
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcia dos Santos Rizzo | + 2028 word(s) | 2028 | 2021-02-07 01:57:25 | | | |
| 2 | Peter Tang | Meta information modification | 2028 | 2021-02-24 12:23:36 | | |
Video Upload Options
Galactomannans are versatile macromolecules with broad industrial potential. The influence of changes in the chemical structures and respective bioactivities of these polysaccharides have been extensively studied. The derivatives obtained by sulfation, complexation, and phosphorylation are the most studied biological properties in galactomannans. The derivatives obtained have shown several pharmacological activities such as antiviral, antimicrobial, anticoagulant, fibrinolytic, chemopreventive, anticancer, antioxidant, chondroprotective, analgesic, immunomodulatory, and antileishmanial.
1. Introduction
The development of new biomaterials and “functionalized polymers,” especially from renewable sources, has practical potential for applications in biomedicine and industry [1]. In this direction, the growing interest in the chemical modification or derivatization of natural polysaccharides to create derivatives with properties adapted to the desired applications is remarkable [2][3]. Molecular modification and improvement of the biologically active properties of polysaccharides have sparked interest about the development of biomedical materials. Therefore, it is important to find the correlation between the chemical structure and the functional activity of the polysaccharides [4][5][6].
Galactomannans are polysaccharides which can be obtained from microorganisms, including fungi and yeasts, but the vast majority originate from plants [7]. Among the plant-derived polysaccharides, galactomannans have received much attention, since these biopolymers can be obtained in their pure form, with a high yield, by aqueous extraction from the endosperm of the seeds of leguminous plants [6][8]. Furthermore, since galactomannans are an abundant plant resource, their production on an industrial scale has been widely targeted [5][9], especially in the food, pharmaceutical, and cosmetic industries [10].
Galactomannans have a heterogeneous polymeric structure, consisting of a main chain of mannose units linked by β-1,4-glycosidic bonds and galactose side units linked to mannose by α-1,6-glycosidic bonds (Figure 1) [7][11]. Galactomannans from different sources differ from each other in the ratio of mannose/galactose units, molecular weight, and the distribution of individual galactose branches in the main chain, which significantly influences their biochemical properties [12][13].
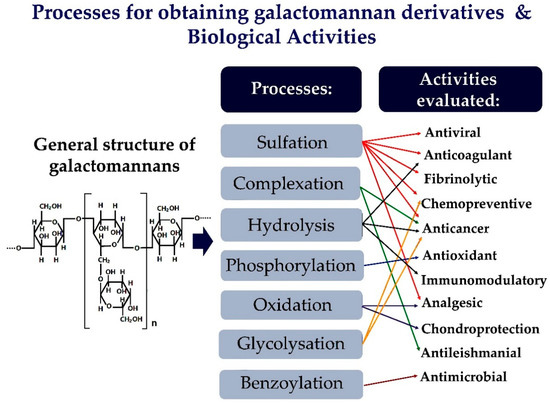
Figure 1. Illustration of the chemical changes of galactomannans and their biological activities evaluated.
In addition, the structural peculiarities of galactomannans make them soluble in water at different temperatures, versatile in their applications, and chemically and biochemically reactive [1]. These factors, combined with the absence of ionic charge in the structure of these polysaccharides, make galactomannans more susceptible to molecular changes than many polysaccharides found in nature. Alterations can be made in order to inherit the characteristics of the native polymer, such as biocompatibility, to expand its technological functionality, improve its intrinsic biological properties, and/or create new functional properties, opening a range of application possibilities in the biomedical areas [12][14][15].
The galactomannans are suitable for used in chemical modification methods, as they have a simple structure. Their derivatives can be easily characterized chemically. Sulfation is an effective, versatile, and simple modification method applied to galactomannans to improve their biological activity. However, other methods can also be used to change the structure of galactomannans [16][17][18]. Thus, Figure 1 provides a schematic representation of the main chemical changes in galactomannans and the various biological activities evaluated.
