
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Crystal Ghantous | + 3606 word(s) | 3606 | 2020-12-08 06:58:47 | | | |
| 2 | Dean Liu | -2123 word(s) | 1483 | 2021-02-24 10:31:42 | | |
Video Upload Options
This review summarizes some of the promising early stage protein and miRNA biomarker candidates as well as the currently used biomarkers for hypertension and other cardiovascular diseases.
1. Introduction
1. 1. Hypertension and Vascular Smooth Muscle Cell Remodeling
VSMCs reside in the tunica media, the middle layer of blood vessels and the thickest layer in arteries. They contract and relax in response to different stimuli in order to regulate blood flow to the tissues that the vessels irrigate. In essential hypertension, small resistance arteries undergo vascular remodeling and become characterized by an increased wall thickness to lumen ratio and a narrower lumen[1][2].
Several molecular mechanisms mediate hypertension-induced vascular remodeling. The force of mechanical stretch exerted by hypertension on the vascular wall promotes the production of reactive oxygen species (ROS)[3], which in turn induce VSMC remodeling[4][5]. The excessive force of stretch mediated by hypertension also causes alterations in the extracellular matrix, activating the RhoA pathway, which in turn promotes actin cytoskeleton remodeling in VSMCs[3]; the hypertension-induced activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and protein kinase B (AKT) also results in vascular remodeling [6][7]. Moreover, caveolae, which are lipid raft invaginations in the plasma membrane, mediate hypertension-induced VSMC modeling via endothelial nitric oxide synthase (eNOS) and endothelin receptor type A (ETA)[8][9][10]. Studies have also shown that angiotensin II type 1 receptor (AT1), platelet-derived growth factor receptor (PDGF-R), and specific ion channels, like voltage-gated calcium channels, are implicated in hypertension-induced VSMC remodeling[6][11][12][13][14] (Figure 1).
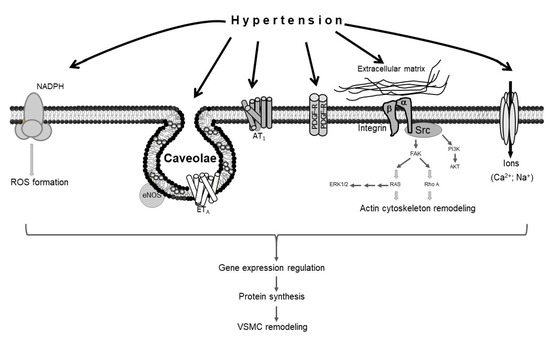
Figure 1. Schematic representation of vascular smooth muscle cell (VSMC) remodeling in response to hypertension. Hypertension stimulates different sensors in the plasma membrane of VSMCs, activating several signaling pathways that lead to VSMC remodeling.
1.2. Hypertension and Endothelial Dysfunction
Endothelial cells are located in the tunica intima layer of blood vessels and form the luminal surface. Blood pressure exerts two types of forces on the endothelial cells: outward mechanical stretch and shear stress. When blood pressure is low, endothelial cells secrete a number of vasoactive molecules, like angiotensin II, endothelin-1, ROS, and prostanoids, which act on VSMCs to promote VSMC contraction and subsequent vasoconstriction[15][16]. In contrast, when blood pressure rises, vasodilator substances like nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factor are produced by endothelial cells[17][18].
The forces exerted by hypertension cause endothelial damage and dysfunction, resulting in reduced production of NO[19][20]. Consequently, blood pressure-induced vasodilation is compromised. Moreover, hypertension-mediated endothelial dysfunction promotes the development of atherosclerosis.
Atherosclerosis is associated with the build-up of an atheromatous plaque, which is mainly composed of oxidized low-density lipoprotein (LDL) and macrophages inside the walls of arteries. It is a risk factor for coronary artery disease, myocardial infarction (MI), hypertension, stroke, and peripheral artery disease[21][22][23][24]. Arterial calcification is associated with atheroma progression and alters the mechanical properties of the vascular wall, thereby increasing the risk of rupture of the atherosclerotic plaque[25]. Discovering distinctive biomarkers that indicate early atherosclerosis development may allow the early detection of atherosclerosis, which in turn would encourage the patient to make healthy lifestyle changes or begin treatment in order to prevent its progression.
