
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stuart A Walters | + 2877 word(s) | 2877 | 2021-02-19 07:52:43 | | | |
| 2 | Camila Xu | Meta information modification | 2877 | 2021-03-18 03:49:53 | | |
Video Upload Options
Horseradish cultivars are highly heterozygous clones and are maintained through asexual propagation, using root cuttings.
1. Introduction
1.1. Horseradish Production Areas
Horseradish (Armoracia rusticana) is a large leaved, hardy perennial herb that is in the Brassicaceae family and native to southeastern Europe and western Asia [1][2]. Although horseradish is produced primarily in the United States and Europe, horseradish is now grown in many different temperate regions of the world. There is now also significant production in some other temperate regions including Canada, China, and South Africa [3][4]. The United States is a large producer of horseradish with the state of Illinois producing the most with about 800 ha [2][5]. The area east of St. Louis, Missouri, is considered the most concentrated horseradish production region in the world [3][5]; and Collinsville, Illinois, advertises itself as the “Horseradish Capital of the World”. Other major horseradish production areas in the United States include Eau Claire, Wisconsin, and Tulelake, California, with smaller-scale production occurring in Minnesota, Michigan, Ohio, Pennsylvania, New Jersey, Connecticut, Massachusetts, Oregon and Washington among others [2][5]. Horseradish is also produced in several European countries including Austria, Germany, Hungary, Czech Republic, and Poland with about 3000 ha in production [2].
1.2. Horseradish Propagation for Field Production
Horseradish is highly heterozygous and will not reproduce true to type from seed. It is tetraploid and the somatic number of chromosomes is 32 (2 n = 4 × = 32). Since horseradish cultivars are highly heterozygous clones, the only way to maintain a particular selection is through asexual propagation, using root cuttings. Thus, horseradish is commercially propagated clonally from root cuttings to maintain specific genotypes [1][2][5]. Horseradish seed is only used for breeding efforts to obtain new genotypes and sexual propagation procedures have been refined for cultivar improvement in Illinois, USA.
1.3. Lack of Horseradish Cultivar Development
The development of new improved horseradish cultivars has been somewhat limited by the lack of viable seed resulting from low fertility of horseradish flowers [2][4]. For many years, horseradish was believed to be sterile and therefore impossible to improve by traditional sexual crosses. Prior to the 20th century, the only way to improve horseradish was to select and plant root cuttings from the most desirable plants [6]. Although horseradish flowers have low fertility, viable seed can be produced in certain instances [6][7][8], and cross breeding unrelated clones, will often result in greater amount of seed production. In Poland, slightly greater numbers of horseradish seed can be obtained through cross-breeding plants from geographically remote areas, although this technique still only results in the production of few seed [9].
Until the 1960s, horseradish production in North America was confined to the production of three cultivars, ‘Common’, ‘Swiss’ and ‘Big Top Western’ [10], although ‘Sass’ was also grown in limited amounts in Illinois due to its high-yielding characteristics [11]. Due to this narrow genetic base, a horseradish breeding program was initiated in Illinois to develop additional cultivars to prevent widespread losses to diseases, insects or other possible calamities [6].
1.4. Overcoming Pollination Barriers for Horseradish Breeding
Although horseradish plants are believed to lack significant seed production due to male-sterility [8][9], Walters et al. [4] found that horseradish clones evaluated in Illinois, USA all produced viable pollen, but differed in their ability to recognize and reject their own pollen and form capsules with viable seed. Additionally, 74% of these horseradish clones evaluated exhibited some level of self-incompatibility, and >80% of self-pollinations resulted in some type of abnormal capsule development containing no seed or a large portion of non-viable seed. Horseradish is generally described as sterile because it normally does not form pods filled with viable seeds or only develops seed in very limited quantities [4].
