| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karine Belguise | + 1235 word(s) | 1235 | 2021-02-08 09:53:43 | | | |
| 2 | Karina Chen | Meta information modification | 1235 | 2021-02-20 07:28:08 | | | | |
| 3 | Karina Chen | Meta information modification | 1235 | 2021-02-20 07:29:32 | | |
Video Upload Options
Protein Kinase C theta (PKCθ) is a serine/threonine kinase that belongs to the novel PKC sub-family. PKCθ has been extensively studied for its role in the immune system where it plays a critical role in T cell activation. Beyond its physiological role in immune responses, increasing evidence implicates PKCθ in the pathology of various diseases, especially autoimmune disorders and cancers. Particularly, in various types of cancers, the high PKCθ expression leads to aberrant cell proliferation, migration and invasion, thereby promoting cancer aggressiveness. The recent development and application of PKCθ inhibitors in the context of auto-immune diseases could benefit the emergence of treatment for cancers in which PKCθ has been implicated.
1. Introduction
The Protein Kinase C (PKC) family is a family of serine/threonine kinases that are involved in various cellular processes for different cell types. The PKC family is classified into three subfamilies: classical (α, βI, βII, γ), novel (δ, ε, η, θ) and atypical (ζ, ι/λ) PKC isoforms. This classification is based on their structure and ability to respond to calcium and/or diacylglycerol (DAG) [1]. Among this family, the novel PKCθ isoform is different from other PKC isoforms since its physiological expression is limited to a few types of cells, such as T cells, platelets and skeletal muscle cells. This specific expression confers to this isoform a central role in the immune system where PKCθ controls T cell activation, survival and differentiation [2]. In skeletal muscle cells, PKCθ regulates muscle cell development, homeostasis and remodeling [3]. Beyond its physiological functions, PKCθ is also involved in the pathology of various diseases. In the context of the immune system and skeletal muscle tissue, the dysregulation of PKCθ activity leads to both autoimmune and inflammatory diseases and to insulin resistance and Type 2 diabetes, respectively [3][4]. In the last decade, growing evidence implicated the PKCθ signaling in the biology of cancer where it controls cancer cell proliferation, migration and invasion at the cytoplasmic or nuclear levels. Here, we discuss this emerging function of PKCθ in cancer by analyzing its diverse modes of action and their consequence on critical biological processes involved in tumorigenesis and cancer progression.
2. PKCθ Structure and Physiological Function
In this section, we provide a brief overview of the PKCθ structure and the PKCθ physiological function mainly in the immune system. For extensive details, the readers can refer to several excellent reviews written by the experts in the field of T cell biology (reviewed in [2][4][5][6][7]).
2.1. PKCθ Structure
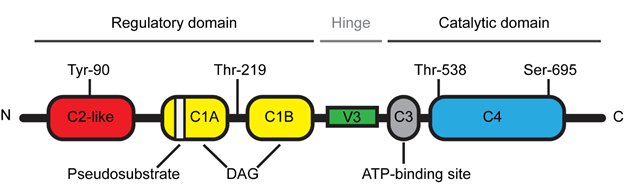
The novel PKCθ isoform is a protein kinase encoded by the PRKCQ gene and composed of 706 amino acids with a molecular weight of approximately 82 kDa [8]. PKCθ is a DAG-dependent but Ca2+-independent, protein kinase whose structure consists of several functional domains that are conserved among the novel PKC subfamily (Figure 1) [1]. The N-terminal regulatory domain contains the C2-like domain, the pseudosubstrate region and the DAG-binding domain (C1A/B) while the C-terminal catalytic domain contains the ATP-binding domain (C3) and the substrate-binding domain (C4). The regulatory and catalytic domains are separated by a hinge region, called the V3 motif, which is unique and highly specific to each PKC isoforms.
Figure 1. Schematic representation of Protein Kinase C theta (PKCθ) structure.
