
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ashley Putman | + 2657 word(s) | 2657 | 2021-02-15 07:36:19 | | | |
| 2 | Catherine Yang | -11 word(s) | 2646 | 2021-02-23 04:49:10 | | |
Video Upload Options
Oxidative stress has been associated with many pathologies, in both human and animal medicine. Damage to tissue components such as lipids is a defining feature of oxidative stress and can lead to the generation of many oxidized products, including isoprostanes (IsoP).
1. Introduction
Oxidative stress, the imbalance between oxidants and antioxidants leading to tissue damage, has been associated with many diseases in both humans and animals [1]. Oxidants are chemically reactive molecules that are responsible for the tissue damage associated with oxidative stress and are composed of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Produced in moderate amounts as a byproduct of energy generation, oxidants are used in numerous cell signaling and immune pathways under physiologic conditions [2]. Antioxidants are designed to counter oxidants and maintain homeostasis, contributing to what is termed redox balance. However, a disruption in the balance that favors excessive oxidant accumulation leads to oxidative stress [2]. Examples of human diseases with an oxidative stress-related component in their pathophysiology include Alzheimer’s disease, type II diabetes, and heart failure [3][4][5]. In veterinary species, oxidative stress has been associated with similar disorders such as canine counterpart of senile dementia of the Alzheimer type, metabolic stress in dairy cattle, and congestive heart failure in dogs [6][7][8]. Despite the evidence supporting oxidative stress as an important contributor to numerous pathologies, no clinical signs of the process are displayed [9]. Furthermore, it can be challenging to distinguish between redox balance and unchecked oxidants, thereby making it necessary to use specific measurements to determine if oxidative stress is occurring. In fact, the need for reliable oxidative stress biomarkers and an understanding of any physiologic roles they may play in disease pathogenesis has become a priority in the research community.
Broadly, a biomarker can be described as “a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes or responses to an exposure or intervention” [10]. Several biomarkers of oxidative stress can be identified due to the tissue damage that occurs as a function of increased interactions between ROS and biological molecules. Common biomarkers generated from the reactions between ROS and nucleic acids, proteins, and lipids include 8-hydroxydeoxyguanosine, protein carbonyls, and isoprostanes (IsoP), respectively [11][12]. However, the most favorable biomarkers must be more than merely measurable; they should also show specificity for a particular process, have prognostic value, or correlate with pathology [13].
Since their discovery in the early 1990s, IsoP have become one of the most widely used biomarkers of in vivo oxidative stress because they are highly sensitive and specific, can be measured noninvasively from numerous biological tissues and fluids, and are chemically stable [14][15]. In addition to IsoP themselves, IsoP metabolites are also valuable biomarkers. The metabolite 2,3-dinor-5, 6-dihydro-8-iso-prostaglandin F2α, for instance, is only generated in the liver from plasma IsoP and has a longer half-life than its parent compound. Therefore, its presence is considered an accurate representation of systemic oxidative stress over time [16]. Indeed, increased IsoP and IsoP metabolites have been associated with many human oxidative stress-related pathologies, including metabolic syndrome and cardiovascular disease [17]. However, while IsoP continuously become more popular in human medicine, studies regarding their potential role in disease pathogenesis remain sparse. Furthermore, literature of IsoP in veterinary species is relatively limited. This review will discuss the history, biosynthesis, and detection of IsoP, followed by current use and knowledge gaps regarding their role in veterinary medicine. Finally, the review will conclude with thoughts on future considerations for IsoP to be used successfully in veterinary medicine.