Sexual propagation procedures to obtain viable seed from clonal crosses of horseradish were initiated in Wisconsin, USA during the late 1940s. Weber [8] found that increased seed development occurred on ‘Common’ horseradish if functional pollen from ‘Bohemian’ was placed on receptive ‘Common’ stigmas; and, this cross was the most effective way of collecting large amounts of viable seed. Although ‘Common’ is completely pollen-sterile, with only about 5% of the ovules containing normal functional gametophytes, the placement of ‘Bohemian’ pollen onto ‘Common’ stigmas induced seed formation [8][12]. In Illinois, USA, the problem of non-viable seed production in horseradish was originally overcome by following the suggestions of Weber [8] by using ‘Common’ as the female parent and ‘Bohemian’, ‘Big Top Western’, and ‘Sass’ as male parents [6]. The various seedlings developed from these crosses has really formed the foundation for the Illinois, USA horseradish breeding program. The continued interbreeding and selection of progeny from these original crosses has led to the development of most cultivars used in Illinois, USA today, with many that are either partially or fully self-compatible. Although horseradish seed can easily be produced today from many of the cultivars, breeding lines or other germplasm materials used in making crosses, the number and viability of seed obtained differs among the specific crosses that are made due to sexual compatibility between different clones.
2. Germplasm Resources
Most of the germplasm available for improvement of horseradish is located in Eastern Europe and Asia. Although only A. rusticana clones have been used for the genetic improvement of horseradish, both of the wild allied species (A. macrocarpa and A. sisymbroides) have potential as sources of donor genes for improvement of the horseradish genome and are also tetraploid. This is especially true for A. macrocarpa since it found in areas where A. rusticana is thought to be native (1). Moreover, A. sisymbrioides could also possibly be used for horseradish improvement, but this species is morphologically distant from the other two species having glaucous leaves and fruits that are oblong and slightly recurved [1]. The current USA germplasm collection is maintained by the Horseradish Growers of Illinois and Southern Illinois University—Carbondale (SIUC) and contains about 35 of the approximate 200 clones that were originally maintained at the University of Illinois [13]. This collection contains clones that were either imported from various locations around the world (primarily Eastern Europe and Russia) or improved clones developed in the U.S. Some of these clones have outstanding root yields and quality characteristics that will be considered for use in future crosses [14]. Other known horseradish germplasm collections include those found in Czech Republic, Denmark, Finland, Hungary, Italy, Norway, and Sweden [15].
Improving Fertility in Horseradish
Although the production and use of seed is most important for horseradish breeding activities to develop new genetic combinations, the lack of adequate seed production has hindered breeding efforts for this crop in most parts of the world. Since cross-pollination is needed to achieve the highest seed production in horseradish [12], the production of fertile lines of horseradish through cross-pollination and selection would greatly stimulate the genetic improvement of this plant [8]. Furthermore, research aimed at obtaining horseradish plants capable of sexual reproduction could result in higher genetic diversity, better adaptation to various environmental conditions, and pest resistance [9].
The problem of non-viable seed was originally overcome in Illinois, USA by Rhodes et al. [6] following the suggestions of Weber [8] that was previously discussed. These original crosses were made in an attempt to combine characters such as improved disease resistance, higher root quality, and increased yields [6]. The continued interbreeding and selection of offspring from these original crosses has led to the development of most of the cultivars used in Illinois, USA today, with many that are either partially or fully self-compatible, exhibiting no issues of male sterility [4]. Although horseradish seed can easily be produced today from many cultivars, breeding lines or other germplasm materials in the Illinois, USA breeding program, the number and viability of seed obtained differs among the crosses that are made.
