| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gui-Li Yang | + 1677 word(s) | 1677 | 2021-01-27 04:09:35 | | | |
| 2 | Dean Liu | -605 word(s) | 1072 | 2021-02-20 08:53:52 | | |
Video Upload Options
Duckweed is the smallest and fastest-growing aquatic plant, and has advantages including simple processing and the ability to grow high biomass in smaller areas.
1. Introduction
Recently, plants have been considered as alternative bioreactors and have burgeoned the field of synthetic biology due to advantages such as product safety, inexpensive production cost, and easy scale-up[1][2][3]. Plant bioreactors are referred to as “chemical factories”, in which genetically modified crops are cultivated to produce biological agents with potential commercial values, such as vaccine antigens, antibodies, nutritional supplements, and industrial enzymes[4]. In the past two decades, some effective expression systems in plants have been widely investigated, and more than 100 recombinant proteins have been produced using diverse plant tissues[5]. For example, cholera toxin B subunit was transferred into potatoes that worked as edible vaccines[6][7]. Marquetblouin et al.[8] obtained the immunodominant antigen of measles virus by transforming it into carrots. Based on these results, transgenic plants might serve as effective expression systems for the production of recombinant proteins in the biopharmaceutical industry[9].
In-depth knowledge of plant molecular biology and gene engineering has led to the production of protein products using “naturalized bioreactors”. A multitude of plants have been utilized to produce recombinant proteins, such as tobacco[10], tomato[11], rice[12], and potato[13]. However, most of them are crops or terrestrial plants that compete with crops for land. Besides, genetically modified plants tend to cause gene drift, which is a great challenge for the industrialization of plant expression systems [14]. Therefore, the selection of a synthetic platform is essential to avoid food crisis and for maintaining the effective yield of recombinant products.
Duckweed is a tiny aquatic plant, which is easy to survive and widely distributed. The exponential growth of duckweed plays a vital role in the rapid production of proteins, resulting in a shorter production cycle[15]. Under certain conditions, the asexual reproduction of duckweed makes it genetically stable without genetic drift. Other desirable characteristics of large-scale production are simple and easy culture conditions[16]. In addition, duckweed is a non-crop plant that probably does not cause a food crisis. Therefore, duckweed could serve as a potential recombinant protein expression system. Dr. Anne-Marie Stomp of North Carolina State University was the first to propose the use of genetically modified duckweed as a carrier to produce recombinant proteins, which is still extensively researched[16][17][18].
2. Biological Characteristics of Duckweed
2.1. Inherent Characteristics of Duckweed
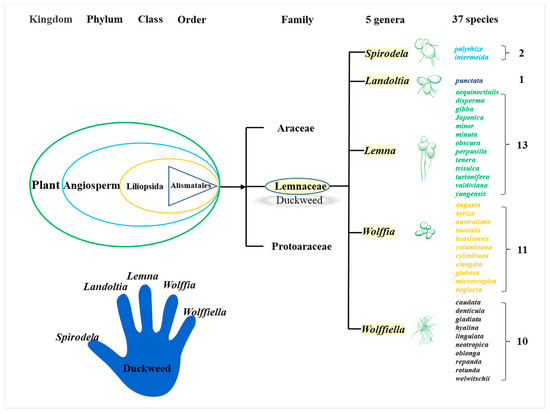
Duckweed is a monocotyledon, belonging to the Lemnaceae family, which consists of five genera (Spirodela, Landoltia, Lemna, Wolffia, and Wolffiella) and 37 species (Figure 1)[19]. Duckweed is the smallest flowerer globally, with a size of only a few millimeters. The biological structure of duckweed is simple, which is always bilobed, obovate, or elliptic [20]. Duckweed usually reproduces asexually with an extremely short cycle. The daughter plants of the duckweed are produced from the budding pouch of the mother plant (Figure 2). The exponential reproduction of duckweed results in a high biomass growth rate. Duckweed is able to adapt to a wide range of pH, and the optimum pH for growth is 4.5~7.2. Duckweed can also survive at temperatures ranging from 2 to 35 °C, with an optimum temperature of 25 °C for growth. These properties contribute to its wide distribution in natural water bodies. It grows in paddy fields, ponds, lakes, and other static waters[19][21].
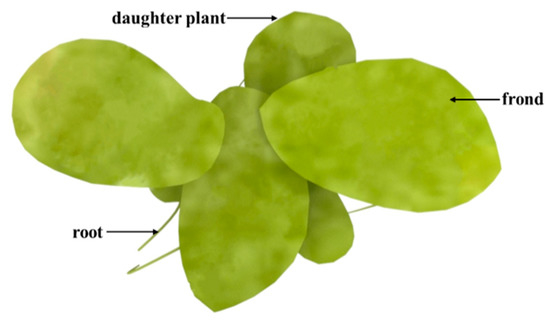
Figure 2. Schematic diagram of Landotia punctata.