2. Biomarkers Reflecting Hypertension Pathogenesis
Since hypertension promotes the development of other CVDs, identifying antecedent, screening, and early stage diagnostic biomarkers is crucial in preventing hypertension-associated CVDs. Biomarkers of hypertension include those that indicate oxidative stress and inflammation since hypertension is associated with these states. Interestingly, adipokines have also emerged as potential biomarkers of hypertension. The following section describes these biomarkers in detail.
2.1. Biomarkers Reflecting Oxidative Stress
Hypertension is highly associated with oxidative stress, which in turn mediates hypertension-induced cardiovascular complications[3][5]. Several molecules have been shown to reflect the oxidative state. For instance, measurements of nitrite (NO2-) and nitrate (NO3-) can be used because they are markers of NO bioavailability. NO2- and NO3- are products of oxidative degradation of NO, which physiologically causes vasodilation and prevents hypertension [26][27] (Table 4). Their increased levels indicate a reduction in NO bioavailability and are thus associated with hypertension.
Table 4. Molecules used as biomarkers of hypertension.
| Molecule | Function/Description | Levels in Hypertension | Reference(s) |
|---|---|---|---|
| Nitrate and nitrite | Physiological reservoir of NO that can be reduced to NO to regulate signal transduction | Elevated | [27][28] |
| Asymmetric dimethylarginine (ADMA) | Inhibits nitric oxide synthase | Elevated | [29] |
| Reactive oxygen species (ROS) | Highly reactive signal transduction molecules that cause nucleic acid, lipid, and protein damage when present in high concentrations (oxidative stress) | Elevated | [30][31] |
| Uric acid | Final oxidation product of purine metabolism | Elevated | [32][33] |
Asymmetric dimethylarginine (ADMA) and uric acid are other biomarkers for hypertension (Table 4). They inhibit the production of NO[34][35], so their increased levels are associated with reduced NO bioavailability and impaired vasodilation[29][36][32]. Moreover, ADMA levels are correlated with acute coronary events and can also be used as a biomarker for adverse cardiac outcomes[37]. ROS are another indicator of hypertension, since hypertension has been shown to directly increase ROS[3][30] (Summarized in Table 4). Although the aforementioned factors are widely used for the diagnosis of hypertension, there is an increasing demand to introduce more biological markers such as proteins and genes to improve the prediction of this condition. According to the Rat Genome Database, hundreds of genes are associated with hypertension (Rat: https://rgd.mcw.edu/rgdweb/elasticResults.html?term=hypertension&chr=ALL&start=&stop=&species=Rat&category=Gene&objectSearch=true;human: https://rgd.mcw.edu/rgdweb/elasticResults.html?term=hypertension&chr=ALL&start=&stop=&species=Human&category=Gene&objectSearch=true).
2.2. Protein Biomarkers Reflecting Inflammation
Since hypertension is associated with vascular inflammation[38], markers of inflammation can be used as biomarkers for hypertension. The cell adhesion molecules vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM), and platelet endothelial cell adhesion molecule (PECAM) allow inflammatory cells to adhere to the vascular wall[39][40][41]. High plasma levels of these molecules have been shown to be associated with hypertension[40][41][42].
Hypertensive patients have higher circulating levels of the inflammatory cytokines IL-1β, IL-10, and tumor necrosis factor-alpha (TNF-α), indicating the potential use of these inflammatory biomarkers as markers for hypertension [43]. Elevated levels of IL-1β, IL-10, and TNF-α are also correlated with increased arterial stiffness associated with hypertension[43]. Moreover, IL-6 and TNF-α levels can be used as independent risk factors for hypertension in healthy individuals[44] (Summarized in Table 5).
