
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Julia Bondareva | + 4987 word(s) | 4987 | 2021-01-13 04:36:21 | | | |
| 2 | Camila Xu | Meta information modification | 4987 | 2021-02-25 10:55:49 | | |
Video Upload Options
Dendrimers are multifunctional polymer compounds with a tree-like shape and a high branching degree.
1. Introduction
One of the long-term goals of basic science is the search for as many new and outstanding substances as possible under the limits of natural laws. Of course, new chemical substances are not synthesized by random approaches with no purpose in mind. Chemists create new materials with the aim that their properties will be scientifically significant or useful for practical purposes. The synthesis of new molecules is achieved by performing chemical reactions, some of which are already well known and must be developed. Thus, another goal of basic science is to develop new synthetic strategies to create exciting and useful substances. One such recently prepared class of substances is dendrimers. Dendrimers have been studied for the past thirty years. The number of publications related to dendrimer research is growing every year, as shown in Figure 1, and this trend is expected to continue.
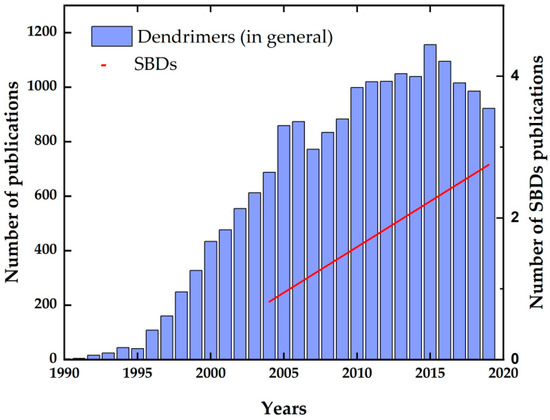
Figure 1. The number of publications related to dendrimers and sulfonimide-based dendrimers (SBDs) according to Scopus 1990–2020.
This growing interest can happen because dendrimers are important in chemistry, biology, medicine, biotechnology, and the pharmaceutical and food industries [1]. Their physicochemical properties, such as monodispersity, multivalency, and globular shape, distinguish them from classic polymers and make them a separate class of molecules. Due to their inner cavities, dendrimers can create a suitable environment for stabilizing nanoparticles, which can be directly synthesized from metal salts [2]. Despite these properties, some commercial products are based on dendrimers or using dendrimers as part of chemical composition [3]; some of them are already on the market, and some are in clinical trials [4][5]. The main reason for this is the dearth of synthetic strategies for preparing dendrimers. The absence of a highly efficient synthetic method and the difficulties associated with purifying increasingly branched macromolecules have inhibited their transfer from academia to industry. Figure 2a [6] shows a two-dimensional model of the dendritic structure in a flat form, consisting of a set of geometric figures representing the kernel, points of branches, and end groups [7]. Thus, many dendrimers have been constructed incorporating different functionalities either at the core, branches, terminals, or a mixture of any two or three of these dendritic units. Currently, there are about 50 dendrimer families, including PAMAM [8], poly(amidoamine-organosilicon) (PAMAMOS) [9], poly(propyleneimine) (PPI) [10], liquid crystal dendrimers [11], chiral dendrimers [12], peptide dendrimers [13], tecto-dendrimer [14], glycodendrimers [15], polyethylene glycol (PEG) dendrimers [16], etc. Since many dendrimers have already been obtained and investigated, the amount of information available in the literature is continuously growing. Therefore, we mainly focus on a specific type of dendrimer molecule, namely, sulfonimide-based dendrimers (SBDs), which contain sulfonimide groups at each branching point. The typical SBD structure is presented in Figure 2b, where a nitrogen atom represents the core and the branching points contain sulfonimide functionalities.
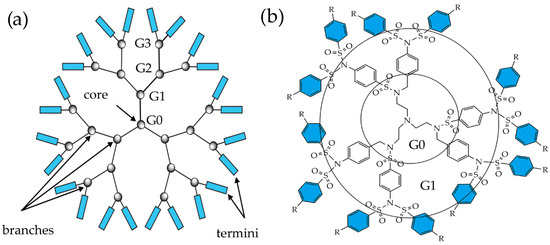
Figure 2. (a) Schematic of the dendrimer structure showing the key components. (b) A schematic of a G1 of SBD in the form of a planar graph.
Each structural part of the dendrimer is responsible for certain properties. The multiplicity of the core and the branching units determine the dendrimer's shape and size. In some cases, the core can assign a particular function to the dendrimer. Increasing the number of branches increases the number of generations, which refers to the number of layers attached to the core. Moreover, the nature of the branching points influences the stability of the molecule itself and the presence of internal voids, which can accommodate guest molecules. The end groups create the molecule's outer shell and give the dendrimer unique properties, such as low/high solubility in different solvents, viscosity, and conformational stability [17]. One of the advantages of dendrimers and other classes of macromolecules is the presence of an enormous number of terminal groups, which exponentially amplify the desired properties. SBDs were chosen as the materials of interest due to their unique properties, which are absent in other similar branched macromolecules. Among polybranched compounds, SBDs possess relatively high melting (120–250 °C) and glass transition temperatures (135–162 °C) [7]. Additionally, aromatic sulfonimides are chemically stable and crystalline. The combination of these properties makes them applicable in situations where the material must be stable in the temperature range of 120–130 °C. Dendrimers' main advantages are the ability to access the desired features through careful design and installing various chemical groups in the dendrimer. Furthermore, SBDs can be considered in surface chemistry as functional surface coatings [18].
2. Properties
2.1. Structure
In terms of size, dendrimers are organic nanoparticles (as liposomes, carbon nanomaterials, and polymer micelles are) [19]. As nanoparticles, their size does not exceed 15 nm, and they show unique size-dependent physical and chemical properties. For example, the dimensions of recently synthesized SBDs up to the G5 do not exceed 5 nm, as shown in Table 1 [20].
Table 1. Characteristics of naphthyl decorated SBDs.
| Generations | Molecular Weight, Da | Dimensions, nm | 2D Graphical Representation |
3D Chemical Structure View 1 |
|---|---|---|---|---|
| G2 | 1334 | 2.6 |  |
 |
| G3 | 2541 | 3.2 | 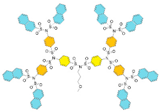 |
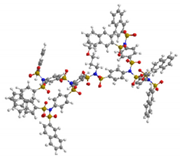 |
| G4 | 5304 | 3.6 | 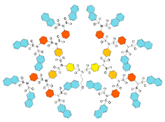 |
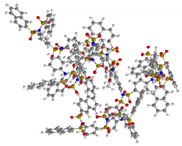 |
| G5 | 10831 | 4.6 | 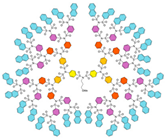 |
 |
1 red—oxygen, yellow—sulfur, blue—nitrogen, grey—carbon, white—hydrogen.