2. History
Isoprostanes are a relatively new oxidative stress biomarker, having only been characterized in vivo in the last 30 years. However, the earliest evidence of IsoP formation surfaced from in vitro work completed by Pryor and Stanley in the 1970s. They found that when methyl linolenate was autoxidized, a bicyclic endoperoxide was formed as a precursor to prostaglandin-like compounds [18]. In the 1980s, it was shown that the lower side chains of prostaglandin D2 (PGD2) will isomerize in aqueous solutions to form isomers of 9α,11β-PGF2α when reduced by 11-ketoreductase [19]. Later attempts to describe the presence of these compounds in vivo lead to the discovery of what are now termed isoprostanes [20]. In the early 1990s, Morrow and colleagues used mass spectrometry to characterize the 9α,11β-PGF2α isomers discovered by Wendelborn et al. [19]. They were prompted by the fact that the peaks generated by the 9α,11β-PGF2α isomers increased around 50-fold when the plasma was analyzed after several months of storage at −20 °C when compared to the plasma that was analyzed immediately after collection [21]. Furthermore, the authors found that the addition of antioxidants to the plasma samples would reduce the formation of F-ring prostaglandin-like compounds. This suggested that the molecules were generated independently of enzymatic pathways. First was the discovery of prostaglandin (PG) F2-like compounds, termed the F2-isoprostanes [21]. Shortly thereafter, Morrow et al. determined that PGE- and PGD-isoprostanes could be generated in vivo as well [22].
Although these earliest discovered IsoP were derived from arachidonic acid, it was soon determined that other polyunsaturated fatty acids could also generate IsoP in the face of interactions with free radicals. Later in the 90s, the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were found to produce F3-IsoP and F4-neuroprostanes, respectively [23][24][25]. Within a decade of the omega-3 IsoP discoveries, the omega-6 fatty acid, adrenic acid, was found to produce its own class of IsoP when undergoing interactions with free radicals [26]. Furthermore, IsoP are not exclusive to animals. Indeed, the plant-based omega-3 fatty acid α-linolenic acid will generate phytoprostanes (PhytoP) [27]. As the name suggests, IsoP are isomers of PG; however, they are synthesized by different mechanisms.
3. Use and Physiological Roles
3.1. Biomarkers of Lipid Peroxidation
An ongoing challenge in veterinary medicine lies in the ability to diagnose disease promptly so that best management and treatment recommendations can be made. Isoprostanes may be a valuable tool to clinical practice in this capacity because current literature regarding the use of IsoP is largely based on their advantageous nature as biomarkers of oxidative stress-related disorders. At this time, a handful of literature can be found associating a subset of relevant veterinary diseases with altered IsoP concentrations affecting major domestic species [8][28][29][30][31][32]. These studies provide important insights as there is a dearth of information regarding threshold IsoP concentrations that may distinguish between health and disease. Mavangira et al. found that plasma and urine free 15-F2t-IsoP were higher in cows afflicted with coliform mastitis when compared to control cows, while milk 15-F2t-IsoP concentrations were lower in coliform mastitis cows as assessed by LC-MS/MS. In contrast, hydrolyzed samples analyzed with a Cell Biolabs ELISA yielded higher total milk 15-F2t-IsoP in coliform mastitis cows than in controls [28]. Colic is a common condition of horses that may result in veterinary intervention in the form of medical therapy or surgery. However, the numerous causes of colic present a challenge in terms of finding a single biomarker that can reliably determine which treatment may be most beneficial and predict prognosis in affected horses. Noschka and colleagues set out to determine if IsoP and the IsoP metabolite 2,3-dinor-5, 6-dihydro-8-iso-prostaglandin F2α from urine samples would be suitable prognostic predictors in various causes of equine colic. After normalizing urine IsoP and 2,3-dinor-5, 6-dihydro-8-iso-prostaglandin F2α concentrations to urine creatinine, the authors found that both increased in horses with colic compared to healthy control horses. Furthermore, urine 2,3-dinor-5, 6-dihydro-8-iso-prostaglandin F2α concentrations were significantly higher in horses that underwent surgical correction and those that did not survive. Therefore, the authors proposed that 2,3-dinor-5, 6-dihydro-8-iso-prostaglandin F2α may be a useful biomarker for determining if a horse with colic will require surgery or will be less likely to survive [30]. Concentrations of urine IsoP were predictive in a different way. Urine IsoP concentrations were significantly higher in medical colic cases compared to control horses, but surgical cases were not significantly different from controls nor medical colic cases. Additionally, urine IsoP concentrations were significantly higher in horses that survived compared to control, while no difference was detected between nonsurvivors and controls or survivors [30].
