| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joo Kheng Goh | + 1711 word(s) | 1711 | 2021-02-12 09:28:39 | | | |
| 2 | Catherine Yang | Meta information modification | 1711 | 2021-02-22 04:51:50 | | | | |
| 3 | Vicky Zhou | Meta information modification | 1711 | 2021-02-22 07:09:35 | | | | |
| 4 | Apinan Soottitantawat | -1 word(s) | 1710 | 2021-02-22 08:05:10 | | | | |
| 5 | Uracha Ruktanonchai | Meta information modification | 1710 | 2021-02-22 09:34:44 | | | | |
| 6 | Uracha Ruktanonchai | Meta information modification | 1710 | 2021-02-22 09:38:13 | | | | |
| 7 | Darren Low | Meta information modification | 1710 | 2021-02-22 13:03:13 | | |
Video Upload Options
Controlled Release Fertilizers (CRF) can be defined as “products containing sources of water-soluble nutrients, the release of which in the soil is controlled by a coating applied to the fertilizer. However, the most significant challenge that persists is the “tailing” effect, which reduces the economic benefits in terms of maximum fertilizer utilization. The high materials cost is also deemed as a significant obstacle restraining the widespread application of CRF in agriculture.
1. Introduction
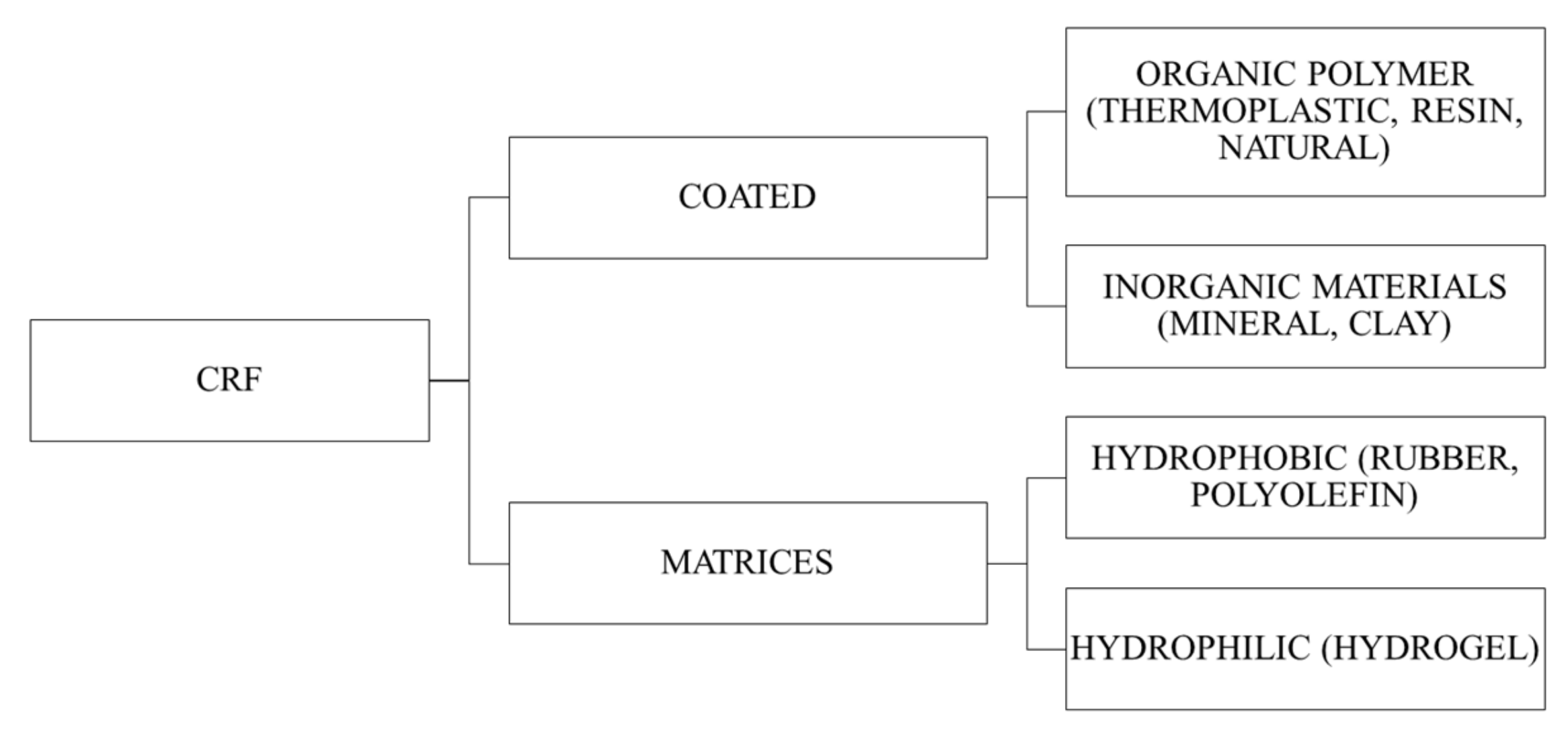
Slow release fertilizer (SRF) and controlled release fertilizer (CRF) are often used interchangeably. SRF is known as “low solubility compounds with a complex/high molecular weight chemical structure that release nutrients through either microbial or chemically decomposable compound” [1], where CRF can be defined as “products containing sources of water-soluble nutrients, the release of which in the soil is controlled by a coating applied to the fertilizer” [2]. SRFs are generally classified into condensation products of urea-aldehydes, fertilizers with a physical barrier (coated or incorporated into matrix) and super granules. CRF is a subset of SRF which falls under the category of fertilizer with a physical barrier. The simplified classification of CRFs is presented in Figure 1.[3][4]
Figure 1. Simplified classification of controlled release fertilizers (CRFs).
Although the required nutrient release rate by CRF varies for each plant depending on the metabolic requirements of the crop for a specified period, the European Standardization Committee (CEN) Task Force has made some recommendations on the criteria of CRF such that the rate of nutrient release must be slower than conventional fertilizer, not more than 15% of the nutrients are released within 24 h, not more than 75% of the nutrients are released within 28 days and at least 75% of the nutrients are released within the stated release time [5]. Some other factors that are required from a CRF include cost-effectiveness, being environmentally friendly and sustainability.