2. Strategies for Obtaining Galactomannan Derivatives
Biological activities of polysaccharides are affected by several factors, among them, monosaccharide composition, molecular weight, molecular structure, functional groups, degree and pattern of substitution, flexibility, and chain configuration [4][19][20]. In addition to the evident biological activity of natural polysaccharides, derivatives obtained by hydrolysis, complexation, or chemical modifications, such as sulfation, phosphorylation, oxidation, and carboxymethylation, were also considered in many studies as successful bioactive derivatives [5][21][22][23].
Among these polysaccharides, galactomannans have been extensively studied for molecular modifications. Galactomannans can be modified mainly by chemical or enzymatic methods. A large number of hydroxyl groups of galactomannans can be altered by etherification, esterification, or oxidation. The changes that add hydrophilic groups to molecules of galactomannans can reduce hydrogen bonds between the molecules and improve solubility and swelling characteristics. Other characteristics that are also improved by the increase in hydrophilicity are the clarity of the gum and the compatibility with electrolytes [24].
The changes in chemical structure and biological activities of galactomannans have been extensively studied. The most studied biological properties in galactomannans are derivatives obtained by complexation or chemical modifications, such as sulfation, oxidation, and phosphorylation. Figure 2 represents the main strategies for obtaining galactomannan derivatives.
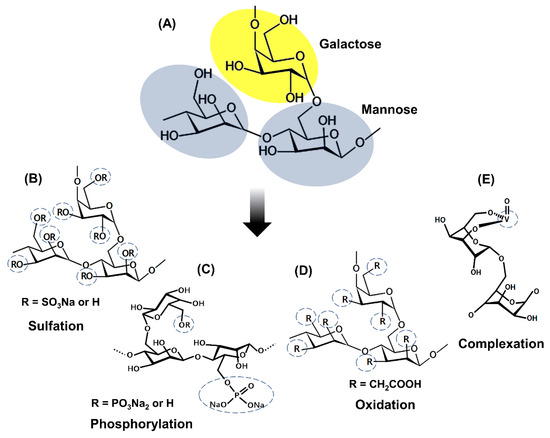
Figure 2. Representation of the general chemical structure of galactomannans (A) and the structure of the derivatives achieved by (B) Sulfation (Adapted from Muschin et al. [5]); (C) Phosphorylation (Adapted from Wang et al. [20]); (D) Oxidation (Adapted from Castro et al. [25]); (E) Complexation with vanadium (Adapted from Adriazola et al. [22]).
Sulfation is the most studied chemical modification in galactomannans. Most studies about sulfation of galactomannans used dimethylformamide as the solvent and SO3– pyridine complex as a sulfating reagent [6][8][9][23][26][27][28][29]. Several studies report preferred sulfation for the 6-CH2 groups due to their higher reactivity with esterifying agents and a degree of sulfating (DS) ranging from 0.4 to 1.85. These sulfated galactomannans are composed of negatively charged sulfate groups that are related to their biological activities [4][5][6][8][9][23][26][27][28][29]. The addition of sulfate groups in the polysaccharide structure improves solubility and enhances the biological properties compared to the non-sulfated polysaccharides. Anticoagulant, antiviral, antioxidant, and antitumor activities are successful examples of sulfated polysaccharides [4][23][28][29].
Polysaccharides can also undergo oxidation in several ways to generate distinct structures. For example, primary alcohol may be oxidized to form the aldehyde or later a carboxylic acid [30]. Protein-free guar gum was oxidized by the method of the TEMPO (2,2,6,6-tetramethylpiperidine-1-oxide radical)/NaBr/NaClO system [25]. This oxidized derivative presents a degree of substitution of 0.36, and the oxidation happened preferentially in the carbon 6 of the mannose residue. FT-IR and 13C NMR spectrum showed the presence of the COOH group in the oxidized molecule.