Table 5. Inflammatory biomarkers of hypertension.
| Inflammatory Mediators | Function/Description | Levels in Hypertension | Reference(s) |
|---|---|---|---|
| Vascular cell adhesion molecule (VCAM) | Endothelial cell surface glycoprotein that allows endothelial cell-leukocyte adhesion in inflammation | Elevated | [45] |
| Intercellular adhesion molecule (ICAM) | Endothelial cell surface glycoprotein that aids in endothelial cell-leukocyte adhesion | Elevated | [46] |
| Platelet endothelial cell adhesion molecule (PECAM) | Cell surface protein of platelets, monocytes, neutrophils, subsets of T cells that aids in leukocyte transendothelial migration, and a constituent of the endothelial intercellular junctions | Elevated | [40] |
| 6-keto-prostaglandin F1a | Stable and active metabolite of prostacyclin that promotes vasodilation and inhibits platelet aggregation | Reduced | [47] |
| C-reactive protein (CRP) | Activates complement and binds to foreign and damaged cells and tissue | Elevated | [48] |
| Tumor necrosis factor (TNF-α) | Pro-inflammatory cytokine involved in apoptosis, cell proliferation, differentiation, and platelet activation | Elevated | [44][49] |
| IL-10, IL-1β | IL-10: Cytokine involved in mediating the inflammatory response, B cell survival, proliferation and antibody production, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activity IL-1β: Cytokine involved in regulating the inflammatory response, cell proliferation, differentiation, apoptosis, and cyclooxygenase-2 induction |
Elevated | [43] |
| IL-6 | Immune response in inflammation | Elevated | [44] |
| P-selectin | Cell adhesion molecule of platelets and endothelial cells that works in the interaction of leukocytes with platelets or endothelial cells | Elevated | [50] |
| Oxidized-LDL | Taken up by macrophages to form foam cells, a key step in atherosclerosis development | Elevated | [47] |
| Renin and prorenin | Renin hydrolyzes angiotensinogen to angiotensin I, while prorenin is its inactive precursor | Elevated | [51] |
| Leptin | - Hormone mainly produced by adipocytes that acts as a satiety factor to increase energy expenditure by signaling at the hypothalamus - Promotes VSMC hypertrophy - Pro-inflammatory cytokine - Regulates puberty, menstrual cycles, and reproductive function |
Elevated | [52][53] |
| Adiponectin | - Insulin-sensitization and fatty acid oxidation - Anti-inflammatory - Cardioprotective |
Reduced | [54] |
2.3. Adipokines as Biomarkers of Hypertension
We and others have shown the significant association between the hormone leptin and hypertension [3][52][53][54][55][56][57]. Leptin is an obesity-associated adipokine that physiologically reduces appetite and increases energy expenditure. Research has shown that hypertensive patients have higher circulating leptin levels[58] and that leptin can be used as a predictor of new-onset hypertension[52]. Moreover, a recent biomedical and proteomics study conducted in our lab has shown that leptin is produced by VSMCs and that its synthesis is upregulated by hypertension (unpublished data and[3]). In turn, leptin contributes to VSMC hypertrophy and promotes atherosclerosis[3][59] (Summarized in Table 5).
Adiponectin is another adipokine that is emerging as a biomarker for hypertension[60](Table 5). This anti-inflammatory protein has been shown to exert cardioprotective effects on the heart by inhibiting pressure overload-induced cardiac hypertrophy and protecting against myocardial injury after ischemia-reperfusion[61][62][63]. Studies have shown that circulating adiponectin levels are reduced in hypertensive patients[54], which may explain the detrimental effects of hypertension on the cardiovascular system. In addition, we have recently shown that adiponectin is not only expressed by adipocytes, but also VSMCs, and that adiponectin supplementation reduces hypertension-induced VSMC hypertrophy[4][64].
References
- Aalkjaer, C.; Heagerty, A.M.; Petersen, K.K.; Swales, J.D.; Mulvany, M.J. Evidence for increased media thickness, increased neuronal amine uptake, and depressed excitation—Contraction coupling in isolated resistance vessels from essential hypertensives. Circ. Res. 1987, 61, 181–186.