3. Horseradish Cultivars
Prior to the 1970s, horseradish production in Illinois, USA had traditionally included only a few varieties including Bohemian types (‘Bohemian’, ‘Swiss’, and ‘Sass’), ‘Big Top Western’ and ‘Common’ [10]. Bohemian types are known to produce smooth and high quality small-size roots, ‘Big Top Western’ produces large, high quality roots that often have a rough or bark-like exterior, and ‘Common’ (or ‘Maliner Kren’) is known for its high quality and large roots, but is highly susceptible to turnip mosaic virus (TuMV) and white rust (Albugo candida) [5]. Bohemian types of horseradish arrived in the United States, especially the Midwest, with Central European immigrants and gave this horseradish the name ‘Bohemian’ meaning ‘Czech.’ While they vary widely in their resistance to diseases, they all have fleshy roots, although smaller than those of ‘Common’. ‘Big Top Western’ is still commercially produced in some areas of Canada and the Midwest USA, and is well known for its disease resistance, particularly to turnip mosaic 1 virus. In the Tule Lake region of northern California, USA ‘Czechoslavakian 1′ is the main clone grown (D. Krizo, 2009 personal communication) and was brought to the area by immigrants from the country now known as the Czech Republic. Additionally, many of these older cultivars, such as ‘Bohemian’, ‘Maliner Kren’, ‘Big Top Western’, and ‘Czech’, are available through various private horticultural gardening companies in the USA.
Currently, there are many cultivars that horseradish growers in Illinois, USA use for planting each year, with new materials released each year from the SIUC breeding program. These cultivars comprise greater than 95% of those that are grown in Illinois, USA today. Certain cultivars are preferred by specific growers, although most will typically grow four to five or more cultivars. Horseradish growers try to limit the number of cultivars that are grown due to problems associated with preserving the genetic purity of each cultivar when several are grown, and then harvested, graded and processed at similar dates. Currently, many of the most widely grown cultivars are those that were recently released from the SIUC breeding program and will be discussed later in the section on new cultivar releases. About 10 to 15 years ago, the most widely grown cultivars were: 15K, 22C, 1038, 1573, 1590, 1722, 7586, D25-E2, and D18-E1 [16][17]. Many of these are still produced, but in small amounts, with a shift toward production of the newer cultivars being released. Horseradish cultivars grown in other regions of North America include ‘Big Top Western’ and H-3 (Minnesota and Wisconsin), ‘Czechoslavakian 1’ (California), and ‘Big Top Western’ and ‘Eastern’ (Canada). However, after a period of about 10 to 15 years, most horseradish cultivars normally “run their course,” as quality, vigor and yielding ability becomes less over time compared to previous years’ plantings, which is similar to that reported for Polish cultivars of horseradish that last on average about 9 years [18].
In other areas of the world, several other named cultivars are used in horseradish production. In Germany, some of the common cultivars used are ‘Baiersdorf’, ‘Hamburger’, ‘Spreewälder’ and ‘Badischer’, with ‘Yugoslavian’ and ‘Steirischer’ grown if a stronger, more pungent taste is desired [19]. ‘Steirischer’ is also the main cultivar grown in the important Styrian horseradish growing region of Austria. The ‘Hungarian’ cultivar is used primarily in Hungary, but also grown in other areas of Eastern Europe and limited amounts in North America. ‘Sindal’ and ‘Yugoslavian’, as well as other selections from the Danish breeding program, are grown in Denmark [20].
4. Horseradish Breeding
4.1. Breeding Objectives
The goal of the Illinois, USA horseradish breeding program is to develop commercially acceptable horseradish cultivars through traditional breeding of Armoracia rusticana clones. New and improved cultivars are selected to have internal root discoloration (IRD) complex resistance, which is caused by several soil-borne pathogens [13][21][22], while also providing high quality and high yielding roots with high numbers of set roots that are used as planting stock the following growing season. This breeding process takes several years to produce a new commercially acceptable cultivar.