2.2. Culture Conditions of Duckweed
For the preservation of germplasm resources, the pure culture of whole duckweed plants has been established[24]. Duckweed can grow on a variety of carbon sources supplied with essential nutrients, although different growth rates are achieved[25]. Sucrose or other sugars supplemented with the medium at concentrations of 0.5%~3% and periodic or continuous artificial light provides duckweed with vigorous growth[26]. The length and replenishment time of the photoperiod differ from species to species of duckweed and depend on the intensity of the light provided. Duckweed prefers stationary culture conditions because the mechanical agitation of the medium deteriorates its growth. The optimum temperature for the laboratory culture also varies among species; however, growth at room temperature is economical for its cultivation[16]. Therefore, high biomass/unit time of genetically homogeneous duckweed can be produced by using appropriate media under the confined environment.
3. Conclusions and Prospects
After Barbara Hoppen first proposed “synthetic biology” to describe the genetically engineered bacteria in 1980[27], a variety of natural products and their precursors have been achieved in microorganisms. Plants, multicellular organisms, possessing abundant inner membrane systems, and organelles with complex temporal and spatial characteristics, provide the necessary foundation for the synthesis of numerous enzymes and metabolites[28]. A plant bioreactor is a plant cell or whole plant processed by transgenic engineering to produce biological derivatives or biological products with a high added value for multiple uses[9]. Previous studies have reported several incidents of the release of genetically modified organisms (GMOs) and contamination of food supply. Therefore, the control of GMO products is an important challenge for utilizing the plant gene expression system. It not only leads to food crisis but also restricts industrialization. Therefore, plants with easy cultivation, simple genetic background, high yield, and low cost should be selected[29]. Duckweeds are an ideal “chassis plant” for synthetic biology, and they provide a promising bioproduction platform to produce polymers, proteins, and small molecules[16][30].
In recent years, duckweed has shown potential in biosynthesis to act as a bioreactor, with advantages such as high protein yield and stable storage of target proteins. It can be used as a biosynthesis platform for the production of vaccines, antibodies, pharmaceutical proteins, and industrial enzyme preparations. An in-depth study of the whole-genome sequencing of duckweeds will help to understand the biology of duckweed species and to use them for producing biomass. The establishment of the complete plant tissue culture system and the genetic transformation system of duckweed effectively combine the theory and application, enabling the rapid development of duckweed synthetic biology. Gene engineering technology has been widely used in the synthetic biology of duckweed. Finally, several researchers have successfully integrated multiple exogenous protein genes into the duckweed genome and made it to express corresponding target products, which is a breakthrough in the biosynthesis of duckweed as a new bioreactor.
To sum up, duckweed is a tiny aquatic angiosperm with great application prospects, which can be regarded as a new bioreactor suitable for various studies and applications. With further improvement in genome sequencing and the genetic transformation system of duckweed, more biological products will be produced by using genetic engineering of duckweed.
References
- Sharma, A.K.; Sharma, M.K. Plants as bioreactors: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 811.
- Rybicki, E.P. Plant-produced vaccines: Promise and reality. Drug Discov. Today 2009, 14, 16–24.
- Goyal, R.; Sharma, R.; Lal, P.; Ramachandran, V.G. Edible vaccines: Current status and future. Indian J. Med. Microbiol. 2007, 25, 93–102.
- He, Z.Q.; Du, X.; Yao, W.; Dai, J. Pharmaceutical proteins produced in plant bioreactor in recent years. Afr. J. Biotechnol. 2008, 7, 4917–4925.
- Tiwari, S.; Verma, P.C.; Singh, P.K.; Tuli, R. Plants as bioreactors for the production of vaccine antigens. Biotechnol. Adv. 2009, 27, 449–467.
- Jani, D.; Meena, L.S.; Rizwan-Ul-Haq, Q.M.; Singh, Y.; Sharma, A.K.; Tyagi, A.K. Expression of cholera toxin B subunit in transgenic tomato plants. Transgenic Res. 2001, 11, 447–454.
- Langridge, W.H.R. A plant-based multicomponent vaccine protects mice from enteric diseases. Nat. Biotechnol. 2001, 19, 548.
- Marquet-Blouin, E.; Bouche, F.; Steinmetz, A.; Muller, C. Neutralizing immunogenicity of transgenic carrot (Daucus carota L.)-derived measles virus hemagglutinin. Plant Mol. Biol. 2003, 51, 459–469.