Phosphorylated polysaccharides exhibit several biological activities, including antioxidant, antitumor, and immunomodulatory properties. Wang et al. [20] phosphorylated the galactomannan chains of Cyamopsis tetragonolobo (guar gum) using POCl3/pyridine and obtained phosphorylated derivatives with the degree of substitution (DS) of 0.35–0.52. They observed an expanded conformation of phosphorylated derivatives with intramolecular hydrogen bonds due to the electrostatic potential of SO3H groups. Experiments with the phosphorylated derivatives with different DS were conducted to evaluate the influence of this parameter on the antioxidant activity.
Hydrolysis is an important strategy for polysaccharide modification. In the enzymatic method, the galactose side chains, or the main mannose chain can be cleaved, and the α-galactosidase and β-mannanase can be used. Compared with the chemical modification method, the enzymatic method is simple to control, and the reaction conditions are milder [24]. The biological activity of the locust bean gum galactomannan hydrolyzed by thermostable β-D-mannanase was evaluated by Chen et al. [31]. In this study, after 24 h of the enzymatic reaction, the weight average molecular weight of galactomannan derivative reduced from 5,580,010 to 3188, and the hydrolysate containing the following manno-oligosaccharides: mannobiose, mannotriose, and mannotetrose.
According to Adriazola et al. [22], complexation with metal ions can make complexes more biocompatible and soluble, less toxic, and endow them with some pharmacological activities, such as antimicrobial and antitumor. Galactomannans can be modified by introducing complexing metals. Vanadium is the metal generally used to complex with galactomannans in order to originate biologically active complexes. Polysaccharides are suitable binders for complexation reactions with the cationic form of vanadium due to the affinity of this metal for hydroxyl groups free of these polyhydroxyl compounds. Complexation of vanadium (IV, V) with monosaccharides is facilitated by the presence of ligands containing vicinal cis-OH groups [19][32]. Anticancer and antileishmanial activities are recognized as examples of the success of galactomannans complexed with vanadium [19][33].
The pharmacological activities of galactomannan derivatives are detailed below.
3. Pharmacological Activities of Galactomannan Derivatives
The intrinsic characteristics of polysaccharides can be changed, for example, by partial hydrolysis and by the removal or introduction of specific chemical groups [34]. When a galactomannan is modified, a biologically inert polymer can be transformed into a biologically active one [35].
Pharmacological activities can be related to changes in the chemical structure of the molecules caused by the type and number of functional groups. Several functional groups, such as -CN, -NO2, -NH2, -OH, -SH, -CHO, -COOH, -COOR, -CONH2, -SO3H, and -PO3H, contribute to drug-receptor interaction. The interaction of a compound with a specific receptor is directly related to a pharmacological effect. Changes in the conformation and flexibility of the molecules can also affect the interaction of molecules with receptors. In addition, several physicochemical properties of a molecule, such as molecular weight, lipophilicity, solubility, permeability, and acid-base ionization, can alter its activity [36][37].
In general, the chemical structure of a molecule determines its physicochemical characteristics and, consequently, determines its absorption, distribution, metabolism, excretion, and toxicity (ADME/Tox) properties and influences the pharmacological effect of the molecule [37].
Table 1 presents the main pharmacological activities of galactomannan derivatives, their source of origin, and the processes for obtaining derivatives.
Table 1. Galactomannan derivatives and assessment of pharmacological activities.