- Arribas, S.M.; Hillier, C.; Gonzalez, C.; McGrory, S.; Dominiczak, A.F.; McGrath, J.C. Cellular aspects of vascular remodeling in hypertension revealed by confocal microscopy. Hypertension 1997, 30, 1455–1464.
- Ghantous, C.M.; Kobeissy, F.H.; Soudani, N.; Rahman, F.A.; Al-Hariri, M.; Itani, H.A.; Sabra, R.; Zeidan, A. Mechanical stretch-induced vascular hypertrophy occurs through modulation of leptin synthesis-mediated ros formation and gata-4 nuclear translocation. Front. Pharm. 2015, 6, 240.
- Nour-Eldine, W.; Ghantous, C.M.; Zibara, K.; Dib, L.; Issaa, H.; Itani, H.A.; El-Zein, N.; Zeidan, A. Adiponectin attenuates angiotensin ii-induced vascular smooth muscle cell remodeling through nitric oxide and the rhoa/rock pathway. Front. Pharm. 2016, 7, 86.
- Zhou, Y.; Zhang, M.J.; Li, B.H.; Chen, L.; Pi, Y.; Yin, Y.W.; Long, C.Y.; Wang, X.; Sun, M.J.; Chen, X.; et al. Ppargamma inhibits vsmc proliferation and migration via attenuating oxidative stress through upregulating ucp2. PLoS ONE 2016, 11, e0154720.
- Yoshizumi, M.; Kyotani, Y.; Zhao, J.; Nakahira, K. Targeting the mitogen-activated protein kinase-mediated vascular smooth muscle cell remodeling by angiotensin ii. Ann. Transl. Med. 2020, 8, 157.
- Liu, P.; Gu, Y.; Luo, J.; Ye, P.; Zheng, Y.; Yu, W.; Chen, S. Inhibition of src activation reverses pulmonary vascular remodeling in experimental pulmonary arterial hypertension via akt/mtor/hif-1
signaling pathway. Exp. Cell Res. 2019, 380, 36–46. - Forrester, S.J.; Elliott, K.J.; Kawai, T.; Obama, T.; Boyer, M.J.; Preston, K.J.; Yan, Z.; Eguchi, S.; Rizzo, V. Caveolin-1 deletion prevents hypertensive vascular remodeling induced by angiotensin ii. Hypertension 2017, 69, 79–86.
- Lian, X.; Matthaeus, C.; Kassmann, M.; Daumke, O.; Gollasch, M. Pathophysiological role of caveolae in hypertension. Front. Med. 2019, 6, 153.
- Titus, A.; Marappa-Ganeshan, R. Physiology, Endothelin; Statpearls: Treasure Island, FL, USA, 2020.
- Hinoki, A.; Kimura, K.; Higuchi, S.; Eguchi, K.; Takaguri, A.; Ishimaru, K.; Frank, G.D.; Gerthoffer, W.T.; Sommerville, L.J.; Autieri, M.V.; et al. P21-activated kinase 1 participates in vascular remodeling in vitro and in vivo. Hypertension 2010, 55, 161–165.
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The role of vascular smooth muscle cells in arterial remodeling: Focus on calcification-related processes. Int. J. Mol. Sci. 2019, 20, 5694.
- Brown, I.A.M.; Diederich, L.; Good, M.E.; DeLalio, L.J.; Murphy, S.A.; Cortese-Krott, M.M.; Hall, J.L.; Le, T.H.; Isakson, B.E. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arter. Thromb. Vasc. Biol. 2018, 38, 1969–1985.
- Cheng, J.; Wen, J.; Wang, N.; Wang, C.; Xu, Q.; Yang, Y. Ion channels and vascular diseases. Arter. Thromb. Vasc. Biol. 2019, 39, e146–e156.
- Vanhoutte, P.M.; Tang, E.H. Endothelium-dependent contractions: When a good guy turns bad! J. Physiol. 2008, 586, 5295–5304.
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br. J. Pharmacol. 2009, 157, 527–536.