1.1. Advantages of CRF
The application of CRF can help to improve NUE and reduce nutrient loss, primarily through nitrate leaching and the volatilization of ammonia and nitrous oxides, which contribute to minimizing environmental pollution. It is also possible to decrease the fertilizer application rate by 20 to 30% of the recommended value to achieve the same yield [5][6]. This can provide economic advantages in terms of saving labor, time, and energy. In addition, when CRF releases nutrients at a desirable rate (preferably in a sigmoidal pattern), it contributes to agronomic safety by reducing the toxicity imposed to plants, especially seedlings [3][4]. This is because the conventional practice of chemical fertilizer application tends to result in the high local concentration of ions, which induces osmotic stress and causes damage to plants [3][4].
1.2. Disadvantages of CRF
There are still no standardized methods to determine the nutrient release rate from CRF in a reliable way. There is also a lack of correlation between data obtained from laboratory studies and the actual nutrient release rate in practical applications that can be made available to consumers [5]. In addition, CRFs have not always been compared to the best fertilizer management practices when reporting about their advantages [7]. Using a CRF such as Sulfur Coated Urea (SCU) in large quantities can increase soil acidity, while polymer-coated CRFs using synthetic materials may be difficult to degrade, which contributes to other forms of pollution. During the application of CRF, nutrients may continue to be released even in the absence of plants due to the tailing effect. The tailing effect occurs after 80–85% of the nutrients are released and the remaining nutrients are released in a prolonged manner [3]. Furthermore, the cost for manufacturing CRF today is still much higher compared to conventional chemical fertilizers, which restricts its widespread use in agriculture [5].
2. Important Factors Affecting CRFs
The release rate of CRFs is generally affected by the size, coating thickness and uniformity, the selection of materials as well as the selection of binder and filler for the formulation. For hydrogels, the temperature, pH, and ionic strength of the environment also affect the nutrient release rate.