| Galactomannan Sources and/or Polysaccharides | Processes for Obtaining Derivatives | Chemical Analysis | Biological Activity/Properties | References |
|---|---|---|---|---|
|
Mimosa scabrella seeds |
Sulfation |
SEC-MALS |
Antiviral |
[26] |
|
Leucaena leucocephala seeds |
Sulfation |
SEC-MALS |
Antiviral |
[26] |
|
Mimosa scabrella seeds |
Sulfation |
FTIR, 13C NMR |
Antiviral |
[8] |
|
Leucaena leucocephala seeds |
Sulfation |
FTIR, GLC |
Antiviral |
[27] |
|
Caesalpinia ferrea seeds |
Sulfation |
FTIR, 1H, 13C NMR |
Antiviral |
[28] |
|
Adenanthera pavonina seeds |
Sulfation |
FTIR, GLC, IV |
Antiviral |
|
|
Adenanthera pavonina L. seeds |
Sulfation |
FTIR, UV-VIS |
Antiviral |
[29] |
|
Caesalpinia ferrea Mart. seeds |
Sulfation |
FTIR, UV-VIS |
Antiviral |
[29] |
|
Dimorphandra gardneriana seeds |
Sulfation |
FTIR, UV-VIS |
Antiviral |
[29] |
|
Trigonella foenum-graecum seeds, Fenugreek gum |
Sulfation |
FTIR, 1H, 13C NMR, OR, GPC, SPR |
Antiviral |
[5] |
|
Cyamopsis tetragonolobus seeds, Guar gum |
Sulfation |
FTIR, 1H, 13C NMR, OR, GPC, SPR |
Antiviral |
[5] |
|
Caesalpinia spinosa, Tara gum |
Sulfation |
FTIR, 1H, 13C NMR, OR, GPC, SPR |
Antiviral |
[5] |
|
Ceratonia siliqua L. seeds, Locust bean gum |
Sulfation |
FTIR, 1H, 13C NMR, OR, GPC, SPR |
Antiviral |
[5] |
|
Cyamopsis tetragonolobus seeds, Guar gum |
Maillard conjugation |
UV-VIS, SDS PAGE |
Antimicrobial |
|
|
Cyamopsis tetragonolobus seeds, Guar gum |
Benzoylation |
FTIR, 13C NMR, XRD, TGA, C, H, N analysis. |
Antimicrobial |
[41] |
|
Leucaena sp. seeds |
Sulfation |
PC, UV-VIS |
Anticoagulant and fibrinolytic |
[10] |
|
Medicago sativa seeds |
Sulfation |
PC, UV-VIS |
Anticoagulant and fibrinolytic |
[10] |
|
Glycine max seed hulls |
Sulfation |
PC, UV-VIS |
Anticoagulant and fibrinolytic |
[10] |
|
Phoenix dactylifera seeds |
Sulfation |
PC, UV-VIS |
Anticoagulant and fibrinolytic |
[10] |
|
Senna macranthera seeds |
Sulfation |
FTIR, 13C NMR, UV-VIS, GPC |
Anticoagulant |
[42] |
|
Cyamopsis tetragonoloba seeds, Guar gum |
Sulfation |
GPC, IV, UV-VIS |
Anticoagulant |
[9] |
|
Trigonella foenum-graecum seeds, Fenugreek gum |
Sulfation |
FTIR, 1H, 13C NMR, SPR |
Anticoagulant |
[5] |
|
Cyamopsis tetragonolobus seeds, Guar gum |
Sulfation |
FTIR, 1H, 13C NMR, OR, GPC, SPR |
Anticoagulant |
[5] |
|
Caesalpinia spinosa, Tara gum |
Sulfation |
FTIR, 1H, 13C NMR, OR, GPC, SPR |
Anticoagulant |
[5] |
|
Ceratonia siliqua L. seeds, Locust bean gum |
Sulfation |
FTIR, 1H, 13C NMR, OR, GPC, SPR |
Anticoagulant |
[5] |
|
Cyamopsis tetragonolobus seeds, Guar gum |
C-glycosylation and sulfation |
UV-VIS, PC |
Chemopreventive |
[35] |
|
Cyamopsis tetragonoloba seeds, Guar gum |
Sulfation |
FTIR, 13C NMR, SEC-MALS |
Antioxidant |
[4] |
|
Adenanthera pavonina L. seeds |
Sulfation |
FTIR, UV-VIS |
Antioxidant |
[29] |
|
Caesalpinia ferrea Mart. seeds |
Sulfation |
FTIR, UV-VIS |
Antioxidant |
[29] |
|
Dimorphandra gardneriana seeds |
Sulfation |
FTIR, UV-VIS |
Antioxidant |
[29] |
|
