, rather than pending from the surface of the support. As in the case of the synthesis of pure silica mesophases, the use of a surfactant in the presence of bi-silylated organosilica precursors allows obtaining materials featuring ordered mesopores, narrow pore size distribution and in which the organic bridges are integral components of the silica network[1].
Although presenting several advantages over grafting and co-condensation, the synthesis of PMOs also bring about challenging aspects, the most notable being the synthesis of the suitable bi-silylated precursors. In fact, alkoxy-silyl groups are highly reactive species and synthesizing complex molecules baring two of such groups and a specific organic bridged group R, is often a challenging task.
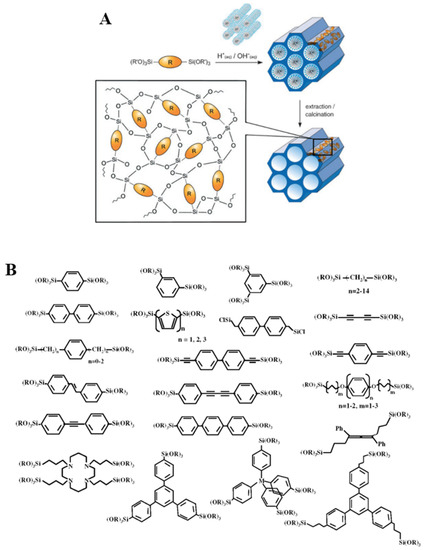
Figure 4. A: General synthetic pathway to PMOs[1]. Reprinted with permission from[1]. Copyright 2006, John Wiley and Sons. B: Representative bridged silsesquioxanes[5]. Reprinted with permission from[5]. Copyright 2008, American Chemical Society.
Nevertheless, bridged polysilsesquioxanes featuring a wide range of bridging groups have been prepared. In 1999, three research groups independently reported on mesoporous organosilica hybrids from bisilylated precursors baring methyl or ethyl groups as organic bridges[3][4][6]. After that, several PMOs were prepared featuring ethylene, ethenylene, phenylene and thiophene as R groups. Rigid unsaturated precursors able to form weak interaction, such as p-p can self-assemble in the synthesis mixture. When hydrolysis/condensation take place, this order can be preserved and layered “crystal-like” structural sub-domains can be included in the final PMO-type materials. The first materials featuring 1,4-phenylene bridges was prepared in 2002. Upon varying the size of the R bridged unit, materials featuring layers of different width were obtained (5.6–11.9 Å)[7]. Building upon these early studies, a variety of organic groups, ranging from hydrocarbons and heteroaromatics to metal complexes, have been successfully incorporated into PMO hybrids (Figure 4B)[1][5][8].
References
- Hybrid Organic-Inorganic Materials;organic–inorganic hybrid composites;porous-materials;catalysis;silica-based materials;sol-gel synthesis;post-synthetic functionalization
- format change
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic Mesoporous Organosilicas with Organic Groups inside the Channel Walls. Nature 1999, 402, 867–871.
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel Mesoporous Materials with a Uniform Distribution of Organic Groups and Inorganic Oxide in Their Frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614.
- Satoru Fujita; Shinji Inagaki; Self-Organization of Organosilica Solids with Molecular-Scale and Mesoscale Periodicities†. Chemistry of Materials 2008, 20, 891-908, 10.1021/cm702271v.
- Brian J. Melde; Brian T. Holland; And Christopher F. Blanford; Andreas Stein; Mesoporous Sieves with Unified Hybrid Inorganic/Organic Frameworks. Chemistry of Materials 1999, 11, 3302-3308, 10.1021/cm9903935.
- Shinji Inagaki; Shiyou Guan; Tetsu Ohsuna; Osamu Terasaki; An ordered mesoporous organosilica hybrid material with a crystal-like wall structure. Nature 2002, 416, 304-307, 10.1038/416304a.
- Norihiro Mizoshita; Takao Tani; Shinji Inagaki; Syntheses, properties and applications of periodic mesoporous organosilicas prepared from bridged organosilane precursors. Chemical Society Reviews 2011, 40, 789-800, 10.1039/c0cs00010h.