The properties of sulfonimide groups are not well understood, and they are described in the literature relative to a tautomer of a sulfonamide group [21][22].
In organic chemistry, a sulfonimide is a functional group consisting of two sulfonyl groups (–SO2) bound to a nitrogen atom from one side and carbon from another side. The compounds are structurally similar to acid anhydrides. The basic nitrogen atom of a sulfonimide with a lone pair can participate in hydrogen bonding [23]. The sulfonyl group is a relatively inactive functional group characterized as weak basic, where a sulfur atom is doubly bonded to two oxygen atoms [24]. The reaction of a sulfonyl chloride with ammonia or an amine leads first to a sulfonamide, and in the case of full nucleophilic substitution, a sulfonimide can be obtained. Sulfone (–SO2) and sulfonamide (–SO2NH) groups can be found in drug-like molecules and are used in pharmaceuticals due to their antibacterial, antimicrobial, antidiabetic, and antiretroviral activities [25].
2.2. Isomeric Structures
The structure, number of branches, and molar weight directly affect the properties of SBDs. According to the topology, dendrimers can be considered as isographic dendrimers, having the same branches (symmetrical structure), or non-isographic dendritic isomers, containing different branches (asymmetric structure). There is some structure-property correlation. Several sulfonimide-based isomers with different molecular weights and shapes have been evaluated to this extent. Several properties such as melting temperature, solubility, chromatographic separation, nuclear magnetic resonance (NMR), spectroscopic, and mass spectrometric characteristics of these compounds were analyzed [7]. The low-molar-weight dendrimers with mostly linear shapes and four to six peripheral groups have high melting points and low solubility in chloroform (CHCl3) [7]. The melting points and solubility of high-molar mass dendrimers with seven, eight, and ten peripheral groups differ noticeably less. Thus, with an increase in dendrimer generation, fewer differences in the properties listed above are observed between dendrimer isomers. The formation of a dense spherical packing of high-generation dendrimers, in which the outer shell mainly determines the properties, is one possible explanation for this behavior.
2.3. Functional Groups
Aromatic SBDs structures contain peripheral methyl and nitro groups that can be chemically converted to carboxylic acids and amines for further chemical or biological modification. From the point of view of medicine, functional groups provide additional binding sites, specific properties, and behaviors responsible for biological activities. For a given drug molecule, its functional groups affect its general solubility in aqueous solutions, the ability to specifically bind to biological targets, mechanism, and duration of action, etc. [26][27]. Carboxylic and amino functional groups may provide an additional opportunity to form a covalent bond with a biological target, enzyme, or drug molecule, or they may participate in the formation of hydrogen bonds [28][29][30][31].
Adding even one chemical group to a given molecule can change some dendrimer properties, such as the solubility, electronegativity, and conformation of the molecule. When a dendrimer structure is functionalized with photoactive units [32][33], it can exhibit moderate fluorescent activity and be used as a photoactive tracer. A dendrimer's properties that contain photoactive chemical parts depend on the interactions with the ground and excited states. For example, it has recently been shown that dendrimers with a cyclam core and naphthyl-based branches exhibit three types of emission bands [34]. Additionally, poly(propylene amine) dendrimers modified on the periphery with fluorescent naphthyl sulfonamide groups can coordinate metal ions [32].
Large alkyl chains or aromatic systems such as naphthalene make the dendrimer lipid-soluble. Changing the number of such groups makes it possible to control the dendrimers' hydrophobicity. Thus, the outer shell of SBDs can be functionalized with naphthalene terminal groups (from four to 32 groups for the G5 dendrimer, which has been synthesized for the first time) [20]. As naphthyl groups and long alkyl chains make the molecule hydrophobic, it is possible to create a water-repellent film [18]. Additionally, the presence of photoactive naphthyl groups, which can form intramolecular or intermolecular bonds under UV radiation, allows the creation of films with different types of binding and packaging. So, under UV irradiation, naphthyl groups can be dimerized with the formation of additional covalent bonds, in contrast to unirradiated UV samples, where the non-covalent bond predominates. Thus, the number of naphthyl groups, the use of UV radiation, and the formation of additional bonds create a dense packing of molecules and lead to different contact angle values, as shown in Figure 3.
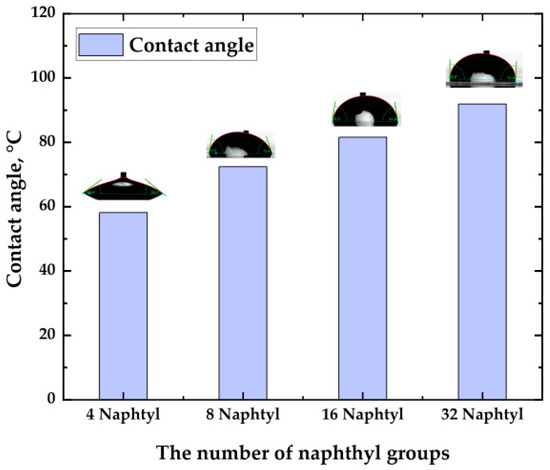
Figure 3. The contact angle depends on the number of naphthyl terminal groups in the dendrimer structure.
2.4. Molar Masses
The focal point of the dendritic polymer can impart some interesting properties. For example, if dendrons carry a polymerizable unit, this could allow solid-state polymerizations [35]. The obtained sulfonimide dendrons, which contain a methacrylate group in the focal point, can be polymerized [36] and reached the molar masses of the G1 and G2 polymers Mw = 1500 and Mw = 1600 kDa, respectively. All such polymers are soluble in polar solvents, such as dimethylsulfoxide (DMSO) and dimethylformamide (DMF), and are insoluble in hexane.
2.5. Solubility
Dendrimers have multifunctional surfaces that allow their solubility and interactions with other molecules to be tuned for different environments. Solubility is crucial for biomedical applications. Dendrimers should be soluble in biologically safe solvents to be applied in the pharmaceutical field. The solvent must be entirely safe for humans and effectively dissolve the dendrimers. One such biologically compatible solvent is water. Water is the most advantageous solvent in many respects, e.g., its availability, biocompatibility, and low price, and it does not require additional stages of removal and cleaning. SBDs are usually insoluble in water. Some dendrimers can be solubilized if a solubilizing agent is added. SBDs are soluble in CHCl3, but this solvent is toxic. Another suitable solvent for SBDs is DMSO. DMSO is considered to have low toxicity that can dissolve both polar and nonpolar compounds. At the same time, DMSO can easily penetrate the skin along with the solute, which may cause undesirable effects. In some cases, binary mixtures of solvents are used, e.g., a popular mixture is DMSO and water. The main limitation with SBDs in this regard is that they are not soluble in water, which makes it difficult to use such materials in biomedical fields. In this context, the development of a method for the aqueous solubilization of these dendrimers by adding solubilizing agents such as cyclodextrins [37] is a target for future studies.