- Giddings, G. Transgenic plants as factories for biopharmaceuticals. Nat. Biotechnol. 2000, 18, 1151.
- Tremblay, R.; Wang, D.; Jevnikar, A.M.; Ma, S. Tobacco, a highly efficient green bioreactor for production of therapeutic proteins. Biotechnol. Adv. 2010, 28, 214–221.
- Yano, M.; Hirai, T.; Kato, K.; Hiwasa-Tanase, K.; Fukuda, N.; Ezura, H. Tomato is a suitable material for producing recombinant miraculin protein in genetically stable manner. Plant Sci. 2010, 178, 469–473.
- Kim, Y.M.; Lee, J.-Y.; Lee, T.; Lee, Y.-H.; Kim, S.-H.; Kang, S.-H.; Yoon, U.-H.; Ha, S.-H.; Lim, S.-H. The suppression of the glutelin storage protein gene in transgenic rice seeds results in a higher yield of recombinant protein. Plant Biotechnol. Rep. 2012, 6, 347–353.
- Jane, B.; Lancet, B.J. Progress in potato-based vaccine for hepatitis B. Lancet 2000, 356, 1661.
- Nguyen, L.V.; Cox, K.M.; Ke, J.S.; Peele, C.G.; Dickey, L.F. Genetic engineering of a Lemna isoleucine auxotroph. Transgenic Res. 2012, 21, 1071–1083.
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.-J. Relative in vitro growth rates of duckweeds (Lemnaceae)–the most rapidly growing higher plants. Plant Biol. 2015, 17, 33–41.
- Stomp, A.M. The duckweeds: A valuable plant for biomanufacturing. Biotechnol. Annu. Rev. 2005, 11, 69.
- Stomp, A.M.; Rajbhandari, N. Genetically Engineered Duckweed. U.S. Patent EP2283721(A3), 31 October 2012.
- Yamamoto, Y.T.; Rajbhandari, N.; Lin, X.; Bergmann, B.A.; Nishimura, Y.; Stomp, A.-M. Genetic transformation of duckweed Lemna gibba and Lemna minor. In Vitro Cell Dev. Biol. Plant 2001, 37, 349–353.
- Les, D.H.; Crawford, D.J.; Landolt, E.; Gabel, J.D.; Kimball, R.T. Phylogeny and systematics of Lemnaceae, the duckweed family. Syst. Bot. 2002, 27, 221–240.
- Klaus, J.A.; Nikolai, B.; Eric, L. Telling duckweed apart: Genotyping technologies for the Lemnaceae. Chin. J. Appl. Environ. Biol. 2013, 19, 1–10.
- Xu, Y.L.; Ma, S.; Huang, M.; Peng, M.; Bog, M.; Sree, K.S.; Appenroth, K.-J.; Zhang, J. Species distribution, genetic diversity and barcoding in the duckweed family (Lemnaceae). Hydrobiologia 2015, 743, 75–87.
- Les, D.H.; Crawford, D.J. Landoltia (Lemnaceae), a new genus of duckweeds. Novon 1999, 9, 530–533.
- An, D.; Li, C.; Zhou, Y.; Wu, Y.; Wang, W. Genomes and transcriptomes of duckweeds. Front. Chem. 2018, 6, 230.
- Hillman, W.S. The Lemnaceae or duckweeds. A review of the descriptive and experimental literature. Bot. Rev. 1961, 27, 221–287.
- Thompson, B.G. The maximization of the productivity of aquatic plants for use in controlled ecological life support systems (CELSS). Acta Astronaut. 1989, 19, 269–273.
- Frick, H. Callogenesis and carbohydrate utilization in Lemna minor 1. J. Plant Physiol. 1991, 137, 397–401.
- Hobom, B. Gene surgery: On the threshold of synthetic biology. Med. Klin. 1980, 75, 834.
- Fischer, R.; Emans, N. Molecular farming of pharmaceutical proteins. Transgenic Res. 2000, 9, 279–299.
- Daniell, H.; Streatfield, S.J.; Wycoff, K. Medical molecular farming: Production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci. 2001, 6, 219–226.
- Dickey, L.; Gasdaska, J.; Cox, K. Expression of Biologically Active Polypeptides in Duckweed. U.S. Patent 6,815,184, 11 September 2004.
- Xu, J.; Zhao, H.; Stomp, A.-M.; Cheng, J.J. The production of duckweed as a source of biofuels. Biofuels 2012, 3, 589–601.
- Wang, W.; Messing, J. Status of duckweed genomics and transcriptomics. Plant Biol. 2014, 17, 10–15.
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective. Chemosphere 2019, 223, 285–309.