
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Soo-min Ok | + 3599 word(s) | 3599 | 2021-02-01 05:07:26 | | | |
| 2 | Peter Tang | Meta information modification | 3599 | 2021-02-23 14:22:46 | | |
Video Upload Options
Candida species are common global opportunistic pathogens that could repeatedly and chronically cause oral mucosa infection and create an inflammatory environment, leading to organ dysfunction. Oral Candida infections may cause temporary or permanent damage to salivary glands, resulting in the destruction of acinar cells and the formation of scar tissue. Restricted function of the salivary glands leads to discomfort and diseases of the oral mucosa, such as dry mouth and associated infection.
1. Introduction
Salivary glands, an essential component to maintaining oral health, are susceptible to a variety of pathologies, including candidiasis. The salivary glands are commonly classified as either major or minor salivary glands based on their sizes, distributions, and functional characteristics [1]. The major salivary glands consist of the parotid, submandibular, and sublingual glands [2], which produce and secrete saliva, moisturize intraoral mucosa and teeth, maintain oral hygiene, and facilitate taste, swallowing, speech, and mastication [3]. The minor salivary glands are distributed throughout oral mucosa surfaces, producing mucous saliva with organic substances, even at night, and protect oral mucosa from injury [4][5][6]. Notably, salivary glands produce high concentrations of the secretory immunoglobulin (Ig) A, which prevents other Igs from being broken down by proteolytic enzymes from microbes [7][8]. These critical functions of saliva are repressed when the salivary glands are damaged by Candida infections.
Candida is a genus of yeast and major human fungal pathogens [9]. Candida species are opportunistic pathogens that could repeatedly and chronically cause oral mucosa infections [10][11]. The most prevalent species found in oral Candida infection is Candida albicans, due to its cell adherence properties and great pathogenic potential [12]. C. albicans is isolated from more than 80% of oral Candida lesions [13]. Other clinically relevant species include Candida glabrata, Candida tropicalis, Candida parapsilosis, Candida kefyr, Candida dubliniensis, Candida lusitaniae, Candida krusei, and Candida guilliermondii [14]. It has been reported that 30–45% of healthy adults carry oral Candida organisms, and 25–80% of adults develop oral candidiasis under the condition of using antibiotics, steroids, or immunosuppressants; impaired salivary gland function; improperly fitted dentures; poor oral hygiene; and a high carbohydrate diet. Additionally, 49–54% of healthy infants carry oral Candida organisms, and 5–7% of infants develop oral candidiasis [15][16]. In general, the most commonly affected populations are middle-aged to elderly people. Prevalence rates as high as 70% have been reported in nursing-home residents [17]. Denture-associated oral candidiasis is frequent and occurs globally. Additionally, females are affected slightly more frequently than males [17]. Oral candidiasis also occurs in immunocompromised patients, with an estimated prevalence of 9–31% of acquired immunodeficiency syndrome (AIDS) patients and 20% of cancer patients [18].
Host inflammatory reaction to Candida infection may negatively affect salivary gland tissue and function. During Candida infection, epithelial leukocyte penetration and subepithelial inflammation are observed in histological examinations [19]. The inflammatory mediators, such as chemokines and cytokines (TNF-α, IL-6, and IL1β), are secreted from oral epithelial cells and phagocytic cells, including neutrophils, macrophages, and dendritic cells [19]. The inflammatory reaction could damage salivary glands in the form of sialectasis, ductal ectasia, and progressive acinar destruction. The sublingual and minor salivary glands are located in the superficial layer of the oral mucosa and may be more vulnerable to inflammatory-mediated damage.
2. Candida Infection and Salivary Gland Function
2.1. Candida Infection Affecting Salivation
In the early stages of oral candidiasis, Candida attaches to the oral mucosa and begins to multiply [20][21]. Several proteins, such as secretory IgA, lactoferrin, histatins, and defensins, downregulate adhesion and multiplication of Candida [10][22][23][24]. Among the proteins, histatins and defensins are particularly effective as antifungal factors, which are produced in epithelial cells and salivary glands [25]. Histatins 1, 3, and 5 are present within saliva, accounting for about 80% of total salivary histatins [26]. Human β-defensin-1 was isolated from both the major and minor salivary glands, especially from ductal cells and not acinar cells [27]. Therefore, when salivation or the antifungal agent levels of saliva are reduced, oral microbial hyperproliferation is permitted and oral candidiasis can more easily manifest [24].