- Feletou, M.; Vanhoutte, P.M. Endothelial dysfunction: A multifaceted disorder (the wiggers award lecture). Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H985–H1002.
- Jia, G.; Durante, W.; Sowers, J.R. Endothelium-derived hyperpolarizing factors: A potential therapeutic target for vascular dysfunction in obesity and insulin resistance. Diabetes 2016, 65, 2118–2120.
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834.
- Taddei, S.; Ghiadoni, L.; Virdis, A.; Buralli, S.; Salvetti, A. Vasodilation to bradykinin is mediated by an ouabain-sensitive pathway as a compensatory mechanism for impaired nitric oxide availability in essential hypertensive patients. Circulation 1999, 100, 1400–1405.
- Garcia de Tena, J. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 353, 429–430; author reply 429–430.
- Li, J.J.; Chen, J.L. Inflammation may be a bridge connecting hypertension and atherosclerosis. Med. Hypotheses 2005, 64, 925–929.
- Elkind, M.S. Inflammation, atherosclerosis, and stroke. Neurologist 2006, 12, 140–148.
- Olin, J.W.; Sealove, B.A. Peripheral artery disease: Current insight into the disease and its diagnosis and management. Mayo Clin. Proc. 2010, 85, 678–692.
- Berliner, J.A.; Navab, M.; Fogelman, A.M.; Frank, J.S.; Demer, L.L.; Edwards, P.A.; Watson, A.D.; Lusis, A.J. Atherosclerosis: Basic mechanisms. Oxidation, inflammation, and genetics. Circulation 1995, 91, 2488–2496.
- Nagababu, E.; Rifkind, J.M. Measurement of plasma nitrite by chemiluminescence. Methods Mol. Biol. 2010, 610, 41–49.
- Casey, D.P.; Beck, D.T.; Braith, R.W. Systemic plasma levels of nitrite/nitrate (nox) reflect brachial flow-mediated dilation responses in young men and women. Clin. Exp. Pharm. Physiol. 2007, 34, 1291–1293.
- Rassaf, T.; Heiss, C.; Hendgen-Cotta, U.; Balzer, J.; Matern, S.; Kleinbongard, P.; Lee, A.; Lauer, T.; Kelm, M. Plasma nitrite reserve and endothelial function in the human forearm circulation. Free Radic. Biol. Med. 2006, 41, 295–301.
- Paiva, H.; Kahonen, M.; Lehtimaki, T.; Alfthan, G.; Viikari, J.; Laaksonen, R.; Hutri-Kahonen, N.; Laitinen, T.; Taittonen, L.; Raitakari, O.T.; et al. Levels of asymmetrical dimethylarginine are predictive of brachial artery flow-mediated dilation 6 years later. The cardiovascular risk in young finns study. Atherosclerosis 2010, 212, 512–515.
- Montezano, A.C.; Touyz, R.M. Molecular mechanisms of hypertension--reactive oxygen species and antioxidants: A basic science update for the clinician. Can. J. Cardiol. 2012, 28, 288–295.
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2011, 34, 5–14.
- Loeffler, L.F.; Navas-Acien, A.; Brady, T.M.; Miller, E.R., 3rd; Fadrowski, J.J. Uric acid level and elevated blood pressure in us adolescents: National health and nutrition examination survey, 1999–2006. Hypertension 2012, 59, 811–817.
- Johnson, R.J.; Kang, D.H.; Feig, D.; Kivlighn, S.; Kanellis, J.; Watanabe, S.; Tuttle, K.R.; Rodriguez-Iturbe, B.; Herrera-Acosta, J.; Mazzali, M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003, 41, 1183–1190.
- Palm, F.; Onozato, M.L.; Luo, Z.; Wilcox, C.S. Dimethylarginine dimethylaminohydrolase (ddah): Expression, regulation, and function in the cardiovascular and renal systems. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3227–H3245.
- Xiao, S.; Wagner, L.; Mahaney, J.; Baylis, C. Uremic levels of urea inhibit l-arginine transport in cultured endothelial cells. Am. J. Physiol. Ren. Physiol. 2001, 280, F989–F995.