4.2. Traditional Breeding Methods for Horseradish
Since horseradish cultivars are propagated asexually using root cuttings, the breeding methods used for horseradish are somewhat similar to other asexually propagated crops. New genetic combinations are made by cross-pollinating cultivars or other germplasm materials. In recent years, the proven cross technique has been primarily used to obtain new genotypes of horseradish, although the polycross technique was also utilized in past years. The proven cross technique procedure consists of crossing two superior commercial horseradish clones under greenhouse conditions and evaluating the resulting progeny for disease tolerance, yields, and root quality characters, with inferior genotypes rouged out over a period of years. The polycross technique involves the natural intercrossing of several advanced clones under field conditions with only the female parent known, and Figure 1 shows developing seed pods in a polycross nursery. This method was used more in the breeding program in past years, since it is the simplest and easiest way to produce high numbers of seed with new genetic combinations, although high amounts of selfing probably occurs. The proven cross method (with specific directed crosses made by hand) has been used in more recent years to target specific genetic combinations. Comparisons of data obtained from the Illinois, USA breeding program for the two methods (polycross: 2007 to 2011, and proven cross: 2009 to 2017) indicate that the proven cross method is more efficient since less progeny are required to evaluate under field evaluations to produce new advanced clones compared to the polycross method (Table 1). However, both of these techniques will result in new, superior genotypes, and can be further repeated by inter-mating of advanced clones that were recently selected.

Figure 1. Horseradish inflorescence with maturing seed pods during mid-spring outdoors in a polycross field block (photo by Dr. Alan Walters).
Table 1. Evaluation of horseradish breeding methods for development of new horseradish cultivars in Illinois, USA.
| Horseradish Cross Method a and Years Utilized to Produce Field Seedlings for Evaluation | Total Number Seedlings Evaluated | Mean Number of Seedlings Evaluated per Year |
Number of New Horseradish Cultivars Released After Field Evaluations |
Percentage of New Horseradish Cultivars Obtained Using Breeding Method |
|---|---|---|---|---|
| Polycross Method: 2007–2011 |
17,500 | 3500 | 9 | 0.051 |
| Proven Cross Method: 2009–2017 |
22,500 | 2500 | 23 | 0.102 |
| - | p = 0.0001 | p = 0.0001 | p = 0.0001 |
a The polycross method is open-pollination of a specific clone, with only female parent known; and, the proven cross method uses directed hand-crossings of clones, with both female and male parents known. Data presented for seedling evaluations and new horseradish cultivars are based on breeder’s notes over that period of time presented for each breeding method. Student’s t-test was used to assess differences between horseradish breeding methods for the efficiency of reducing seedling numbers in the initial field evaluation, and production of new, vigorous commercially adaptable horseradish cultivars.
Two-year old roots (or crowns) from selected horseradish plant materials are used to develop new genetic combinations since these will produce flowers. The year after significant crown development has occurred, that is from set root (~1.3 cm diam.) to large primary root (>2.5 cm diam.), horseradish plants will naturally flower during the early spring. For intercross blocks, horseradish plants will flower the spring after they are placed into the ground during mid-spring the previous year. Many crowns used for making specific targeted crosses under greenhouse conditions are harvested during the autumn and placed into cold storage for about 3 months since they can be forced to flower under greenhouse conditions, if previously provided at least two months of cold treatment at 0 to 5 °C. These crowns are removed from cold storage and are usually planted into large pots in early- to mid-March, with flowers appearing three to four weeks later. Seeds are ready to harvest by mid-May and mid-June for those obtained from the greenhouse and field, respectively. Ripened seed pods are collected, with seeds cleaned and placed into seed packets, which are then placed into plastic containers and stored at 2 to 5 °C until planting. Horseradish crown development is an ongoing process that must be completed each year to have materials to either inter-cross in the field or to hand-cross under greenhouse conditions.