Salivary flow showed a significantly negative correlation between stimulated salivary flow rates and Candida colony-forming units (CFUs) in the patients with xerostomia [28]. This trend also appeared in patients with reduced salivation after radiation therapy [29]. Antifungal therapy for candidiasis patients can expect to relieve pain, redness, and oral mucosa atrophy. Notably, antifungal therapy often increases the amount of saliva by removing Candida. A clinical study investigated the effects of Candida elimination on stimulating whole salivary flow rate [30]. The patients with successful elimination of Candida showed significantly increased stimulated whole salivary flow rate, whereas patients with unsuccessful elimination of Candida did not show increased stimulated whole salivary flow rate. Sympathetic stimuli, like acute pain and stress from Candida infection, can reduce salivary flow rate. In other words, parasympathetic stimuli result in increased saliva flow rate; on the other hand, sympathetic stimuli result in more viscous saliva secretions [31][32]. On the basis of this evidence, researchers have suggested that the increased stimulated whole salivary flow rate after treatment was the result of reduced sympathetic stimulation by oral pain reduction [30]. The study states that a decrease in sympathetic stimulation could lead to changed watery salivary secretion. However, 13.5% of the patients with successful elimination of Candida did not show increased stimulated whole salivary flow rate [30]. The unrestored salivary flow rate may be a result of salivary gland destruction from the Candida infection, and the salivary glands could not restore function even after successful Candida treatment [33][34].
2.2. Candida Infection and Host Immune Response
Oral Candida infection on salivary glands causes host immune responses by activation of T lymphocytes. The T cells mediate inflammation by stimulating the production of inflammatory cytokines, such as TNF-α, IL-1ß, and IL-6. These T cells also stimulate the production of inflammatory chemokines and recruit neutrophils and macrophages. The rapid and localized induction of these cytokines form the first line of defense that limits the transmission of invading Candida. However, recurrent or chronic infections can provide an elevated inflammatory environment, leading to organ dysfunction [35] TNF-α and IL-1ß play well-known roles in the pathogenesis of chronic inflammatory diseases. These cytokines may affect salivary gland damage [36][37][38]. The role of these cytokines in the etiology has been determined experimentally in Sjögren’s syndrome with dry mouth [39]. TNF-α suppresses the transcription of Aquaporin-5 and destroys human salivary gland acinar cells. Aquaporin-5 is critical for saliva production and a specific channel protein found in the acinar cells that allows for rapid transcellular migration of water in response to an hydrostatic/osmotic pressure gradient [40][41]. There is a cycle of destruction where Candida causes immune mediated salivary gland destruction, following reduced salivary flow and consequent Candida infection.
3. Diagnosis of Oral Candidiasis
3.1. Tentative Diagnosis Using Clinical Features and Characteristics
The diagnosis of oral candidiasis can usually be made through a complete medical history and physical examination [42]. Most commonly, candidiasis demonstrates as acute pseudomembranous candidiasis, acute atrophic candidiasis, chronic atrophic candidiasis, chronic hyperplastic candidiasis, angular cheilitis, or median rhomboid glossitis (Figure 1) [43]. (a) Pseudomembranous candidiasis accounts for approximately 35% of oral candidiasis cases. In these cases, the pseudomembrane can be easily removed, revealing underlying mucosa, with minimal bleeding. The pseudomembranous white matter consists of debris, fibrin, and exfoliated epithelium invaded by Candida and its hyphae. Acute pseudomembranous candidiasis can be chronic, either intermittently or constantly affecting the patient. The condition may occur in infants, immune-compromised patients (leukemia and AIDS), or patients taking medication such as antibiotics, immunosuppressants, or topical corticosteroids [18][44][45]. (b) Acute atrophic candidiasis, also known as erythema candidiasis, is usually associated with a burning sensation in oral mucosa. It presents as a raw-looking red lesion and occurs prior to the formation of the pseudomembrane or appears after the removal of the pseudomembrane. Acute atrophic candidiasis usually occurs on the dorsal surface of tongue and is characterized by absent papilla due to the use of topical antibiotics or systemic long-term corticosteroids or antibiotics [18][44][45]. (c) Chronic atrophic candidiasis, referred to AS “denture stomatitis”, is usually associated with wearing dentures and inhibited salivary flow. It appears as erythematous inflammation and edema in denture occluded areas. These lesions are caused by dentures rubbing against the oral mucosa, creating a moist and warm environment that is ideal for the growth of Candida. Chronic atrophic candidiasis can be symptomatic, causing soreness and burning, or asymptomatic and only found on routine examination [46][47]. (d) Chronic hyperplastic candidiasis is a rare type of oral candidiasis and appears as a rough or nodular lesion, which complicates the diagnosis by differentiating from oral cancer. It typically appears as white patches on the commissures of the oral mucosa. The main cause of chronic hyperplastic candidiasis is C. albicans, but other systemic co-factors, such as vitamin deficiency and generalized immune suppression, may contribute. Clinically, the lesions are asymptomatic and regress after proper antifungal treatment and correction of underlying nutritional deficiencies or other co-factors. If the lesion is not treated, it can develop into dysplasia or carcinoma [48]. (e) Candida-associated angular cheilitis is inflammatory fissures that emanate from the commissure of the mouth. Angular cheilitis is frequently found in the clinic, including cases involving a combination of Candida and bacterial organisms. Signs and symptoms may include bleeding, blisters, cracks, crusts, itchiness, pain, redness, and swelling. Predisposing factors can be loss of vertical height in the denture wearer, habitual lip licking, mouth breathing, or nutritional deficiencies, particularly with vitamin B12 or iron [49]. (f) Median rhomboid glossitis is a term used to describe the area of a smooth, red, flat, or raised nodule in the middle of the dorsal surface of the tongue. The affected area of the tongue usually does not have a normal coating of filiform papilla covering the entire upper surface of the tongue. High levels of Candida can be discovered from these lesions, which are associated with the frequent use of steroid inhalers or cigarettes [18][44][45].