SBDs and dendrons of all generations bearing peripheral tosyl- and 2-naphthyl sulfonyl groups exhibit very high solubility in both polar aprotic and nonpolar aprotic solvents (except for hexane). Their solubility in DMF, tetrahydrofuran (THF), CHCl3, and benzene are in the range of 150–250 mg/mL [6]. Nosyl-decorated branched sulfonimides have poor solubility in the above-mentioned solvents at room temperature except for G1 species and Janus-type dendrimers. Compounds with peripheral nitro groups can be dissolved in hot THF and hot CHCl3. As mentioned above, all the compounds have low or no solubility in methanol (MeOH) and ethanol (EtOH). Oligosulfonimides bearing peripheral amino groups are soluble in alcohols or mixtures of alcohols with either chlorinated solvents or benzene.
2.6. Voids and Cavities
Additionally, the presence of internal voids is an important parameter of dendrimers. Internal cavities can serve as reservoirs to store small molecules and nanoparticles, allowing dendrimers to participate in host-guest chemistry. Figure 4 shows the example of G3 and G4 naphthyl decorated SBDs, and their internal cavities are marked with red circles.
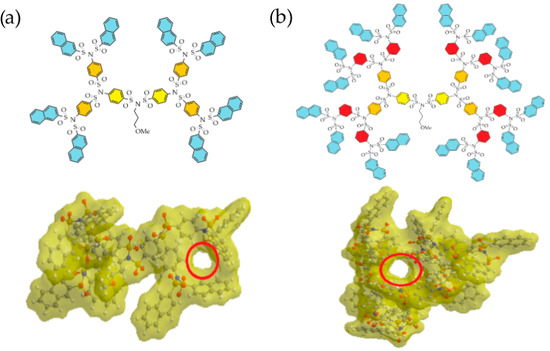
Figure 4. (a) Planar representation of a G3 SBD and its 3D architecture with a Connolly molecular surface. (b) Planar representation of a G4 SBD and its 3D architecture with a Connolly molecular surface.
Dendrimers can interact with low molecular weight drug molecules in several ways: physical encapsulation, covalent binding through terminal chemical groups, and electrostatic interaction [38]. The possibility of using all these binding methods is explained by the dendrimer’s structural features, such as the presence of internal cavities for placing the drug inside the dendrimer structure and the existence of many surface functional groups for covalent conjugation with the drug substance.
3. Synthesis
Branched cascade molecules called dendrimers were first synthesized in 1978 [39]. Since then, dendrimer molecules have received extensive attention in organic, supramolecular, and polymer chemistry. For SBDs, two synthetic approaches are often used, which are known as divergent and convergent strategies, but several new techniques have also been applied in recent years.
3.1. Divergent Technique
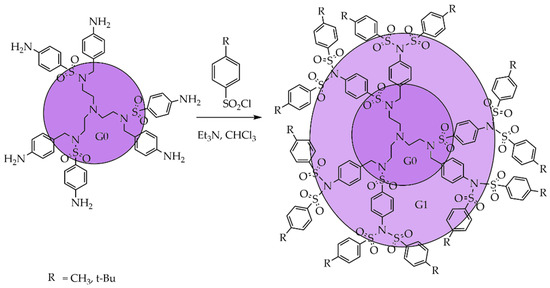
The first attempt at the synthesis of SBDs was reported by Prof. Vögtle et al., who obtained sulfonamide-substituted G2-G4 polypropylene amine (POPAM) dendrimers by N-monosulfonylation and N-bissulfonylation [40]. Prof. Vögtle et al. proposed using sulfonic acid chlorides (sulfonyl chlorides) for the mono- and bifunctionalization of dendrimers. Arylsulfonyl chlorides are commercially available, making these dendrimers synthetically accessible and increasing the potential structural variability of dendritic sulfonimides. They reported the first N-sulfonylation of poly(propyleneimine) (PPI) dendrimers and suggested using this method to synthesize higher-generation SBDs [32].
The next synthetic approach for obtaining dendrimers with sulfonimide units at the branching points was reported by Prof. Vögtle et al. in 2004 [41]. Prof. Vögtle et al. developed a method that allows the controlled functionalization of oligoamines such as tris(2-aminoethyl) amine by persulfonylation using sulfonyl chlorides. Thus, the sulfonylation reaction can be controlled by changing the reaction conditions, for example, the amount and nature of the reagents, as well as the time and temperature of the reaction. The selectivity of the persulfonylation has opened up possibilities for the synthesis of new types of SBDs with more complex structures and different functional groups through sulfonylation of the amine, by not only the addition method 1→1 but also using the more effective way 1→2 [42]. The selectivity of the sulfonylation reaction may also depend on the nature of the base used; for example, the reaction of tris (2-aminoethyl) amine with arylsulfonyl chlorides in dichloromethane (CH2Cl2) or CHCl3 in the presence of a base catalyst leads to the formation of sulfonamides according to the addition scheme 1→1. The same reaction can lead to sulfonimide according to the 1→2 addition scheme if cesium carbonate is used as the base. The yields of sulfonimide depend on the nature of the sulfonyl chloride. Along with the influence of the base’s nature, sulfonyl chloride's choice affects the sulfonylation reaction. Scheme 1 shows that the sulfonylation of a sulfonamide with six terminal amino groups using sulfonyl chlorides containing methyl and tert-butyl groups in the presence of triethylamine unexpectedly allowed the sulfonylation of all external amino groups. Thus, using the 1→2 branching scheme will enable one to quickly and efficiently obtain chemically stable dendrimers with sulfonimide branches.
Scheme 1. Controllable per-functionalization (persulfonylation) of dendritic oligoamines.
The commercial availability and widespread popularity of polypropylene-amine dendrimers (POPAM) [43] and polyamidoamine dendrimers (PAMAM) [44] are the main factors for studying persulfonylation using these types of dendrimers to obtain higher-generation products. The interaction of the G1 POPAM dendrimers with tosyl chloride was carried out in the presence of cesium carbonate as a base to produce sulfonimide, as shown in Scheme 2 [45]. In addition to the target product, by-products of incomplete substitution of the hydrogen atom of the amino group can also form with the formation of a mixture of sulfonamides. Since the molecular weight of the unsubstituted products is compared to the target dendrimer, sulfonamides can be easily separated using conventional silica gel column chromatography.
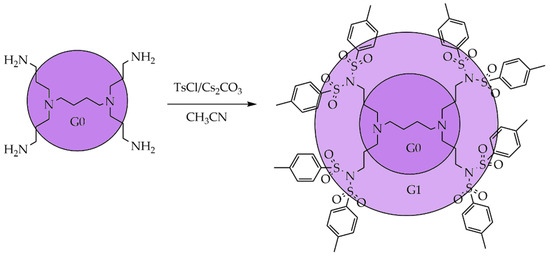
Scheme 2. Persulfonylation of a G1 polypropylene-amine (POPAM) dendrimer.