- Juonala, M.; Viikari, J.S.; Alfthan, G.; Marniemi, J.; Kahonen, M.; Taittonen, L.; Laitinen, T.; Raitakari, O.T. Brachial artery flow-mediated dilation and asymmetrical dimethylarginine in the cardiovascular risk in young finns study. Circulation 2007, 116, 1367–1373.
- Valkonen, V.P.; Paiva, H.; Salonen, J.T.; Lakka, T.A.; Lehtimaki, T.; Laakso, J.; Laaksonen, R. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet 2001, 358, 2127–2128.
- Harrison, D.G.; Guzik, T.J.; Lob, H.E.; Madhur, M.S.; Marvar, P.J.; Thabet, S.R.; Vinh, A.; Weyand, C.M. Inflammation, immunity, and hypertension. Hypertension 2011, 57, 132–140.
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552.
- Rodrigues, S.F.; de Oliveira, M.A.; dos Santos, R.A.; Soares, A.G.; de Cassia Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Hydralazine reduces leukocyte migration through different mechanisms in spontaneously hypertensive and normotensive rats. Eur. J. Pharm. 2008, 589, 206–214.
- De Ciuceis, C.; Amiri, F.; Brassard, P.; Endemann, D.H.; Touyz, R.M.; Schiffrin, E.L. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin ii-infused macrophage colony-stimulating factor-deficient mice: Evidence for a role in inflammation in angiotensin-induced vascular injury. Arter. Thromb. Vasc. Biol. 2005, 25, 2106–2113.
- Preston, R.A.; Ledford, M.; Materson, B.J.; Baltodano, N.M.; Memon, A.; Alonso, A. Effects of severe, uncontrolled hypertension on endothelial activation: Soluble vascular cell adhesion molecule-1, soluble intercellular adhesion molecule-1 and von willebrand factor. J. Hypertens. 2002, 20, 871–877.
- Barbaro, N.R.; Fontana, V.; Modolo, R.; De Faria, A.P.; Sabbatini, A.R.; Fonseca, F.H.; Anhe, G.F.; Moreno, H. Increased arterial stiffness in resistant hypertension is associated with inflammatory biomarkers. Blood Press 2015, 24, 7–13.
- Bautista, L.E.; Vera, L.M.; Arenas, I.A.; Gamarra, G. Independent association between inflammatory markers (c-reactive protein, interleukin-6, and tnf-alpha) and essential hypertension. J. Hum. Hypertens. 2005, 19, 149–154.
- Shalia, K.K.; Mashru, M.R.; Vasvani, J.B.; Mokal, R.A.; Mithbawkar, S.M.; Thakur, P.K. Circulating levels of cell adhesion molecules in hypertension. Indian J. Clin. Biochem. 2009, 24, 388–397.
- Oguz, M.M.; Oguz, A.D.; Sanli, C.; Cevik, A. Serum levels of soluble icam-1 in children with pulmonary artery hypertension. Tex. Heart Inst. J. 2014, 41, 159–164.
- Kuklinska, A.M.; Mroczko, B.; Musial, W.J.; Usowicz-Szarynska, M.; Sawicki, R.; Borowska, H.; Knapp, M.; Szmitkowski, M. Diagnostic biomarkers of essential arterial hypertension: The value of prostacyclin, nitric oxide, oxidized-ldl, and peroxide measurements. Int. Heart J. 2009, 50, 341–351.
- Hage, F.G. C-reactive protein and hypertension. J. Hum. Hypertens. 2014, 28, 410–415.
- Mehaffey, E.; Majid, D.S.A. Tumor necrosis factor-alpha, kidney function, and hypertension. Am. J. Physiol. Ren. Physiol. 2017, 313, F1005–F1008.
- Yang, P.; Liu, Y.F.; Yang, L.; Wei, Q.; Zeng, H. Mechanism and clinical significance of the prothrombotic state in patients with essential hypertension. Clin. Cardiol. 2010, 33, E81–E86.