2.1. Temperature
An increase in temperature reduces the duration of lag period and increases the linear rate of release [8]. Emami et al. [9] explained that as the temperature in the environment (soil) increases, the solubility of nutrients within the polymer and diffusion rate also increases, as diffusion coefficient is a function of temperature. In addition, pore size also increases with increasing temperature due to higher swelling, which results in higher release rates. It was also mentioned that as temperature increases by 15 °C, the release rate doubles. Bi et al. [10] reported that more rapid diffusion occurs at a temperature of 37 °C compared to 25 °C. They highlighted that the difference in temperature affects the degradation behavior in enzymatic environments. Similar findings were also reported by Uzoh et al. [11]. The temperature dependence of the linear release rate is represented by Equation (1):
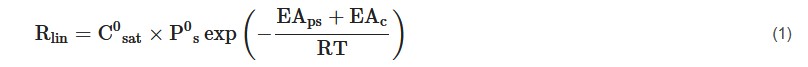
2.2. pH
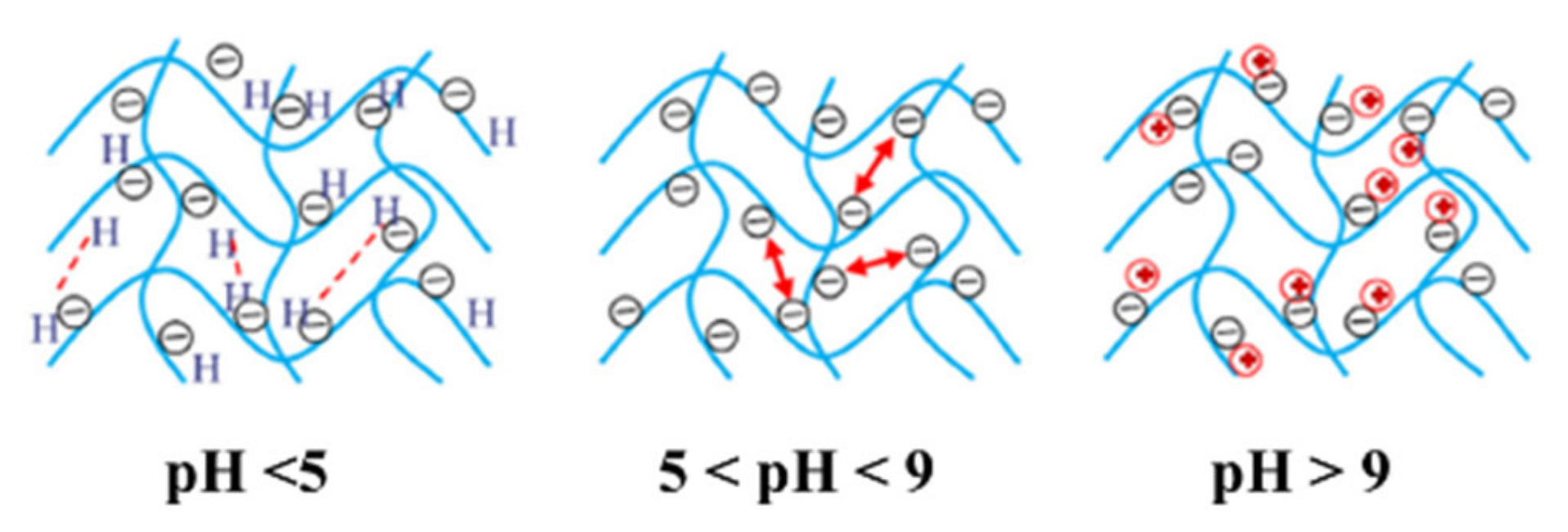
The acidic or alkaline nature of the release medium has a significant effect on the interactions of chemical species in the granule and the diffusion coefficient of the ions [12]. Emami et al. [9], Uzoh et al. [11], Rashidzadeh and Olad [13], Wen et al. [14], and Salimi et al. [15] reported that at an acidic environment (pH 2–5), there is a high concentration of H+ ions. This causes most of the carboxylate anions (COO−) to be protonated and prevents anion-anion electrostatic repulsion in the network, decreasing the swelling capacity. Similarly, at an alkaline environment (>pH 9), the presence of Na+ ions in the solution shields the COO− anion and prevents anion-anion electrostatic repulsion. Between pH 5–9, or in a more neutral condition, the swelling capacity was expected to be the highest as the COOH groups are converted to COO− ions, which maximizes electrostatic repulsion. Olad et al. [16] added that hydrogels are smart materials and respond well to pH as this work shows on-off swelling behaviors between pH 8 and pH 2, where slower release is observed in lower pHs with decreased swelling. The general behavior of hydrogel in different pH levels is shown in Figure 2.