2.2. PKCθ Function in the Immune System
Due to the high expression levels of PKCθ in T lymphocytes, extensive research has studied the biological function of this novel PKC isoform in the immune system. The generation and analysis of PKCθ-deficient mice have unraveled the selective role of PKCθ in the T cell immune response [9][10]. While PKCθ is critical for the T helper (Th)2- and Th17-mediated responses, the Th1- and cytotoxic T cell-driven responses remain relatively intact in the absence of PKCθ [4][7]. However, a few studies reported that some specific Th1 responses were altered in PKCθ deficient mice [11][12]. T lymphocyte activation is a central step of the T cell immune response during which T cell interacts with an antigen-presenting cell (APC) [4]. This cell-cell junction forms a well-organized and dynamic structure called the immunological synapse [13]. Following this T cell-APC interaction, cytoplasmic PKCθ is translocated to the membrane at the immunological synapse [6] and this specific and critical relocalization is highly dependent on the unique V3 motif of PKCθ [14]. In addition, other events are also required for the proper localization and activation of PKCθ at the immunological synapse. Concerning the PKCθ localization, several studies indicated that the lck-mediated phosphorylation of PKCθ at tyr-90 participated in the PKCθ recruitment to the immunological synapse [14][15] and a report from Thuille et al. suggested that the PKCθ autophosphorylation at thr-219 was required for the cell membrane localization of PKCθ [16]. Moreover, the data from Cartwright et al. suggested that PKCθ required its active kinase domain in order to be maintained at the immunological synapse [17]. More recently, Wang et al. reported that the sumoylation of PKCθ upon T cell activation was involved in the specific localization of PKCθ and in the organization of the immunological synapse [18]. Concerning the PKCθ activation, the phosphorylation at Thr-538 in the activation loop regulates the PKCθ activity by maintaining PKCθ in an active conformation and thus this phosphorylation has been used as a marker reflecting the PKCθ activation [19]. GCK-like kinase (GLK, MAP4K3) has been identified as one kinase capable of directly phosphorylating this Thr-538 residue during the T cell activation [20]. Moreover, the auto-phosphorylation at Ser-695 induced during T cell activation is also required for the PKCθ kinase activity [19][21].
Once translocated to the immunological synapse, PKCθ integrates various signaling cascades that conduct to the activation of important transcription factors, including Nuclear Factor κB (NF-κB), Activating Protein 1 (AP-1) and, to a lesser extent, Nuclear Factor of Activated T-cells (NFAT) [5]. This transcriptional machinery then induces the production of interleukin-2, a cytokine essential for the T cell proliferation [5]. Moreover, the PKCθ function is not only limited to the activation of signaling pathways that leads to the transcriptional regulation of gene expression. For example, PKCθ has been involved in the actin cytoskeletal reorganization that occurs during the formation of the immunological synapse and the related polarization of activated T cells [18][22][23]. PKCθ can also enter the nucleus of activated T cells to directly bind to the chromatin in order to regulate the expression of immune response genes and microRNAs involved in the cytokine regulation [24].
2.3. Implication of PKCθ in Immunological Disorders
As a selective regulator of the Th2 and Th17 immune responses, the perturbation of PKCθ expression and activity leads to the development of Th2-driven inflammatory diseases and Th17-mediated autoimmune diseases. Indeed, PKCθ is highly expressed and activated in these immunological disorders [4]. Studies from the PKCθ-deficient mice showed that the PKCθ suppression decreased the T cell inflammatory response in autoimmunity, allergy and allograft rejection [4]. Therefore, the therapeutic use of specific PKCθ inhibitors could provide an interesting approach for these PKCθ-dependent pathologies [25]. Clinical studies using sotrastaurin (AEB071) as the PKCθ inhibitor showed some encouraging results in the context of immunosuppressive therapy for autoimmune diseases such as psoriasis and organ transplantation [4][26]. However, sotrastaurin is not specific to PKCθ and it also shows strong and specific inhibitory activity against PKCα and PKCβ and to a lesser extend against PKCδ, PKCε and PKCη. It thus suggests that sotrastaurin would inhibit not only the PKCθ-mediated functions but also the functions from other PKCs [27]. Therefore, current research works aim to develop more selective PKCθ inhibitors [28][29]. These inhibitors are currently tested in mouse models and further studies are needed to validate them in the clinical trials.
References
- Steinberg, S.F. Structural basis of protein kinase C isoform function. Physiol. Rev. 2008, 88, 1341–1378.