Cyamopsis tetragonoloba seeds, Guar gum |
Phosphorylation |
FTIR, 13C NMR, XPS, GC–MS, SEC-MALS |
Antioxidant |
[20] |
|
Schizolobium amazonicum seeds |
Sulfation |
FTIR, HPSEC, 13C NMR, GLC, UV-VIS |
Anticancer |
[6] |
|
Cyamopsis tetragonolobus seeds, Guar gum |
C-glycosylation and sulfation |
UV-VIS, PC |
Anticancer |
[35] |
|
Cyamopsis tetragonolobus seeds, Guar gum |
C-glycosylation |
UV-VIS, PC |
Anticancer |
[35] |
|
Schizolobium amazonicum seeds |
Partial hydrolysis |
FTIR, HPSEC, GLC, 13C NMR, 51V NMR, PT |
Anticancer |
[34] |
|
Schizolobium amazonicum seeds |
Partial hydrolysis and complexation with oxovanadium |
FTIR, HPSEC, GLC, 13C NMR, 51V NMR, PT |
Anticancer |
[34] |
|
Mimosa scabrella seeds |
Complexation with oxovanadium |
FTIR, HPSEC, GLC, 51V NMR, PT |
Anticancer |
[19] |
|
Cyamopsis tetragonoloba seeds, Protein-free guar gum |
Sulfation |
FTIR, 1H, 13C NMR, IV, PT, GPC |
Analgesia and chondroprotection |
[25] |
|
Cyamopsis tetragonoloba seeds, Protein-free guar gum |
Oxidation |
FTIR, 1H, 13C NMR, IV, PT, GPC |
Analgesia and chondroprotection |
[25] |
|
Ceratonia siliqua L. seeds, Locust bean gum |
Hydrolysis |
HPSEC, HPLC |
Immunomodulatory |
[38] |
|
Mimosa scabrella seeds |
Complexation with oxovanadium |
FTIR, PT, 51V NMR, GC-MS |
Immunomodulatory |
[22] |
|
Mimosa scabrella seeds |
Complexation with oxovanadium |
FTIR, PT, 51V NMR, GC-MS |
Antileishmanial |
[22] |
|
Ramalina celastri |
Complexation with vanadium |
FTIR, PT |
Immunomodulatory |
[39] |
|
Ramalina celastri |
Complexation with vanadium |
FTIR, PT |
Antileishmanial |
[39] |
|
Ramalina celastri |
Complexation with oxovanadium |
PT, 51V NMR |
Immunomodulatory |
[33] |
|
Ramalina celastri |
Complexation with oxovanadium |
PT, 51V NMR |
Antileishmanial |
[33] |
13C NMR: Carbon-13 (C13) nuclear magnetic resonance; FTIR: Fourier Infrared spectroscopy; GC-MS: Gas Chromatography Mass Spectrometry; GLC: Gas–liquid chromatography; GPC: Gel permeation chromatography; 1H: Proton nuclear magnetic resonance; HPSEC: high-performance size exclusion chromatography; IV: intrinsic viscosity; OR: Optical rotation; PC: Paper chromatography; PT: potentiometric titration; SEC: Size-exclusion chromatography; SDS PAGE: SDS Slab polyacrylamide gel electrophoresis; SEC-MALS: Size Exclusion Chromatography with Multiangle light scattering; SPR: plasmon resonance; TGA: Thermo gravimetric analysis; UV-VIS: ultraviolet–visible spectrophotometry; 51V NMR: Vanadium-51 nuclear magnetic resonance; XPS: X-ray photoelectron spectroscopy; XRD: X-ray diffraction.
References
- Rossi, B.; Campia, P.; Merlini, L.; Brasca, M.; Pastori, N.; Farris, S.; Melone, L.; Punta, C.; Galante, Y.M. An aerogel obtained from chemo-enzymatically oxidized fenugreek galactomannans as a versatile delivery system. Carbohydr. Polym. 2016, 144, 353–361.
- Bassi, P.; Kaur, G. Fenugreek gum derivatives with improved bioadhesion and controlled drug release: In vitro and in vivo characterization. J. Drug Deliv. Sci. Technol. 2015, 29, 42–54.