2.3. Ionic Strength
From the studies mentioned in the previous section, swelling capacity in a salt solution (containing NaCl, KCl, CaCl2, FeCl3) was also shown to be significantly lower compared to distilled water. This is attributed to the difference in osmotic pressure, which decreases due to the charge screening effect of the cations which shields the COO− anions and reduces the repulsive force. The swelling capacity decreases in the order of Na+ > K+ > Ca2+ > Fe3+ [13][14][15]. With increasing charge (multivalent cations), it will form complexes with the carboxylate groups, which results in cross-linking points. This avoids the expansion of the hydrogel network, reducing swelling and the release rate.
2.4. Granule Radius and Coating Thickness
Shaviv et al. [17] presented a mathematical model to predict the three different stages of release. It was reported that the product of the radius and coating thickness is proportional to the lag period, while it is inversely proportional to the release rate in the linear and decay phase. The study suggested that by either increasing the radius or coating thickness, the lag period can be prolonged, and the release rate can be slowed down in both the linear and decay phases. Increasing the radius of the CRF is generally preferred in the interest of economic feasibility. However, there is always an optimum granule size required for the proper distribution of nutrients in the root zone.
3. Conclusions and Future Outlook
There is a growing number of researchers utilizing natural polymers for the formulation of CRFs. Binders and fillers play an important role in nutrient release pattern of CRFs as they can form compact structures, altering the properties of pore size and interacting with urea, which favors adsorption. Hydrophobic materials and coatings are essential for achieving controlled release properties of CRFs. The temperature, pH, and ionic strength of the environment significantly govern the nutrient release behavior. Mechanistic, empirical, and semi-empirical approaches have been commonly used for modelling nutrient release from CRFs. It should be mentioned that CRF is a broad field of study that is constantly changing and evolving, with multiple aspects yet to be examined and reviewed. Thus, the focus of future works can be narrowed down to focus on the utilization and formulation of low-cost biodegradable materials as a blend or with suitable binders that favor adsorption and provide sufficient hydrophobicity. Field testing under different environmental conditions is also needed to validate the results and to study the tailing effect of release towards the end-of-life of CRFs. The formulation of procedures and methods for upscaling should also be developed with the goal of bring CRFs to a point of practical and commercial application.
There are many patents located by Scopus that were filed on the invention of CRFs. They mainly focused on creating effective formulations to improve physical properties and increase the utilization rate of fertilizer nutrients. Recent patents showed that the majority of the inventions employed biodegradable coating materials such as polylactic acid (PLA), okara (soy pulp), linseed, polyurea and corn starch hydrogel [18][19][20][21][22], while some involved the use of polyurethane and resin . Fillers ranging from silicate, gypsum, corn starch, microcrystalline cellulose, bentonite, and other bio-based additives are often incorporated in the formulations. These CRFs are typically produced using different encapsulation techniques such as spray coating, drum coating and fluidized bed. The reported applications of CRF focus mainly on plant growth promotion of field crops such as wheat, rice, corn and cotton, and vegetables such as choy sum as well as ornamental plants. CRFs possess slow nutrient release patterns that match the needs of these crops. The patent findings demonstrate the commercial potential of CRFs as an alternative to existing agricultural fertilizers.
References
- Shaviv, A. Controlled release fertilizers. In Proceedings of the IFA International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt, Germany, 28–30 June 2005.
- AAPFCO. Official Publication No. 48; Association of American Plant Food Control Officials, Inc.: West Lafayette, IA, USA, 1995.
- Shaviv, A. Advances in controlled-release fertilizers. Adv. Agron. 2001, 71, 1–49.