Small animals are afflicted with a variety of oxidative stress-related disorders, presenting an opportunity for use of IsoP. Viviano and Van der Wielen supported that dogs with systemic illness undergo more oxidative stress than healthy controls through the use of biomarkers such as urine F2-IsoP concentrations [31]. Spinal cord and cardiovascular disease, common conditions seen in veterinary medicine, are associated with oxidative damage [29]. Dogs with intervertebral disk disease undergoing surgery had increased urine F2-IsoP: creatinine ratios compared to healthy dogs undergoing surgery, both before and after the surgical procedure. Interestingly, dogs that had more severe neurological signs as a result of their disc disease had lower urine F2-IsoP concentrations than those with less severe neurologic signs [29]. Dogs with congestive heart failure had significantly higher 15-F2t-IsoP than healthy controls [8]. Chronic kidney disease is also a common ailment in small animals, but can be difficult to diagnose in its early stages due to lack of a sensitive biomarker that can detect the disease before significant organ damage has occurred [33]. Furthermore, oxidative stress has been implicated in the progression of human chronic kidney disease, but had not been thoroughly investigated in cats prior to a study completed by Whitehouse and colleagues [32]. Cats with stage 1 chronic kidney disease had significantly higher urine F2-IsoP concentrations than cats in stages 2-4. In contrast to the increased concentrations of IsoP that are associated with more advanced stages of chronic kidney disease in humans, cats in the more severe stages 3 or 4 had lowest urinary IsoP concentrations, even when compared to healthy controls [32]. Differences between human and animal studies illustrate the need to understand species-specific IsoP concentrations so that IsoP may be used effectively in veterinary medicine.
As generation of ROS occurs during normal physiological processes, hence leading to lipid peroxidation, IsoP are also produced routinely in clinically healthy individuals [34]. However, only a few studies describe basal IsoP concentrations in apparently healthy animals. The modern dairy cow undergoes major physiological transitions throughout a typical lactation cycle, especially at the onset and cessation of lactation. For instance, tremendous amounts of energy are required for the copious milk synthesis typical of a modern dairy cow at the onset of lactation, suggesting a necessary increase in ROS production via the mitochondria [35]. A concurrent decrease in antioxidant intake during early lactation can thereby lead to ROS accumulation and lipid peroxidation. Kuhn et al. documented dynamic concentrations of 15-F2t-IsoP throughout the lactation cycles of clinically healthy cattle. Indeed, plasma and urine concentrations of the IsoP were significantly lower in mid-lactation cows compared to those in either early or late lactation. However, milk concentrations were significantly lower during early lactation compared to mid- or late lactation [36]. Additional work completed by our lab demonstrated that clinically healthy Holstein cows experience changes in IsoP concentrations throughout the physiological transition from lactating to non-lactating as well. In fact, each of the 7 detected IsoP exhibited its own unique expression profile throughout the transition. Intriguingly, the most well-studied IsoP, 15-F2t-IsoP, did not reach statistical significance after adjusting for multiple comparisons, although a relative increase in concentrations was noted for 2 d after abrupt cessation of lactation [37]. Our study demonstrated that other IsoP besides 15-F2t-IsoP may be biologically relevant and deserve further study investigating their role in the host. During times of intense exercise, and therefore increased oxygen consumption, Hinchcliff et al. unsurprisingly determined that healthy sled dogs completing 58 km runs on 3 consecutive days had significantly higher plasma concentrations of IsoP than healthy control dogs that did not run at all [38]. Determining the concentrations that may be seen during both disease and health in animals provides a first step in understanding how changes in the concentrations may be impacting the host.