- Braz, L.; Grenha, A.; Corvo, M.C.; Lourenço, J.P.; Ferreira, D.; Sarmento, B.; Costa, A.M.R. Synthesis and characterization of Locust Bean Gum derivatives and their application in the production of nanoparticles. Carbohydr. Polym. 2018, 181, 974–985.
- Wang, X.; Wang, J.; Zhang, J.; Zhao, B.; Yao, J.; Wang, Y. Structure-antioxidant relationships of sulfated galactomannan from guar gum. Int. J. Biol. Macromol. 2010, 46, 59–66.
- Muschin, T.; Budragchaa, D.; Kanamoto, T.; Nakashima, H.; Ichiyama, K.; Yamamoto, N.; Shuqin, H.; Yoshida, T. Chemically sulfated natural galactomannans with specific antiviral and anticoagulant activities. Int. J. Biol. Macromol. 2016, 89, 415–420.
- Cunha de Padua, M.M.; Cadena, S.M.S.C.; Petkowicz, C.L.O.; Martinez, G.R.; Noleto, G.R. Galactomannan from Schizolobium amazonicum seed and its sulfated derivatives impair metabolism in HepG2 cells. Int. J. Biol. Macromol. 2017, 101, 464–473.
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Nagar, B.J.; Naikwadi, N.N.; Variya, B.C. Galactomannan: A versatile biodegradable seed polysaccharide. Int. J. Biol. Macromol. 2013, 60, 83–92.
- Chrestani, F.; Sierakowski, M.R.; Uchoa, D.E.A.; Nozawa, C.; Sassaki, G.L.; Gorin, P.A.J.; Ono, L. In vitro antiherpetic and antirotaviral activities of a sulfate prepared from Mimosa scabrella galactomannan. Int. J. Biol. Macromol. 2009, 45, 453–457.
- Mestechkina, N.M.; Shcherbukhin, V.D.; Bannikova, G.E.; Varlamov, V.P.; Drozd, N.N.; Tolstenkov, A.S.; Makarov, V.A.; Tikhonov, V.E. Anticoagulant activity of low-molecular-weight sulfated derivatives of galactomannan from Cyamopsis tetragonoloba (L.) seeds. Appl. Biochem. Microbiol. 2008, 44, 111–116.
- Hussein, M.M.D.; Helmy, W.A.; Salem, H.M. Biological activities of some galactomannans and their sulfated derivatives. Phytochemistry 1998, 48, 479–484.
- Dos Santos, V.R.F.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Relationship between galactomannan structure and physicochemical properties of films produced thereof. J. Food Sci. Technol. 2015, 52, 8292–8299.
- Almeida, R.R.; Magalhães, H.S.; Souza, J.R.R.; Trevisan, M.T.S.; Vieira, Í.G.P.; Feitosa, J.P.A.; Araújo, T.G.; Ricardo, N.M.P.S. Exploring the potential of Dimorphandra gardneriana galactomannans as drug delivery systems. Ind. Crop. Prod. 2015, 69, 284–289.
- Ponzini, E.; Natalello, A.; Usai, F.; Bechmann, M.; Peri, F.; Müller, N.; Grandori, R. Structural characterization of aerogels derived from enzymatically oxidized galactomannans of fenugreek, sesbania and guar gums. Carbohydr. Polym. 2019, 207, 510–520.
- Betancur-Ancona, D.; Pacheco-Aguirre, J.; Castellanos-Ruelas, A.; Chel-Guerrero, L. Microencapsulation of papain using carboxymethylated flamboyant (Delonix regia) seed gum. Innov. Food Sci. Emerg. Technol. 2011, 12, 67–72.
- Bassi, P.; Kaur, G. Bioadhesive vaginal drug delivery of nystatin using a derivatized polymer: Development and characterization. Eur. J. Pharm. Biopharm. 2015, 96, 173–184.
- Xie, J.-H.; Wang, Z.-J.; Shen, M.-Y.; Nie, S.-P.; Gong, B.; Li, H.-S.; Zhao, Q.; Li, W.-J.; Xie, M.-Y. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016, 53, 7–15.