4.3. Horseradish Breeding Cycle
The typical horseradish breeding cycle (from seed development to grower distribution of a new cultivar) normally takes about 8 years in Illinois, USA (Figure 2). The typical procedure used for horseradish cultivar development is: (1) year 1—seed development and collection from a particular hand cross in greenhouse between two known superior clones or an intercross block in field with unknown male parent; (2) year 2—seedling production in greenhouse and transplanting in field for evaluation under commercial field conditions; (3) years 3, 4, and 5—chosen seedlings are further evaluated and selected under field conditions; (4) year 6—field increase of clones that made it through the field evaluation and selection process, with growers making the final determination to cultivar status; (5) year 7—tissue culture and field increase of new cultivars (done the same year); and (6) year 8—new cultivars available to commercial growers for field planting.
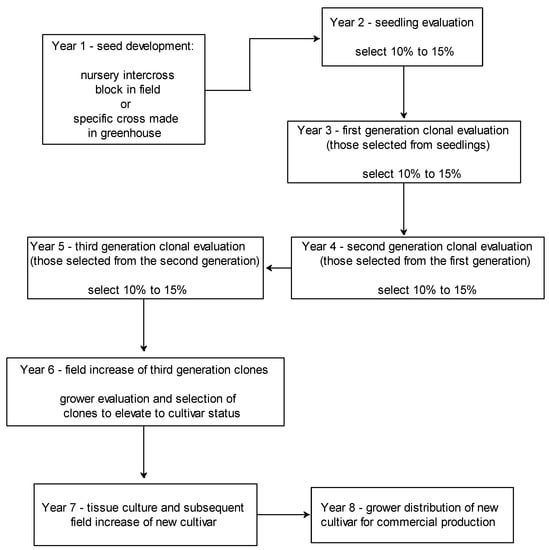
Figure 2. Typical breeding cycle for horseradish and duration required to produce new a new clonal cultivar. Horseradish roots are selected based on lack of internal root discoloration, high root biomass and quality (smooth root surface with few fine roots), horizontal root development in the soil opposed to vertical growth, and high set root production.
During each annual field selection cycle that occurs from years 2 to 5 in the breeding process, about 10% to 15% of horseradish materials are selected each year from those evaluated and passed on to the next stage (Figure 2). Field selections are primarily based upon IRD resistance, root quality (smooth roots), set root production, and yield potential. A clone showing any symptoms of IRD during the selection process is generally discarded from the program, unless it shows great potential in either yield or root quality characters. Once a superior clone has been identified after surviving several years in the breeding program, it must be multiplied rapidly to achieve high plant numbers in a relatively short period of time; and, this is accomplished through in vitro propagation methods followed by subsequent rooting under mist and then field increase [23][24].
References
- Sampliner, D.; Miller, A.J. Ethnobotany of Horseradish (Armoracia rusticana, Brassicaceae) and Its Wild Relatives (Armoracia spp.): Reproductive Biology and Local Uses of in Their Native Ranges. Econ. Bot. 2009, 63, 303–313.
- Shehata, A.; Mulwa, R.M.S.; Babadoost, M.; Uchanski, M.; Norton, M.A.; Skirvin, R.; Walters, S.A. Horseradish: Botany, Horticulture, Breeding. Hortic. Rev. 2009, 35, 221–261.
- Walters, S.A. Horseradish Tolerance to Internal Root Discoloration. Mod. Concepts Dev. Agron. 2019, 5.
- Walters, S.A.; Bernhardt, P.; Joseph, M.; Miller, A.J. Pollination and Sterility in Horseradish. Plant Breed. 2016, 135, 735–742.
- Walters, S.A.; Wahle, E.A. Horseradish Production in Illinois. HortTechnology 2010, 20, 267–276.
- Rhodes, A.M.; Courter, J.W.; Shurtleff, M.C.; Vandemark, J.S. Improving Horseradish Through Breeding. Ill. Res. 1965, 7, 17.
- Moravec, J. The Possibilities to Improve Yield and Quality of Horseradish (Armoracia rusticana Lam.). Vyzk. Ustav Zelin. Olomouc Bull. 1963, 7, 1–9.
- Weber, W.W. Seed Production in Horseradish. J. Hered. 1949, 40, 223–227.