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous overview on the controlled release fertilizers. Adv. Chem. 2014, 2014, 363071.
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture; IFA, International Fertilizer Industry Association: Berlin, Germany, 2010.
- Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Atares, S.; García, C.; Zotarelli, L.; San Bautista, A.; Vicente, O. Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice. Plants 2020, 9, 1183.
- Lammel, J. Cost of the different options available to the farmers: Current situation and prospects. In Proceedings of the IFA International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt, Germany, 28–30 June 2005.
- Du, C.-W.; Zhou, J.-M.; Shaviv, A. Release characteristics of nutrients from polymer-coated compound controlled release fertilizers. J. Polym. Environ. 2006, 14, 223–230.
- Emami, N.; Razmjou, A.; Noorisafa, F.; Korayem, A.H.; Zarrabi, A.; Ji, C. Fabrication of smart magnetic nanocomposite asymmetric membrane capsules for the controlled release of nitrate. Environ. Nanotechnol. Monit. Manag. 2017, 8, 233–243.
- Bi, S.; Barinelli, V.; Sobkowicz, M.J. Degradable Controlled Release Fertilizer Composite Prepared via Extrusion: Fabrication, Characterization, and Release Mechanisms. Polymers 2020, 12, 301.
- Uzoh, C.F.; Onukwuli, O.D.; Ozofor, I.H.; Odera, R.S. Encapsulation of urea with alkyd resin-starch membranes for controlled N2 release: Synthesis, characterization, morphology and optimum N2 release. Process. Saf. Environ. 2019, 121, 133–142.
- Basu, S.; Kumar, N.; Srivastava, J. Modeling NPK release from spherically coated fertilizer granules. Simul. Model. Pract. Theory 2010, 18, 820–835.
- Rashidzadeh, A.; Olad, A. Slow-released NPK fertilizer encapsulated by NaAlg-g-poly (AA-co-AAm)/MMT superabsorbent nanocomposite. Carbohydr. Polym. 2014, 114, 269–278.
- Wen, P.; Han, Y.; Wu, Z.; He, Y.; Ye, B.-C.; Wang, J. Rapid synthesis of a corncob-based semi-interpenetrating polymer network slow-release nitrogen fertilizer by microwave irradiation to control water and nutrient losses. Arab. J. Chem. 2017, 10, 922–934.
- Salimi, M.; Motamedi, E.; Motesharezedeh, B.; Hosseini, H.M.; Alikhani, H.A. Starch-g-poly (acrylic acid-co-acrylamide) composites reinforced with natural char nanoparticles toward environmentally benign slow-release urea fertilizers. J. Environ. Chem. Eng. 2020, 8, 103765.
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. C 2018, 90, 333–340.
- Shaviv, A.; Raban, S.; Zaidel, E. Modeling controlled nutrient release from polymer coated fertilizers: Diffusion release from single granules. Environ. Sci. Technol. 2003, 37, 2251–2256.
- Ambrose, R.P.K.; Wassgren, C.R.; Pai, D.; Chen, Y. Layer-Wise Agglomerated Urea Granules. U.S. Patent 16/704,342, 11 June 2020.
- Adhikari, R.; Muster, T.H.; Freischmidt, G. Controlled Release Granular Fertiliser. U.S. Patent 16/337,608, 30 January 2020.
- Li, J.; Jingling, Z.; Song, X.; Ong, C.N.; Loh, C.S.; Tan, W.K. Production of Nutrigel Materials from Soya Waste. U.S. Patent 16/643,177, 20 August 2020.
- Kannan, G.; Posada, C.; Haigh, J.; Harper, T.; Kanagalingam, S. Coated Granular Fertilizers, Methods of Manufacture Thereof, and Uses Thereof. U.S. Patent 10,233,133, 19 March 2019.
- Flores, J.; Bosley, M.A.; Rifai, S.; Mahoney, R.P.; Hajduk, D.; Casado Portilla, R.; Newton, T.; Soane, D.S. Nontoxic Coating Concentrates for Agricultural Uses. U.S. Patent 16/696,029, 23 July 2020.