3.2. Vascular Regulation
Although IsoP are sensitive and specific biomarkers of oxidative stress, research also is focused on determining potential physiological roles in the host. Vascular endothelium dysfunction is a primary component of oxidative stress and as such, the interaction between IsoP and the vasculature has gathered a considerable amount of research attention [39][40][41][42][43][44][45][81]. Most of the research supports that a predominant role of IsoP is vasoconstriction, which has been demonstrated in numerous species and tissue types [40]. Early evidence of vasoconstriction was established by directly infusing rat kidneys with 15-F2t-IsoP, which revealed a substantial dose-dependent decrease in glomerular filtration rate and renal plasma flow in vivo, both at the whole kidney and single nephron level [41]]. Both 15-F2t-IsoP and 15-E2t-IsoP were shown to cause sustained and dose-dependent coronary vasoconstriction in isolated guinea pig hearts, sometimes decreasing coronary flow by 50% [42]. Vasoconstriction can be strongly attenuated by infusing or treating cells concomitantly with a thromboxane A2 receptor (TP) antagonist (SQ29548), suggesting that IsoP exert their action through TP [41][42]. In fact, numerous studies have supported IsoP as a ligand for TP and Figure 1 summarizes proposed biological consequences as a result of IsoP-TP interaction [43][44]. Isoprostanes are also capable of vasodilation, although this biological effect is often eclipsed by vasoconstriction and is dependent on many factors including the IsoP isomer, concentration, species, vascular bed, and whether or not a ligand is bound to TP [40][45]. For example, 15-E2t-IsoP relaxed porcine pulmonary and coronary arteries that had been pre-treated with the TP agonist U46619. Conversely, none of the 6 IsoP tested (including 15-E2t-IsoP) relaxed pulmonary veins pre-constricted by U46619 [45].
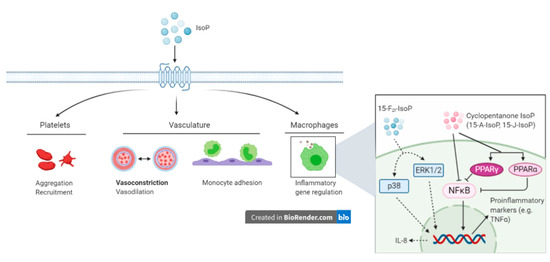
3.3. Inflammatory Effects
Outside the scope of vascular regulation, a limited number of studies have investigated other biological roles of IsoP. The intimate relationship between inflammation and oxidative stress suggests that IsoP may serve as an inflammatory mediator. Indeed, 15-F2t-IsoP is capable of inhibiting monocyte adhesion to human dermal microvascular endothelial cells, with maximal inhibition being noted at a concentration of 1 µM. However, in human umbilical vein endothelial cells, 15-F2t-IsoP enhanced monocyte adhesion at 10−10 to 10−8 M [46]. To characterize the mechanism by which 15-F2t-IsoP caused the suppression of monocyte adhesion to endothelial cells, the authors investigated several direct and indirect effects the IsoP may have. Of the proposed mechanisms, 15-F2t-IsoP inhibited monocyte adhesion induced by tumor necrosis factor alpha (TNFα), had inhibitory effects comparable to those seen with a TP agonist, and appeared to be working through the p38 and c-Jun N-terminal kinases (JNK) pathway via a TP-mediated mechanism. Furthermore, 15-F2t-IsoP appears to encourage the production of a secondary inhibitor of monocyte adhesion, which acts in a TP-independent manner [46]. Scholz and coworkers discovered that 8-isoP also increase interleukin-8 expression in human macrophages [47]. Cyclopentenone IsoP (15-A2-IsoP and 15-J2-IsoP) are also capable of modulating macrophage inflammatory actions. The cyclopentenone IsoP abrogated inflammatory responses in both RAW 267.4 murine macrophages and primary macrophages via blocking translocation of nuclear factor kappa B (NFκB) to the nucleus. Downstream effects of this blockade included inhibition of nitrite production, which was abrogated by performing glutathione adduction [48]. Interestingly, F2-IsoP does not inhibit nitrite production, which implicates the cyclopentenone moiety as being responsible for the anti-inflammatory responses. The cyclopentenone IsoP diverge in their actions under some circumstances though, highlighted by the potent activation of PPARγ by 15-J2-IsoP but not 15-A2-IsoP [48]. Additional evidence for the anti-inflammatory effects of IsoP has been offered for 15-A3t-IsoP, 14-A4t-NeuroP, and 4-F4t-NeuroP [49][50]. Given the impact IsoP have demonstrated in macrophages, it would likely be beneficial to investigate their effects on other cells that participate in the inflammatory response, such as other leukocytes and endothelial cells.