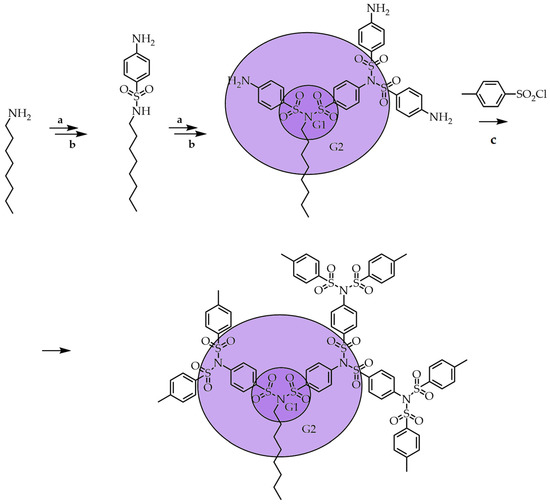
Interestingly, subjecting the G2 POPAM dendrimer to the same sulfonylation procedure with tosyl chloride does not lead to the expected products. Under these conditions, the G2 POPAM dendrimer's further sulfonylation yielded a complex mixture of products consisting of various sulfonylated fragments not subject to separation and purification. Similar to POPAM dendrimers, outer shell PAMAM dendrimers contain aliphatic amino groups. However, the G2 PAMAM dendrimers' sulfonylation with sulfonyl chlorides in the presence of cesium carbonate resulted in octasulfonamide rather than hexadecasulfonimide. The method proved to be of limited use because it could only be applied for the peripheral decoration of G1 branched oligoamines. The partial persulfonylation of the oligoamines makes product separation difficult. Therefore, future investigations on the sulfonylation of amine-terminated dendrimers of other sulfonyl chlorides may lead to the development of new approaches to selective functionalization with the creation of new dendritic structures with useful properties. Based on the previous N-bis-sulfonylation (persulfonylation) results, Oleg Lukin et al. described the synthesis of a newly designed type of dendron that carries sulfonimide functional groups at every branching point. The selectivity of the primary amines persulfonylation in combination with a repeating methodology allows the creation of selectively decorated and shape-changing dendritic sulfonimide structures from simple building blocks [6]. Taking into account the limitations of the peripheral decoration of aliphatic amines, Oleg Lukin et al. employed an amine persulfonylation, which results in a 1→2 branching addition in a repeating manner that would lead to dendritic sulfonimides. The synthetic procedures are shown in Scheme 3 [6]. The main reactions involved are the persulfonylation of amines and the reduction of nitroaromatic compounds.
Scheme 3. Synthesis of symmetrical oligo SBD (a) NO2C6H4SO2Cl, Et3N, CH2Cl2, reflux; (b) H2, 10% Pd/C, C6H6/C2H5OH (c) CH3C6H4SO2Cl, Et3N, CH2Cl2, reflux.
Oligosulfonimides were synthesized in two steps: by treating N-octylamine with sulfonyl chloride with the nitro group in the presence of triethylamine and catalytic reduction of the nitro moieties to the corresponding amines. Octylamine was selected as the core molecule because of intermediate sulfonimides' good solubility compared to similar primary amines with different alkyl chains [46]. The octylamine was persulfonylated in one step using an excess of the corresponding sulfonyl chloride.
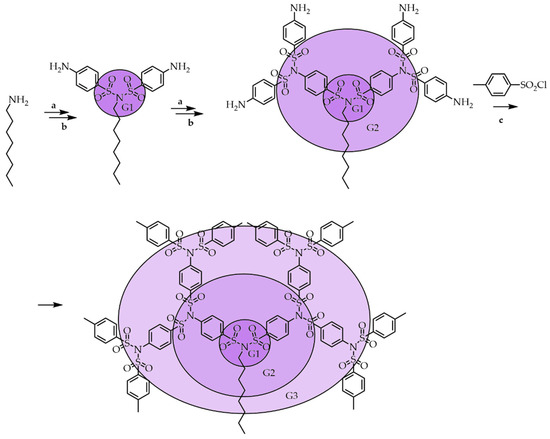
In the last step, the dendrimer was functionalized by persulfonylation with chemically inert tosyl chloride, giving a G3 symmetrical dendrimer with methyl groups at the ends. Thus, the divergent approach shown in Scheme 3 is one of the most effective methods for producing repeating branched structures based on sulfonimide with various functional groups at the ends. Another advantage of this method is the simplicity of the procedure for purification of the obtained dendrimers by simple recrystallization in suitable solvents with a satisfactory yield. The synthesis of SBDs is attractive from the commercial availability of various arylsulfonyl chlorides, which allows one to quickly obtain different types of high-generation SBDs decorated with the desired functional groups. Compared with previous works, this new one-step persulfonylation for primary amines by only heating with an excess of both the arylsulfonyl chlorides and bases affords much better yields than the reaction requiring a longer reaction time with cesium carbonate in acetonitrile. The ability to control the sulfonylation reaction allows one to obtain dendrimers having incompletely substituted branches in their structure; the presence of such defects can be programmed for certain tasks. In Scheme 4 [6], at the first stage, the only sulfonamide was prepared from octylamine and then further sulfonylated with a large excess of sulfonyl chloride, which led to full persulfonylation. The repetition of these steps afforded unsymmetrical sulfonimide.
Scheme 4. Synthesis of SBDs with structural asymmetry. (a) 4-NO2C6H4SO2Cl, Et3N, CH2Cl2 (b) SnCl2, HCl, EtOH, reflux; (c) CH3C6H4SO2Cl, Et3N, CH2Cl2, reflux.