- Jan Danser, A.H. Renin and prorenin as biomarkers in hypertension. Curr. Opin. Nephrol. Hypertens. 2012, 21, 508–514.
- Asferg, C.; Mogelvang, R.; Flyvbjerg, A.; Frystyk, J.; Jensen, J.S.; Marott, J.L.; Appleyard, M.; Jensen, G.B.; Jeppesen, J. Leptin, not adiponectin, predicts hypertension in the copenhagen city heart study. Am J Hypertens. 2010, 23, 327–333.
- Shankar, A.; Xiao, J. Positive relationship between plasma leptin level and hypertension. Hypertension 2010, 56, 623–628.
- Kim, D.H.; Kim, C.; Ding, E.L.; Townsend, M.K.; Lipsitz, L.A. Adiponectin levels and the risk of hypertension: A systematic review and meta-analysis. Hypertension 2013, 62, 27–32.
- Ghantous, C.M.; Azrak, Z.; Hanache, S.; Abou-Kheir, W.; Zeidan, A. Differential role of leptin and adiponectin in cardiovascular system. Int. J. Endocrinol. 2015, 2015, 534320.
- Kaisar, O.M.; Johnson, D.W.; Prins, J.B.; Isbel, N. The role of novel biomarkers of cardiovascular disease in chronic kidney disease: Focus on adiponectin and leptin. Curr. Cardiol. Rev. 2008, 4, 287–292.
- Soudani, N.; Ghantous, C.M.; Farhat, Z.; Shebaby, W.N.; Zibara, K.; Zeidan, A. Calcineurin/nfat activation-dependence of leptin synthesis and vascular growth in response to mechanical stretch. Front. Physiol. 2016, 7, 433.
- de Haro Moraes, C.; Figueiredo, V.N.; de Faria, A.P.; Barbaro, N.R.; Sabbatini, A.R.; Quinaglia, T.; Ferreira-Melo, S.E.; Martins, L.C.; Demacq, C.; Junior, H.M. High-circulating leptin levels are associated with increased blood pressure in uncontrolled resistant hypertension. J. Hum. Hypertens. 2013, 27, 225–230.
- Schafer, K.; Halle, M.; Goeschen, C.; Dellas, C.; Pynn, M.; Loskutoff, D.J.; Konstantinides, S. Leptin promotes vascular remodeling and neointimal growth in mice. Arter. Thromb. Vasc. Biol. 2004, 24, 112–117.
- Ebrahimi-Mamaeghani, M.; Mohammadi, S.; Arefhosseini, S.R.; Fallah, P.; Bazi, Z. Adiponectin as a potential biomarker of vascular disease. Vasc. Health Risk Manag. 2015, 11, 55–70.
- Shibata, R.; Sato, K.; Pimentel, D.R.; Takemura, Y.; Kihara, S.; Ohashi, K.; Funahashi, T.; Ouchi, N.; Walsh, K. Adiponectin protects against myocardial ischemia-reperfusion injury through ampk- and cox-2-dependent mechanisms. Nat. Med. 2005, 11, 1096–1103.
- Shibata, R.; Ouchi, N.; Ito, M.; Kihara, S.; Shiojima, I.; Pimentel, D.R.; Kumada, M.; Sato, K.; Schiekofer, S.; Ohashi, K.; et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 2004, 10, 1384–1389.
- Gonon, A.T.; Widegren, U.; Bulhak, A.; Salehzadeh, F.; Persson, J.; Sjoquist, P.O.; Pernow, J. Adiponectin protects against myocardial ischaemia-reperfusion injury via amp-activated protein kinase, akt, and nitric oxide. Cardiovasc. Res. 2008, 78, 116–122.
- Ghantous, C.M.; Farhat, R.; Djouhri, L.; Alashmar, S.; Anlar, G.; Korashy, H.M.; Agouni, A.; Zeidan, A. Molecular mechanisms of adiponectin-induced attenuation of mechanical stretch-mediated vascular remodeling. Oxidative Med. Cell. Longev. 2020, 2020, 6425782.