References
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511.
- Mavangira, V.; Sordillo, L. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 2018, 116, 4–14.
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol. Med. 2001, 7, 548–554.
- Polidori, M.C.; Praticó, D.; Savino, K.; Rokach, J.; Stahl, W.; Mecocci, P. Increased F2 isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J. Card. Fail. 2004, 10, 334–338.
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585.
- Skoumalová, A.; Rofina, J.; Schwippelova, Z.; Gruys, E.; Wilhelm, J. The role of free radicals in canine counterpart of senile dementia of the Alzheimer type. Exp. Gerontol. 2003, 38, 711–719.
- Contreras, G.A.; Strieder-Barboza, C.; Raphael, W. Adipose tissue lipolysis and remodeling during the transition period of dairy cows. J. Anim. Sci. Biotechnol. 2017, 8, 1–12.
- Freeman, L.M.; Rush, J.E.; Milbury, P.E.; Blumberg, J.B. Antioxidant Status and Biomarkers of Oxidative Stress in Dogs with Congestive Heart Failure. J. Vet. Intern. Med. 2005, 19, 537–541.
- Abuelo, A.; Hernández, J.; Benedito, J.; Castillo, C. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal 2013, 7, 1374–1378.
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource; Food and Drug Administration; National Institutes of Health: Silver Springs, MD, USA, 2016.
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of Oxidative Damage in Human Disease. Clin. Chem. 2006, 52, 601–623.
- Fenga, C.; Gangemi, S.; Teodoro, M.; Rapisarda, V.; Golokhvast, K.; Docea, A.O.; Tsatsakis, A.M.; Costa, C. 8-Hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to low-dose benzene. Toxicol. Rep. 2017, 4, 291–295.
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170.
- Milne, G.L.; Musiek, E.S.; Morrow, J.D. F2-isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers 2005, 10 (Suppl. 1), S10–S23.
- Janicka, M.; Kot-Wasik, Á.; Kot, J.; Namieśnik, J. Isoprostanes-Biomarkers of Lipid Peroxidation: Their Utility in Evaluating Oxidative Stress and Analysis. Int. J. Mol. Sci. 2010, 11, 4631–4659.
- Roberts, L.J.; Morrow, J.D. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000, 28, 505–513.
- Van ’t Erve, T.J.; Kadiiska, M.B.; London, S.J.; Mason, R.P. Classifying oxidative stress by F2-isoprostane levels across human diseases: A meta-analysis. Redox Biol. 2017, 12, 582–599.
- Pryor, W.A.; Stanley, J.P. Suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymic production of prostaglandin endoperoxides during autoxidation. J. Org. Chem. 1975, 40, 3615–3617.
- Wendelborn, D.F.; Seibert, K.; Roberts, L.J. Isomeric prostaglandin F2 compounds arising from prostaglandin D2: A family of icosanoids produced in vivo in humans. Proc. Natl. Acad. Sci. USA 1988, 85, 304–308.
- Roberts, L.J., 2nd; Fessel, J.P. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Chem. Phys. Lipids 2004, 128, 173–186.
- Morrow, J.D.; Harris, T.M.; Roberts, L.J. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: Analytical ramifications for measurement of eicosanoids. Anal. Biochem. 1990, 184, 1–10.
- Morrow, J.D.; Minton, T.; Mukundan, C.R.; Campbell, M.D.; Zackert, W.; Daniel, V.C.; Badr, K.F.; Blair, I.; Ii, L.J.R. Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J. Biol. Chem. 1994, 269, 4317–4326.