Intermediate sulfonamide can be sulfonylated at the arylamine position or at the sulfonamide position with different sulfonyl chlorides to obtain an asymmetrically branched structure. The first molecule of sulfonyl chloride reacts with the amino group. Then the second molecule of sulfonyl chloride reacts in the same position, even if the sulfonamide position is present in the molecule. This selectivity can be explained based on fundamental organic chemistry; the amino group attached to the aromatic ring is more nucleophilic than the sulfonamide (–SO2NH) because its nitrogen lone pair is delocalized in the amide bond. Due to unsymmetrical synthesis with different functionalities, dendrimers with similar interiors but different exteriors called Janus-type dendrimers [47] were obtained. This synthetic approach has the advantage of having a wide range of affordable, inexpensive sulfonyl chloride reagents that can produce chemically stable SBDs of various designs that do not require sophisticated purification methods. Despite this advantage, several shortcomings were limiting the synthesis of SBDs. For example, with an increase in dendrimer generation, its molecular weight also increases, which can significantly reduce the product’s solubility and prevent further synthesis and the target product's purification procedure. In addition, the catalytic reduction reaction was not fully reproducible and led to an increase in the number of defects and by-products. The reduction of p-nosyldecorated dendritic sulfonimides with tin chloride (SnCl2) instead of Pd or Raney-Ni catalysts in boiling EtOH gives a full reduction but with subsequent cleavage of the p-aminobenzenesulfonyl groups to quantitatively form the corresponding amino sulfonamides. It was found that the use of a mixture of EtOH and CH2Cl2 in equal proportions is the way to the pure reduction of nitro groups. To reach a higher generation of SBDs, Oleg Lukin et al. investigated the influence of different arylsulfonyl chlorides under the same reaction conditions. Investigations indicated that para- and meta-substituted arylsulfonyl chlorides that lead to G3 and G4 compounds are usually high yielding. The reactivity of aromatic and aliphatic sulfonamides and aromatic amines is different and decreases with the increasing aliphatic part: ArSO2NHAr ‘> ArNH2> ArSO2NH-Alk [48].
In 2010, a group of chemists described the synthesis of G1 and G2 methacrylate monomers containing sulfonimide units at each branch and their radical polymerization to the corresponding sulfonimide-based polymers [36].
Scheme 5 [36] highlights the main reactions, including the sulfonylation of amino groups and the reduction of nitro groups and esters. The G1 monomers’ syntheses started from commercially available ester, which was sulfonylated with arylsulfonyl chlorides. The ester groups were cleanly reduced with diisobutylaluminum hydride (DIBAL-H), affording corresponding alcohols. In the last step, methacrylate was added by reacting alcohols with methacrylic acid chloride (MAC). The synthesis procedures of monomers of the G2 and G3 were similar to the above. These procedures can be repeated, leading to a G3 monomer MG3(CH3). For the polymerization reaction, the monomers were dissolved in DMF, and the solution was heated under nitrogen in the presence of azoisobutyronitrile (AIBN). The highest G5 SBD decorated with 32 naphthyl terminal groups was prepared using divergent techniques [20] with two steps, similar to that mentioned in Scheme 3. 3-Methoxypropylamine was used as the starting material and persulfonylated with a p-nosyl sulfonyl chloride. The nitroaromatic compounds were reduced with tin (II) chloride because other reducing reagents, such as Fe/NH4Cl, Zn/CH3COOH, and others, led to mixtures of products. The two-stage approach has significantly increased the production of higher-generation SBDs. Thus, the G3 was obtained on a scale of 7 g in one synthetic cycle, while the species of the G4 were obtained on a scale of 5 g, and the G5 SBD shown in Table 1 was obtained on a 2 g scale [20].
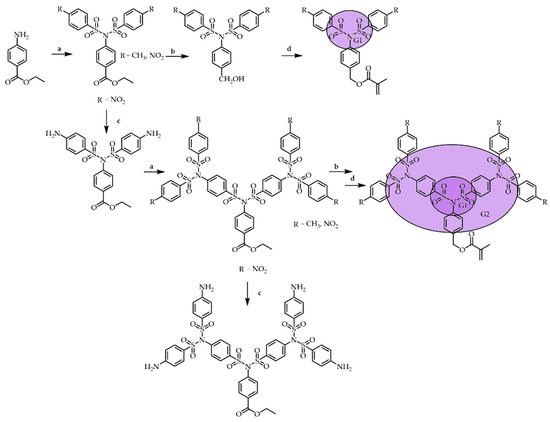
Scheme 5. Synthesis of macromonomers MG1 and MG2. (a) ArSO2Cl, Et3N, CH2Cl2 (b) diisobutylaluminum hydride (DIBAL-H), tetrahydrofuran (THF) (c) SnCl2/HCl, EtOH: dichloromethane (DCM); (d) MAC, trimethylamine (TEA), THF.
3.2. Convergent Technique
Typically, SBDs, as well-known commercially available dendrimers such as PAMAM and POPAM, are synthesized using a divergent procedure [49], which has the disadvantage of significantly reducing the yield with increasing dendrimer generation and the accumulation of statistical defects in higher generations. A convergent procedure is usually used to simplify the synthesis and purification and provide better yields of high-generation dendrimers. A convergent approach to the preparation of dendrimer structures is based on the production of branched compounds bearing the active functional group, which can be involved in covalent attachment with the core molecule. A vast number of multifunctional compounds can be used as cores for dendrimers, such as biphenyl, terphenyl, pentaphenylene, and triazine core molecules. Scheme 6 [6] shows the use of tosyl-decorated sulfonimide dendrons to synthesize terphenyl-cored dendrimers using double Suzuki cross-coupling (SCC) reaction.
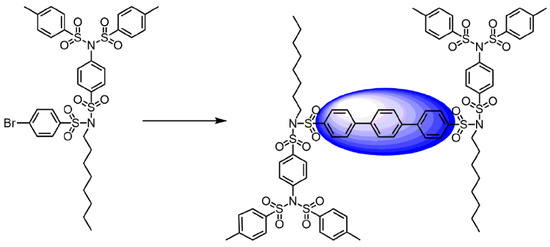
Scheme 6. Synthesis of a terphenyl-cored SBDs using 1,4-phenylene diboronic acid diester, Pd[(P(pTol)3]3, THF/H2O.
Surprisingly, sulfonimide derivatives with nitroaromatic groups on the periphery do not react under Suzuki conditions. Although there were several reports of dendrimers obtained by such a one-stage assembly method, they did not receive further development due to the following restrictions: getting hyperbranched polymers [50] with an increase in molecular weight (>2 kDa) instead of dendrimers, or poor chemical stability [51]. Thus, the development of simple methods for obtaining structurally advanced high molecular weight dendrimers remains a problem. Using the previously reported stepwise persulfonylation of octylamine with various sulfonyl chlorides by sequential reactions, a G4 dendron with a 4-bromobiphenylsulfonyl unit in the focal position was achieved. The double cross-coupling reaction of the dendrons with 1,4-phenylenediboronic acid can give the pentaphenylene cored dendrimer with a molar mass exceeding 10 kDa [52].
Rozhkov et al. proposed the synthesis of new branched arylsulfonyl chlorides, which can be further used as reagents for the convergent synthesis of SBDs [53]. As shown in Scheme 7, the synthesis begins with the target molecule, in which the more reactive nitro groups located in the ortho position are used for substitution. The para position’s remaining nitro group was reduced to the amine group using tin (II) chloride. The amine was persulfonylated in a single step with tosyl chloride to give the sulfonimide, which was further converted to bis-sulfonyl chloride with a chlorinating reagent.