- Hayashi; K.; Altman, A. Protein kinase C theta (PKCtheta): A key player in T cell life and death. Pharmacol. Res. 2007, 55, 537–544.
- Marrocco, V.; Fiore, P.; Madaro, L.; Crupi, A.; Lozanoska-Ochser, B.; Bouché, M. Targeting PKCtheta in skeletal muscle and muscle diseases: Good or bad? Biochem. Soc. Trans. 2014, 42, 1550–1555.
- Zhang; Y.E.; Kong, K.F.; Altman, A. The yin and yang of protein kinase C-theta (PKCtheta): A novel drug target for selective immunosuppression. Adv. Pharmacol. 2013, 66, 267–312.
- Isakov; N.; Altman, A. Protein kinase C(theta) in T cell activation. Annu. Rev. Immunol. 2002, 20, 761–794.
- Kong; F.K.; Altman, A. In and out of the bull’s eye: Protein kinase Cs in the immunological synapse. Trends Immunol. 2013, 34, 234–342.
- Marsland, J.B.; Kopf, M. T-cell fate and function: PKC-theta and beyond. Trends Immunol. 2008, 29, 179–185.
- Baier, G.; Telford, D.; Giampa, L.; Coggeshall, K.M.; Bitterlich, G.B.; Isakov, N.; Altman, A. Molecular cloning and characterization of PKC theta, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J. Biol. Chem. 1993, 268, 4997–5004.
- Pfeifhofer, C.; Kofler, K.; Gruber, T.; Tabrizi, N.G.; Lutz, C.; Maly, K.; Leitges, M.; Baier, G. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J. Exp. Med. 2003, 197, 1525–1535.
- Sun, Z.; Arendt, C.W.; Ellmeier, W.; Schaeffer, E.M.; Sunshine, M.J.; Gandhi, L.; Annes, J.; Petrzilka, D.; Kupfer, A.; Schwartzberg, P.L.; et al., PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 2000, 404, 402–407.
- Nishanth, G.; Burkiewicz, M.S.; Händel, U.; Kliche, S.; Wang, X.; Naumann, M.; Deckert, M.; Schlüter, D. Protective Toxoplasma gondii-specific T-cell responses require T-cell-specific expression of protein kinase C-theta. Infect. Immun. 2010, 78, 3454–3464.
- Ohayon, A.; Golenser, J.; Sinay, R.; Tamir, A.; Altman, A.; Pollack, Y.; Isakov, N. Protein kinase C theta deficiency increases resistance of C57BL/6J mice to Plasmodium berghei infection-induced cerebral malaria. Infect. Immun. 2010, 78, 4195–4205.
- Grakoui, A.; Bromley, S.K.; Sumen, C.; Davis, M.M.; Shaw, A.S.; Allen, P.M.; Dustin, M.L. The immunological synapse: A molecular machine controlling T cell activation. Science 1999, 285, 221–227.
- Kong, K.O.; Yokosuka, T.; Balancio, A.J.C.; Isakov, N.; Saito, T.; Altman, A. A motif in the V3 domain of the kinase PKC-theta determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat. Immunol. 2011, 12, 1105–1112.
- Liu, Y.; Witte, S.; Liu, Y.C.; Doyle, M.; Elly, C.; Altman, A. Regulation of protein kinase Ctheta function during T cell activation by Lck-mediated tyrosine phosphorylation. J. Biol. Chem. 2000, 275, 3603–3609.
- Thuille, N.; Heit, I.; Fresser, F.; Krumböck, N.; Bauer, B.; Leuthaeusser, S.; Dammeier, S.; Graham, C.; Copeland, T.D.; Shaw, S.; et al., Critical role of novel Thr-219 autophosphorylation for the cellular function of PKCtheta in T lymphocytes. EMBO J. 2005, 24, 3869–3880.
- Cartwright; G.N.; Kashyap, A.K.; Schaefer, B.C. An active kinase domain is required for retention of PKCtheta at the T cell immunological synapse. Mol. Biol. Cell 2011, 22, 3491–3497.