- Zhang, Z.; Wang, H.; Chen, T.; Zhang, H.; Liang, J.; Kong, W.; Yao, J.; Zhang, J.; Wang, J. Synthesis and structure characterization of sulfated galactomannan from fenugreek gum. Int. J. Biol. Macromol. 2019, 125, 1184–1191.
- Barddal, H.P.O.; Faria, F.A.M.; Nogueira, A.V.; Iacomini, M.; Cipriani, T.R. Anticoagulant and antithrombotic effects of chemically sulfated guar gum. Int. J. Biol. Macromol. 2020, 45, 604–610.
- Noleto, G.R.; Petkowicz, C.L.O.; Mercê, A.L.R.; Noseda, M.D.; Méndez-Sánchez, S.C.; Reicher, F.; Oliveira, M.B.M. Two galactomannan preparations from seeds from Mimosa scabrella (bracatinga): Complexation with oxovanadium (IV/V) and cytotoxicity on HeLa cells. J. Inorg. Biochem. 2009, 103, 749–757.
- Wang, J.; Yang, T. Synthesis and characterization of phosphorylated galactomannan: The effect of DS on solution conformation and antioxidant activities. Carbohydr. Polym. 2014, 113, 325–335.
- Lavazza, M.; Formantici, C.; Langella, V.; Monti, D.; Pfeiffer, U.; Galante, Y.M. Oxidation of galactomannan by laccase plus TEMPO yields an elastic gel. J. Biotechnol. 2011, 156, 108–116.
- Adriazola, I.O.; Amaral, A.E.; Amorim, J.C.; Correia, B.L.; Petkowicz, C.L.O.; Mercê, A.L.R.; Noleto, G.R. Macrophage activation and leishmanicidal activity by galactomannan and its oxovanadium (IV/V) complex in vitro. J. Inorg. Biochem. 2014, 132, 45–51.
- Godoi, A.M.; Faccin-Galhardi, L.C.; Lopes, N.; Nozawa, C.; Almeida, R.R.; Ricardo, N.M.P.S.; Linhares, R.E.C. Characterization and antiherpetic activity of native and chemically sulfated polysaccharide from Adenanthera pavonina. Curr. Pharm. Biotechnol. 2015, 16, 1024–1031.
- Ba, J.; Gao, Y.; Xu, Q.; Qin, M. Research Development of Modification of Galactomannan Gums from Plant Resources. Adv. Mater. Res. 2012, 482–484, 1628–1631.
- Castro, R.R.; Silva, C.M.M.; Nunes, R.M.; Cunha, P.L.R.; Paula, R.C.M.; Feitosa, J.P.A.; Girão, V.C.C.; Pompeu, M.M.L.; Leite, J.A.D.; Rocha, F.A.C. Structural characteristics are crucial to the benefits of guar gum in experimental osteoarthritis. Carbohydr. Polym. 2016, 150, 392–399.
- Ono, L.; Wollinger, W.; Rocco, I.M.; Coimbra, T.L.M.; Gorin, P.A.J.; Sierakowski, M.R. In vitro and in vivo antiviral properties of sulfated galactomannans against yellow fever virus (BeH111 strain) and dengue 1 virus (Hawaii strain). Antivir. Res. 2003, 60, 201–208.
- Gemin, E.; Ferreira, C.E.O.; Sierakowski, M.R.; Jorge, T.R.; Joineau, M.E.G.; Ono, L. In vitro anti-HSV-1 activity of a chemically sulfated galactomannan from Leucaena leucocephala seeds. J. Basic Appl. Pharm. Sci. 2010, 31, 165–170.
- Lopes, N.; Faccin-Galhardi, L.C.; Espada, S.F.; Pacheco, A.C.; Ricardo, N.M.P.S.; Linhares, R.E.C.; Nozawa, C. Sulfated polysaccharide of Caesalpinia ferrea inhibits herpes simplex virus and poliovirus. Int. J. Biol. Macromol. 2013, 60, 93–99.