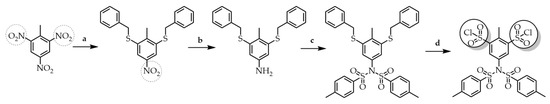
Scheme 7. Synthesis of bis-sulfonyl chloride. (a) benzyl mercaptan, K2CO3, DMF (b) SnCl2, i-PrOH (c) CH3C6H4SO2Cl, Et3N, CH2Cl2 (d) N-chlorosuccinimide, HCl, CH3COOH.
The new sulfonimide branched arylsulfonyl chlorides, which can be useful reagents for the convergent synthesis of multifunctionalized core compounds, were obtained in good yield and purity. [53] The convergent route reaches its limit at G3 species, whereas the divergent approach can prepare higher-generation sulfonimide-based dendrons.
3.3. Click Chemistry
Tewari et al. synthesized azide-functionalized persulfonylated termini with sulfonimide groups, as outlined in Scheme 8 [54], which were linked together using click chemistry [55]. However, only the G1 dendrimers were obtained using this procedure, emphasizing the initial divergent or convergent approaches' importance.
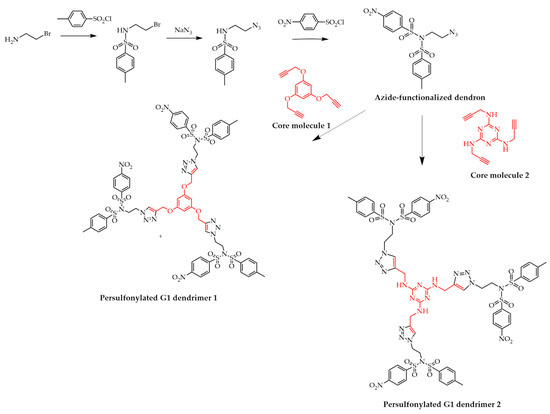
Scheme 8. Synthesis of persulfonylated azide-functionalized dendron and the convergent synthesis of persulfonylated G1 dendrimers.
The synthesis of dendrimers and dendrons with sulfonimide units at each branch point is based on a series of repeating sulfonylation reactions of amines and sulfonamides followed by reduction of the nitro group into an amino group. Such a combination allows precise control of the form of dendrimers, the number of branches in each generation, and their peripheral design with functional groups. Using the selectivity inherent in reactions eliminates the group's sophisticated protection strategy. Currently, dendrimers' construction and functionalization have been achieved through a set of high yielding, fast, inexpensive, and operationally simple. Therefore, sulfone-based moieties in that category, especially N-monosulfonylation and N-bissulfonylation-based reactions, fulfill all the high-efficiency criteria and cost-effective novel dendrimer synthesis. Nevertheless, the synthesis of SBDs is limited to the G5 and requires further development. Since there has been exponential growth in newly synthesized chemical compounds [56], their preparation methods are still essential. Research and development aimed at improving the synthesis have not lost their relevance. The lack of chemical diversity of SBDs and the limited synthesis methods currently available show that the chemistry of SBDs has not been sufficiently studied. In addition to increasing the diversity of structures and increasing the yield of synthesis reactions, special attention must be paid to dendrimers with large aromatic and form-stable branches. It is expected that such rigid, inert structures will have high melting and glass transition temperatures, opening up new possibilities for chemical modification and application. Despite numerous works on the synthesis of dendrimers, much work remains to be done in this regard. As indicated by the number of works and the results obtained, the divergent method remains the preferred synthesis method today, but this does not reduce other approaches' importance. There is still a need for even higher generations of all types of dendrimers, as well as for high-performance and optimized syntheses of individual families of dendrimers to access targeted mono- and multifunctionalized products. The development of various new and innovative building blocks for the synthesis of dendrimers is also important.
4. Purification and Separation
SBDs are easy to handle and do not require difficult purification procedures. The purification process improves the material's monodispersity by removing the molecules with defects in their structures. This is important because the presence of defect structures due to incomplete synthetic reactions or by-processes during the divergent synthesis can affect the observed material properties. Notably, chromatographic purification can be used for SBDs starting with the G3 species; all dendrimeric molecules of lower generations require recrystallization [48]. As already mentioned, these dendrimers are mostly obtained by divergent techniques, which can lead to defects in high-generation dendrimers occurring from incomplete addition reactions during the synthesis or from side processes. Therefore, at some stages, a mixture of products with different molecular weights can be obtained. Some techniques can be used for the separation of a mixture of dendrimers, such as gel permeation chromatography (GPC), high-performance liquid chromatography (HPLC), and simple thin-layer chromatography (TLC) [57]. GPC is impractical for the separation of mixtures of isographic and non-isographic isomers. Unlike GPC, HPLC with a preparative column can be used to separate isographic isomers but with very low efficiency, and it is not applicable for non-isographic isomers. The same situation is seen for silica gel thin-layer chromatography (TLC). Non-isographic isomers have an equal Rf value, and separation is impossible [7]. In contrast, a mixture of the isographic isomers showed noticeable separation on a TLC plate. This implies that the isographic isomers have different hydrodynamic volumes and different polarities and affinities for the stationary phase.
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123746269.
- Liu, X.; Gregurec, D.; Irigoyen, J.; Martinez, A.; Moya, S.; Ciganda, R.; Hermange, P.; Ruiz, J.; Astruc, D. Precise localization of metal nanoparticles in dendrimer nanosnakes or inner periphery and consequences in catalysis. Nat. Commun. 2016, 7, 1–8, doi:10.1038/ncomms13152.
- Walter, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593–4609, doi:10.1039/c2cs35062a.
- McCarthy, T.D.; Karellas, P.; Henderson, S.A.; Giannis, M.; O’Keefe, D.F.; Heery, G.; Paull, J.R.A.; Matthews, B.R.; Holan, G. Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2005, 2, 312–318, doi:10.1021/mp050023q.
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150, doi:10.4103/0975-7406.130965.
- Lukin, O.; Gramlich, V.; Kandre, R.; Zhun, I.; Felder, T.; Schalley, C.A.; Dolgonos, G. Designer Dendrimers: Branched Oligosulfonimides with Controllable Molecular Architectures. J. Am. Chem. Soc. 2006, 128, 8964–8974, doi:10.1021/ja061606b.
- Schubert, D.; Corda, M.; Lukin, O.; Brusilowskij, B.; Fiškin, E.; Schalley, C.A. A topological view of isomeric dendrimers. Eur. J. Org. Chem. 2008, 4148–4156, doi:10.1002/ejoc.200800408.
- Najafi, F.; Salami‐Kalajahi, M.; Roghani‐Mamaqani, H.; Kahaie‐Khosrowshahi, A. A comparative study on solubility improvement of tetracycline and dexamethasone by poly(propylene imine) and polyamidoamine dendrimers: An insight into cytotoxicity and cell proliferation. J. Biomed. Mater. Res. Part A 2020, 108, 485–495, doi:10.1002/jbm.a.36830.