- Nourooz-Zadeh, J.; Halliwell, B.; Änggård, E. Evidence for the Formation of F3-Isoprostanes during Peroxidation of Eicosapentaenoic Acid. Biochem. Biophys. Res. Commun. 1997, 236, 467–472.
- Nourooz-Zadeh, J.; Liu, E.H.; Änggård, E.; Halliwellb, B. F4-Isoprostanes: A Novel Class of Prostanoids Formed during Peroxidation of Docosahexaenoic Acid (DHA). Biochem. Biophys. Res. Commun. 1998, 242, 338–344.
- Roberts, L.J., 2nd; Montine, T.J.; Markesbery, W.R.; Tapper, A.R.; Hardy, P.; Chemtob, S.; Dettbarn, W.D.; Morrow, J.D. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 1998, 273, 13605–13612.
- VanRollins, M.; Woltjer, R.L.; Yin, H.; Morrow, J.D.; Montine, T.J. F2-Dihomo-isoprostanes arise from free radical attack on adrenic acid. J. Lipid Res. 2008, 49, 995–1005.
- Parchmann, S.; Mueller, M.J. Evidence for the Formation of Dinor Isoprostanes E1from α-Linolenic Acid in Plants. J. Biol. Chem. 1998, 273, 32650–32655.
- Mavangira, V.; Mangual, M.; Gandy, J.; Sordillo, L. 15-F2t-Isoprostane Concentrations and Oxidant Status in Lactating Dairy Cattle with Acute Coliform Mastitis. J. Vet. Intern. Med. 2015, 30, 339–347.
- McMichael, M.A.; Ruaux, C.G.; Baltzer, W.I.; Kerwin, S.C.; Hosgood, G.; Steiner, J.M.; Williams, D.A. Concentrations of 15F2tisoprostane in urine of dogs with intervertebral disk disease. Am. J. Vet. Res. 2006, 67, 1226–1231.
- Noschka, E.; Werre, S.R.; Crisman, M.V.; Thatcher, C.D.; Milne, G.L.; Dahlgren, L.A. Implications of urine F2-isoprostane metabolite concentration in horses with colic and its potential use as a predictor for surgical intervention. Equine Vet. J. 2011, 43, 34–41.
- Viviano, K.; VanderWielen, B. Effect of N -Acetylcysteine Supplementation on Intracellular Glutathione, Urine Isoprostanes, Clinical Score, and Survival in Hospitalized Ill Dogs. J. Vet. Intern. Med. 2013, 27, 250–258.
- Whitehouse, W.; Quimby, J.; Wan, S.; Monaghan, K.; Robbins, R.; Trepanier, L.A. Urinary F2-Isoprostanes in Cats with International Renal Interest Society Stage 1-4 Chronic Kidney Disease. J. Vet. Intern. Med. 2017, 31, 449–456.
- Chen, H.; Avital, Y.; Bruchim, Y.; Aroch, I.; Segev, G. Urinary heat shock protein-72: A novel marker of acute kidney injury and chronic kidney disease in cats. Veter- J. 2019, 243, 77–81.
- Downey, L.A.; Simpson, T.; Timmer, J.; Nolidin, K.; Croft, K.; Wesnes, K.A.; Scholey, A.; Deleuil, S.; Stough, C. Impaired verbal episodic memory in healthy older adults is marked by increased F 2 -Isoprostanes. Prostaglandins Leukot. Essent. Fat. Acids 2018, 129, 32–37.
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2014, 99, 1003–1016.
- Kuhn, M.J.; Mavangira, V.; Gandy, J.C.; Sordillo, L. Production of 15-F-isoprostane as an assessment of oxidative stress in dairy cows at different stages of lactation. J. Dairy Sci. 2018, 101, 9287–9295.
- Putman, A.; Brown, J.; Gandy, J.; Wisnieski, L.; Sordillo, L. Changes in biomarkers of nutrient metabolism, inflammation, and oxidative stress in dairy cows during the transition into the early dry period. J. Dairy Sci. 2018, 101, 9350–9359.