- Wang, X.D.; Gong, Y.; Chen, Z.L.; Gong, B.N.; Xie, J.J.; Zhong, C.Q.; Wang, Q.L.; Diao, L.H.; Xu, A.; Han, J.; et al., TCR-induced sumoylation of the kinase PKC-theta controls T cell synapse organization and T cell activation. Nat. Immunol. 2015, 16, 1195–1203.
- Liu, Y.; Graham, C.; Li, A.; Fisher, R.J.; Shaw, S. Phosphorylation of the protein kinase C-theta activation loop and hydrophobic motif regulates its kinase activity, but only activation loop phosphorylation is critical to in vivo nuclear-factor-kappaB induction. Biochem. J. 2002, 361, 255–265.
- Chuang, H.C.; Lan, J.L.; Chen, d.; Yang, C.Y.; Chen, Y.M.; Li, J.P.; Huang, C.Y.; Liu, P.E.; Wang, X.; Tan, T.H. The kinase GLK controls autoimmunity and NF-kappaB signaling by activating the kinase PKC-theta in T cells. Nat. Immunol. 2011, 12, 1113–1118.
- Czerwinski, R.; Aulabaugh, A.; Greco, R.M.; Olland, S.; Malakian, K.; Wolfrom, S.; Lin, L.; Kriz, R.; Stahl, M.; Huang, Y.; et al. Characterization of protein kinase C theta activation loop autophosphorylation and the kinase domain catalytic mechanism. Biochemistry 2005, 44, 9563–9573.
- Britton, G.J.; Ambler, R.; Clark, D.J.; Hill, E.V.; Tunbridge, H.M.; McNally, K.E.; Burton, B.R.; Butterweck, P.; Peyton, C.S.; O’Neil, L.A.H.; et al., PKCtheta links proximal T cell and Notch signaling through localized regulation of the actin cytoskeleton. Elife 2017, 6, e20003.
- Quann, E.J.; Liu, X.; Bonnet, G.A.; Huse, M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat. Immunol. 2011, 12, 647–654.
- Sutcliffe, E.L.; Bunting, K.L.; He, Y.Q.; Li, J.; Phetsouphanh, C.; Seddiki, N.; Zafar, A.; Hindmarsh, E.J.; Parish, C.R.; Kelleher, A.D.; et al., Chromatin-associated protein kinase C-theta regulates an inducible gene expression program and microRNAs in human T lymphocytes. Mol. Cell 2011, 41, 704–719.
- Kwon, M.Y.; Wang, R.; Ma, J.; Sun, Z. PKC-theta is a drug target for prevention of T cell-mediated autoimmunity and allograft rejection. Endocr. Metab. Immune Disord. Drug Targets 2010, 10, 367–372.
- Sleiman, R.H.; Hamze, A.B.; Reslan, L.; Kobeissy, H.; Dbaibo, G. The Novel PKCtheta from Benchtop to Clinic. J. Immunol. Res. 2015, 2015, 348798.
- Evenou, J.P.; Wagner, J.; Zenke, G.; Brinkmann, V.; Wagner, K.; Kovarik, J.; Welzenbach, K.A.; Schmidt, G.W.; Guntermann, C.; Towbin, H.; et al., The potent protein kinase C-selective inhibitor AEB071 (sotrastaurin) represents a new class of immunosuppressive agents affecting early T-cell activation. J. Pharmacol. Exp. Ther. 2009, 330, 792–801.
- Kunikawa, S.; Tanaka, A.; Takasuna, Y.; Tasaki, M.; Chida, N. Discovery of 2,4-diamino-5-cyanopyrimidine derivatives as protein kinase C theta inhibitors with mitigated time-dependent drug-drug interactions. Bioorg. Med. Chem. 2019, 27, 790–799.
- Wang, J.; Jin, W.; Zhou, X.; Li, J.; Xu, C.; Ma, Z.; Wang, J.; Qin, L.; Zhou, B.; Ding, W.; et al., Identification, Structure-Activity Relationships of Marine-Derived Indolocarbazoles, and a Dual PKCtheta/delta Inhibitor with Potent Antipancreatic Cancer Efficacy. J. Med. Chem. 2020, 63, 12978–12991.