- Dvornic, P.R. PAMAMOS: The first commercial silicon-containing dendrimers and their applications. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2755–2773, doi:10.1002/pola.21368.
- Najafi, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Kahaie-Khosrowshahi, A. Effect of grafting ratio of poly(propylene imine) dendrimer onto gold nanoparticles on the properties of colloidal hybrids, their DOX loading and release behavior and cytotoxicity. Colloids Surf. B Biointerfaces 2019, 178, 500–507, doi:10.1016/j.colsurfb.2019.03.050.
- Choi, J.S.; Joo, D.K.; Kim, C.H.; Kim, K.; Park, J.S. Synthesis of a barbell-like triblock copolymer, poly(L-lysine) dendrimer-block-poly(ethylene glycol)-block-poly(L-lysine) dendrimer, and its self-assembly with plasmid DNA. J. Am. Chem. Soc. 2000, 122, 474–480, doi:10.1021/ja9931473.
- Weng, Z.; Zaera, F. Synthesis of Chiral Dendrimer-Encapsulated Nanoparticle (DEN) Catalysts. Top. Catal. 2018, 61, 902–914, doi:10.1007/s11244-018-0955-9.
- Cao, Y.; Nguyen, G.K.T.; Chuah, S.; Tam, J.P.; Liu, C.F. Butelase-Mediated Ligation as an Efficient Bioconjugation Method for the Synthesis of Peptide Dendrimers. Bioconjugate Chem. 2016, 27, 2592–2596, doi:10.1021/acs.bioconjchem.6b00538.
- Chen, F.; Kong, L.; Wang, L.; Fan, Y.; Shen, M.; Shi, X. Construction of core-shell tecto dendrimers based on supramolecular host-guest assembly for enhanced gene delivery. J. Mater. Chem. B 2017, 5, 8459–8466, doi:10.1039/c7tb02585h.
- Yang, S.; Zhang, H.; Liu, Q.; Sun, S.; Lei, P.; Zhao, Z.; Wu, L.; Wang, Y. The synthesis and biological evaluation of chondroitin sulfate e glycodendrimers. Future Med. Chem. 2019, 11, 1403–1415, doi:10.4155/fmc-2019-0011.
- Li, X.; Takeda, K.; Yuba, E.; Harada, A.; Kono, K. Preparation of PEG-modified PAMAM dendrimers having a gold nanorod core and their application to photothermal therapy. J. Mater. Chem. B 2014, 2, 4167–4176, doi:10.1039/c4tb00132j.
- Gupta, A.; Dubey, S.; Mishra, M.; Gupta, M.A. Unique Structures, Properties and Applications of Dendrimers. J. Drug Deliv. Ther. 2018, 8, 328–339, doi:10.22270/jddt.v8i6-s.2083.
- Bondareva, J.; Luchkin, S.; Dagesyan, S.; Egorov, A.; Evlashin, S.; Lukin, O. Covalent and noncovalent films made up of sulfonimide-based dendrimers. Appl. Surf. Sci. 2020, 146345, doi:10.1016/j.apsusc.2020.146345.
- Rajabi, M.A.; Mousa, S. Lipid Nanoparticles and their Application in Nanomedicine. Curr. Pharm. Biotechnol. 2016, 17, 662–672, doi:10.2174/1389201017666160415155457.
- Bondareva, J.; Rozhkov, V.; Kachala, V.V.; Fetyukhin, V.; Lukin, O. An optimized divergent synthesis of sulfonimide-based dendrimers achieving the fifth generation. Synth. Commun. 2019, 49, 3536–3545, doi:10.1080/00397911.2019.1676909.
- Chourasiya, S.S.; Patel, D.R.; Nagaraja, C.M.; Chakraborti, A.K.; Bharatam, P.V. Sulfonamide: Vs. sulfonimide: Tautomerism and electronic structure analysis of N-heterocyclic arenesulfonamides. New J. Chem. 2017, 41, 8118–8129, doi:10.1039/c7nj01353a.
- Lill, S.O.N.; Broo, A. Molecule VI: Sulfonimide or sulfonamide? Cryst. Growth Des. 2014, 14, 3704–3710, doi:10.1021/cg500916u.
- Rothermel, K.; Žabka, M.; Hioe, J.; Gschwind, R.M. Disulfonimides versus Phosphoric Acids in Brønsted Acid Catalysis: The Effect of Weak Hydrogen Bonds and Multiple Acceptors on Complex Structures and Reactivity. J. Org. Chem. 2019, 84, 13221–13231, doi:10.1021/acs.joc.9b01811.
- Hornback, J.M. Organic Chemistry; Thomson: Boston, MA, USA 2005; ISBN 9780534389512.
- Craig, C.R.; Stitzel, R.E. Modern Pharmacology with Clinical Applications; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; ISBN 9780781737623.
- Boucher, B.A.; Hevener, K.E. Chapter 6: Medicinal Chemistry. In The APhA Complete Review for the Foreign Pharmacy Graduate Equivalency Examination, 2nd ed.; The American Pharmacists Association: Washington, DC, USA, 2018; doi:10.21019/9781582122984.ch6.
- Harrold, M.W.; Zavod, R.M. Basic Concepts in Medicinal Chemistry. Drug Dev. Ind. Pharm. 2014, 40, 988–988, doi:10.3109/03639045.2013.789908.
- Alfei, S.; Castellaro, S.; Taptue, G.B. Synthesis and NMR characterization of dendrimers based on 2, 2-bis-(hydroxymethyl)-propanoic acid (bis-HMPA) containing peripheral amino acid residues for gene transfection. Org. Commun. 2017, 10, 144–177, doi:10.25135/acg.oc.22.17.06.034.
- Alfei, S.; Castellaro, S. Synthesis and characterization of polyester-based dendrimers containing peripheral arginine or mixed amino acids as potential vectors for gene and drug delivery. Macromol. Res. 2017, 25, 1172–1186, doi:10.1007/s13233-017-5160-3.
- Alfei, S.; Catena, S. Synthesis and characterization of fourth generation polyester-based dendrimers with cationic amino acids-modified crown as promising water soluble biomedical devices. Polym. Adv. Technol. 2018, 29, 2735–2749, doi:10.1002/pat.4396.
- Schito, A.M.; Alfei, S. Antibacterial Activity of Non-Cytotoxic, Amino Acid-Modified Polycationic Dendrimers against Pseudomonas aeruginosa and Other Non-Fermenting Gram-Negative Bacteria. Polymers 2020, 12, 1818, doi:10.3390/polym12081818.