- Hinchcliff, K.W.; Reinhart, G.A.; DiSilvestro, R.; Reynolds, A.; Blostein-Fujii, A.; Swenson, R.A. Oxidant stress in sled dogs subjected to repetitive endurance exercise. Am. J. Vet. Res. 2000, 61, 512–517.
- Verma, S.; Buchanan, M.R.; Anderson, T.J. Endothelial Function Testing as a Biomarker of Vascular Disease. Circulation 2003, 108, 2054–2059.
- Bauer, J.; Ripperger, A.; Frantz, S.; Ergün, S.; Schwedhelm, E.; Benndorf, R.A. Pathophysiology of isoprostanes in the cardiovascular system: Implications of isoprostane-mediated thromboxane A2receptor activation. Br. J. Pharmacol. 2014, 171, 3115–3131.
- Takahashi, K.; Nammour, T.M.; Fukunaga, M.; Ebert, J.; Morrow, J.D.; Roberts, L.J.; Hoover, R.L.; Badr, K.F. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J. Clin. Investig. 1992, 90, 136–141.
- Möbert, J.; Becker, B.F.; Zahler, S.; Gerlach, E. Hemodynamic Effects of Isoprostanes (8-Iso-Prostaglandin F2α and E2) in Isolated Guinea Pig Hearts. J. Cardiovasc. Pharmacol. 1997, 29, 789–794.
- Audoly, L.P.; Rocca, B.; Fabre, J.E.; Koller, B.H.; Thomas, D.; Loeb, A.L.; Coffman, T.M.; Fitzgerald, G.A. Cardiovascular responses to the isoprostanes iPF(2alpha)-III and iPE(2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation 2000, 101, 2833–2840.
- Benndorf, R.A.; Schwedhelm, E.; Gnann, A.; Taheri, R.; Kom, G.; Didié, M.; Steenpass, A.; Ergün, S.; Böger, R.H. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: A potential link between oxidative stress and impaired angiogenesis. Circ. Res. 2008, 103, 1037–1046.
- Villamor, E.; González-Luis, G. Age-related changes in isoprostane-mediated relaxation of piglet blood vessels. Front. Biosci. 2010, 2, 369–379.
- Kumar, A.; Kingdon, E.; Norman, J. The isoprostane 8-iso-PGF 2α suppresses monocyte adhesion to human microvascular endothelial cells via two independent mechanisms. FASEB J. 2004, 19, 1–24.
- Scholz, H.; Yndestad, A.; Damås, J.K.; Wæhre, T.; Tonstad, S.; Aukrust, P.; Halvorsen, B. 8-Isoprostane increases expression of interleukin-8 in human macrophages through activation of mitogen-activated protein kinases. Cardiovasc. Res. 2003, 59, 945–954.
- Musiek, E.S.; Gao, L.; Milne, G.L.; Han, W.; Everhart, M.B.; Wang, D.; Backlund, M.G.; Dubois, R.N.; Zanoni, G.; Vidari, G.; et al. Cyclopentenone Isoprostanes Inhibit the Inflammatory Response in Macrophages. J. Biol. Chem. 2005, 280, 35562–35570.
- Bosviel, R.; Joumard-Cubizolles, L.; Chinetti, G.; Bayle, D.; Copin, C.; Hennuyer, N.; Duplan, I.; Staels, B.; Zanoni, G.; Porta, A.; et al. DHA-derived oxylipins, neuroprostanes and protectins, differentially and dose-dependently modulate the inflammatory response in human macrophages: Putative mechanisms through PPAR activation. Free. Radic. Biol. Med. 2017, 103, 146–154.
- Brooks, J.D.; Musiek, E.S.; Koestner, T.R.; Stankowski, J.N.; Howard, J.R.; Brunoldi, E.M.; Porta, A.; Zanoni, G.; Vidari, G.; Morrow, J.D.; et al. The fatty acid oxidation product 15-A3t-Isoprostane is a potent inhibitor of NFκB transcription and macrophage transformation. J. Neurochem. 2011, 119, 604–616.