- Winiarczyk, K.; Tchόrzewski, D.; Bednara, J. Development of the Male Gametophyte of an Infertile Plant, Armoracia rusticana Gaertn. Plant Breed. 2007, 126, 433–439.
- Rhodes, A.M. Horseradish Problems and Research in Illinois. In Crop Resources; Siegler, D.S., Ed.; Academic Press: New York, NY, USA, 1977; pp. 137–146.
- Courter, J.W.; Rhodes, A.M. Historical Notes on Horseradish. Econ. Bot. 1969, 23, 156–164.
- Stokes, G.W. Seed Development and Failure in Horseradish. J. Hered. 1955, 46, 15–21.
- Atibalentja, N.; Eastburn, D.M. Verticillium dahliae Resistance in Horseradish Germplasm from the University of Illinois Collection. Plant Dis. 1998, 82, 176–180.
- Walters, S.A. Horseradish Germplasm Evaluation—2018. In Proceedings of the 2020 Horseradish Growers School, Collinsville, IL, USA, 23 January 2020; University of Illinois Extension, Madison-St. Claire Unit: Collinsville, IL, USA, 2020; pp. 7–10.
- Wedelsbäck Bladh, K.; Liljeroth, E.; Poulsen, G.; Yndgaard, F.; Brantestam, A.K. Genetic Diversity in Nordic Horseradish, Armoracia rusticana, as Revealed by AFLP Markers. Genet. Resour. Crop Evol. 2014, 61, 383–394.
- Dorris, F.; Walters, S.A.; Wahle, E.A. Horseradish Variety Survey—2006. In Proceedings of the 2007 Horseradish Growers School, Collinsville, IL, USA, 25 January 2007; Univ. Illinois Extension, Madison-St. Claire Unit: Edwardsville, IL, USA, 2007; pp. 11–13.
- Dorris, F.; Walters, S.A.; Wahle, E.A. Horseradish Variety Survey—2007. In Proceedings of the 2008 Horseradish Growers School, Collinsville, IL, USA, 31 January 2008; University of Illinois Extension, Madison-St. Claire Unit: Edwardsville, IL, USA, 2008; pp. 11–13.
- Braun-Młodecka, U. Evaluation of the Average Age of Vegetable Varieties as the Measure of Varietal Replacement on the Polish Market in the Year, 1988–2000. Electron. J. Pol. Agric. Univ. 2003, 6. Available online: (accessed on 17 January 2021).
- Vogel, G. Handbuch des Speziellen Gemüsebaus; Ulmer: Stuttgart, Germany, 1996; p. 1128.
- Kjeldsen Bjørn, G.; Villebro, J. Improved Quality of Horseradish; Danish Agricultural Research Internal Report No. 183; Danish Institute of Agricultural Sciences: Årslev, Denmark, 2003.
- Babadoost, M.; Chen, W.; Bratsch, A.D.; Eastman, C.E. Verticillium longisporum and Fusarium solani: Two New Species in the Complex of Internal Discoloration of Horseradish Roots. Plant Pathol. 2004, 53, 669–676.
- Eastburn, D.M.; Chang, R.J. Verticillium dahliae: A Causal Agent of Root Discoloration of Horseradish in Illinois. Plant Dis. 1994, 78, 496–498.
- Norton, M.; Uchanski, M.; Scoggins, K.; Skirvin, R. Tissue Culture Project Progress. In Proceedings of the 2001 Horseradish Growers School, Collinsville, IL, USA, 25 January 2001; University of Illinois Extension, Madison-St. Claire Unit: Edwardsville, IL, USA, 2001; pp. 18–20.
- Uchanski, M.; Skirvin, R.M.; Norton, M.A. The Use of In Vitro Thermotherapy to Obtain Turnip Mosaic Virus-Free Horseradish Plants. Acta Hortic. 2004, 631, 175–179.