- Marques, M.M.M.; Morais, S.M.; Silva, A.R.A.; Barroso, N.D.; Pontes Filho, T.R.; Araujo, F.M.D.C.; Vieira, I.G.P.; Lima, D.M.; Guedes, M.I.F. Antiviral and Antioxidant Activities of Sulfated Galactomannans from Plants of Caatinga Biome. Evid. Based Complement. Altern. Med. 2015, 2015, 1–8.
- Cumpstey, I. Chemical Modification of Polysaccharides. Int. Sch. Res. Not. Org. Chem. 2013, 2013, 1–27.
- Chen, W.L.; Chen, H.L.; Guo, G.W.; Huang, Y.C.; Chen, C.Y.; Tsai, Y.; Huang, K.F.; Chao-Hsun Yang, C.H. Locust bean gum galactomannan hydrolyzed by thermostable β-D-mannanase may reduce the secretion of pro-inflammatory factors and the release of granule constituents. Int. J. Biol. Macromol. 2018, 114, 181–186.
- Noleto, G.R.; Mercê, A.L.R.; Iacomini, M.; Gorin, P.A.J.; Soccol, V.T.; Oliveira, M.B. Effects of a lichen galactomannan and its vanadyl (IV) complex on peritoneal macrophages and leishmanicidal activity. Mol. Cell. Biochem. 2002, 233, 73–83.
- Amaral, A.E.; Petkowicz, C.L.O.; Mercê, A.L.R.; Iacomini, M.; Martinez, G.R.; Rocha, M.E.M.; Cadena, S.M.S.C.; Noleto, G.R. Leishmanicidal activity of polysaccharides and their oxovanadium (IV/V) complexes. Eur. J. Med. Chem. 2015, 90, 732–741.
- Cunha-de Padua, M.M.; Cadena, S.M.S.C.; Petkowicz, C.L.O.; Martinez, G.R.; Rocha, M.E.M.; Mercê, A.L.R.; Noleto, G.R. Toxicity of native and oxovanadium (IV/V) galactomannan complexes on HepG2 cells is related to impairment of mitochondrial functions. Carbohydr. Polym. 2017, 173, 665–675.
- Gamal-Eldeen, A.M.; Amer, H.; Helmy, W.A. Cancer chemopreventive and anti-inflammatory activities of chemically modified guar gum. Chem. Biol. Interact. 2006, 161, 229–240.
- Andrews, P.R.; Craik, D.J.; Martin, J.L. Functional Group Contributions to Drug-Receptor Interactions. J. Med. Chem. 1984, 27, 1648–1657.
- Mao, F.; Ni, W.; Xu, X.; Wang, H.; Wang, J.; Ji, M.; Li, J. Chemical Structure-Related Drug-Like Criteria of Global Approved Drugs. Molecules 2016, 21, 75.
- Godoi, A.M.; Faccin-Galhardi, L.C.; Lopes, N.; Rechenchoski, D.Z.; Almeida, R.R.; Ricardo, N.M.P.S.; Nozawa, C.; Linhares, R.E.C. Antiviral activity of sulfated polysaccharide of Adenanthera pavonina against Poliovirus in HEp-2 Cells. Evid. Based Complement. Altern. Med. 2014, 2014, 712634.
- Nakamura, S.; Kato, A.; Kobayashit, K. Bifunctional Lysozyme-Galactomannan Conjugate Having Excellent Emulsifying Properties and Bactericidal Effect. J. Agric. Food Chem. 1992, 40, 735–739.
- Nakamura, S.; Kato, A. Multi-functional biopolymer prepared by covalent attachment of galactomannan to egg-white proteins through naturally occurring Maillard reaction. Nahrung 2000, 44, 201–206.
- Das, D.; Ara, T.; Dutta, S.; Mukherjee, A. New water-resistant biomaterial biocide film based on guar gum. Bioresour. Technol. 2011, 102, 5878–5883.
- Pires, L.; Gorin, P.A.J.; Reicher, F.; Sierakowski, M.R. An active heparinoid obtained by sulphation of a galactomannan extracted from the endosperm of Senna macranthera seeds. Carbohydr. Polym. 2001, 46, 165–169.