- Pina, F.; Passaniti, P.; Maestri, M.; Balzani, V.; Vögtle, F.; Gorka, M.; Lee, S.K.; Van Heyst, J.; Fakhrnabavi, H. Ground and excited-state electronic interactions in poly(propylene amine) dendrimers functionalized with naphthyl units: Effect of protonation and metal complexation. ChemPhysChem 2004, 5, 473–480, doi:10.1002/cphc.200301030.
- Mallakpour, S.; Zandi, H. Synthesis and Characterization of Novel Photoactive Polyamides Containing Naphthalene Moieties. Iran. Polym. J. 2006, 15, 8 (74); 619–927.
- Saudan, C.; Balzani, V.; Ceroni, P.; Gorka, M.; Maestri, M.; Vicinelli, V.; Vögtle, F. Dendrimers with a cyclam core. Absorption spectra, multiple luminescence, and effect of protonation. Tetrahedron 2003, 59, 3845–3852, doi:10.1016/S0040-4020(03)00434-4.
- Wegner, V.G. Topochemische reaktionen von monomeren mit konjugierten dreifachbindungen. II. Mitt.: Eine topochemische farb- und vernetzungsreaktion bei polymeren mit konjugierten acetylengruppen im grundbaustein. Die Makromol. Chem. 1970, 134, 219–229, doi:10.1002/macp.1970.021340119.
- Abdel-Rahman, M.A.; Schweizer, B.W.; Lukin, O.; Zhang, A.; Schlüter, A.D. Dendronized Polymers with Aromatic Sulfonimide Dendrons. Macromol. Chem. Phys. 2010, 211, 1538–1549, doi:10.1002/macp.201000031.
- Jansook, P.; Kurkov, S.V.; Loftsson, T. Cyclodextrins as solubilizers: Formation of complex aggregates. J. Pharm. Sci. 2010, 99, 719–729, doi:10.1002/jps.21861.
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307, doi:10.1016/j.progpolymsci.2013.07.005.
- Buhleier, E.; Wehner, W.; Vogtle, F. Cascade-chain-like and nonskid-chain-like syntheses of molecular cavity topologies. Synthesis 1978, 2, 155–158.
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry: Concepts, Syntheses, Properties, Applications; John Wiley and Sons: Hoboken, NJ, USA, 2009; ISBN 9783527320660.
- Vögtle, F.; Fakhrnabavi, H.; Lukin, O. Controllable, selective per-functionalization of dendritic oligoamines. Org. Lett. 2004, 6, 1075–1078, doi:10.1021/ol030130n.
- Newkome, G.R.; George R.; Moorefield, C.N.; Charles N.; Vögtle, F. Dendrimers and Dendrons: Concepts, Syntheses, Applications; Wiley-VCH: Weinheim, Germany, 2001; ISBN 9783527299973.
- de Brabander-van den Berg, E.M.M.; Meijer, E.W. Poly(propylene imine) Dendrimers: Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenations. Angew. Chem. Int. Ed. Engl. 1993, 32, 1308–1311, doi:10.1002/anie.199313081.
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic macromolecules: Synthesis of starburst dendrimers. Macromolecules 1986, 19, 2466–2468, doi:10.1021/ma00163a029.
- Vögtle, F.; Fakhrnabavi, H.; Lukin, O.; Müller, S.; Friedhofen, J.; Schalley, C.A. Towards a selective functionalization of ammo-terminated dendrimers. Eur. J. Org. Chem. 2004, 4717–4724, doi:10.1002/ejoc.200400472.
- De Christopher, P.J.; Adamek, J.P.; Lyon, G.D.; Klein, S.A.; Baumgarten, R.J. Simple Deaminations. V. Preparation and Some Properties of N-Alkyl-N,N-disulfonimides. J. Org. Chem. 1974, 39, 3525–3532, doi:10.1021/jo00938a017.
- Caminade, A.M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.P. “Janus” dendrimers: Syntheses and properties. New J. Chem. 2012, 36, 217–226, doi:10.1039/c1nj20458k.
- Lukin, O.; Schubert, D.; Müller, C.; Corda, M.; Kandre, R. Persulfonylation of amines applied to the synthesis of higher generation dendrimers. J. Org. Chem. 2008, 73, 3562–3565, doi:10.1021/jo800179m.
- Antoni, P.; Nyström, D.; Hawker, C.J.; Hult, A.; Malkoch, M. A chemoselective approach for the accelerated synthesis of well-defined dendritic architectures. Chem. Commun. 2007, 2249–2251, doi:10.1039/b703547k.
- Okaniwa, M.; Takeuchi, K.; Asai, M.; Ueda, M. One-pot synthesis of dendritic poly(amide-urea)s via Curtius rearrangement. 2. Synthesis and characterization of dendritic poly(amide-urea)s. Macromolecules 2002, 35, 6232–6238, doi:10.1021/ma020388d.
- Ruiz, J.; Lafuente, G.; Marcen, S.; Ornelas, C.; Lazare, S.; Cloutet, E.; Blais, J.C.; Astruc, D. Construction of giant dendrimers using a tripodal building block. J. Am. Chem. Soc. 2003, 125, 7250–7257, doi:10.1021/ja021147o.
- Bergamini, G.; Ceroni, P.; Balzani, V.; Kandre, R.; Lukin, O. Dendrimers with a pentaphenylene core: A photophysical study. ChemPhysChem 2009, 10, 265–269, doi:10.1002/cphc.200800597.
- Rozhkov, V.V.; Kolotylo, M.V.; Onys’Ko, P.P.; Lukin, O. Synthesis of sulfonimide-based branched arylsulfonyl chlorides. Tetrahedron Lett. 2016, 57, 308–309, doi:10.1016/j.tetlet.2015.12.003.
- Khanam, S.; Pandey, S.K.; Rai, S.K.; Verma, D.; Jasinski, J.P.; Pratap, S.; Tewari, A.K. Synthesis of N,N -Bis-Sulfonylated and N -Alkyl- N -Sulfonylated G1 Dendrimers via Click Reaction: Application of Thiocarbamide based Cu I Catalysts. ChemistrySelect 2017, 2, 6370–6374, doi:10.1002/slct.201701591.
- Khanam, S.; Rai, S.K.; Tewari, A.K. Advancement in the sulfone-based dendrimers : From synthesis to application Advancement in the sulfone-based dendrimers : From synthesis to application. Adv. Mater. Lett. 2017, 8, 1005–1019, doi:10.5185/amlett.2017.1609.
- Binetti, R.; Costamagna, F.M.; Marcello, I. Exponential growth of new chemicals and evolution of information relevant to risk control. Ann. Ist. Super. Sanita. 2008, 44, 13–15, PMID: 18469371.
- Mullen, D.G.; Desai, A.; Van Dongen, M.A.; Barash, M.; Baker, J.R.; Holl, M.M.B. Best practices for purification and characterization of PAMAM dendrimer. Macromolecules 2012, 45, 5316–5320, doi:10.1021/ma300485p.