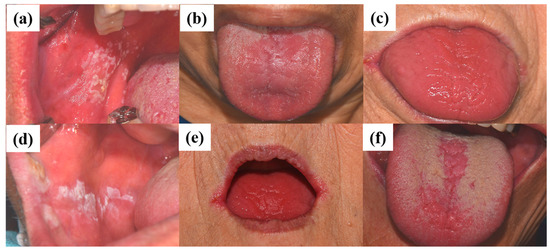
Figure 1. Clinical manifestations of oral candidiasis: (a) acute pseudomembranous candidiasis, (b) acute atrophic candidiasis, (c) chronic atrophic candidiasis, (d) chronic hyperplastic candidiasis, (e) angular cheilitis, and (f) median rhomboid glossitis. Clinical photographs were taken under patients’ informed-consent agreement, with approved Institutional Review Board, PNUDH-2017-026, from the Pusan National University Dental Hospital.
3.2. Definite Diagnosis Using Cytology and Culture
Diagnosis can be confirmed by smear, oral rinse sample, whole saliva sample, culture, or oral biopsy [42]. Specimens for cytology can be obtained by scraping the lesion with a tongue blade. PAS staining of specimens reveals the existence of Candida hyphae and budding yeast. Moreover, 10% potassium hydroxide (KOH), gram, and methylene blue staining can be used instead of PAS. The sensitivity of smear is 51.6%, which is less than that of sample (oral rinse or whole saliva sample) culture. Candida species at a low concentration of 200 to 500 cells per milliliter of saliva could be detected using cell culture method rather than cytology method. Of the asymptomatic healthy population carry Candida in the oral cavity. Therefore, it is necessary to identify a threshold amount of Candida species (>270 CFU/mL), to distinguish oral candidiasis from oral carriage [50]. A definitive diagnosis of candidiasis requires the confirmation of tissue invasion by Candida, using biopsy with PAS staining. Biopsies are always required in hyperplastic candidiasis in order to discard the existence of epithelial dysplasia [51].
4. Prevention and Treatments of Oral Candidiasis
4.1. Prevention
Clinicians should notice that patients with immunocompromised disease, such as AIDS and diabetes, or individuals who have the risk factors of usage of medication (antibiotics, steroids, or immunosuppressants), impaired salivary gland function, dentures, poor oral hygiene, or a high-carbohydrate diet can develop candidiasis easily. Therefore, periodical oral examinations, oral hygiene instruction, and periodic prophylaxis could prevent oral candidiasis. Oral hygiene includes cleaning the tongue with a tongue cleaner, cleaning teeth and dentures with a toothbrush [52], and rinsing oral mucosa with chlorhexidine. In addition, dentures should be removed at night and meticulously washed and soaked in a disinfectant solution, such as chlorhexidine and sodium hypochlorite (1%) [15][53]. To reduce the destruction of salivary glands due to repetitive candidiasis, periodical oral examinations with prophylaxis and proper oral hygiene instruction should be recommended and practiced.
4.2. Treatments of Candida Infection
The treatment of oral candidiasis is based on four basic principles [52][54]: Assess the Candida infection type, diagnose the infection early and accurately, correct the predisposing factor or underlying disease, and administer antifungal agents appropriately. In order to select the proper medications, studies consider factors including local or systematic approach, type of Candida, clinical findings [44], and medication efficacy and toxicity [55]. Commonly used antifungal medications are included in Table 1.
Table 1. Summary of the antifungal medications and their side effects.
| Drug | Formulation | Dose | Side Effect |
|---|---|---|---|
|
Amphotericin B |
Infusion 50 mg |
100–200 mg/6 h |
Renal, cardiovascular, spinal, and neurological |
|
Nystatin |
Suspension 60 mL |
4–6 mL/6 h |
Well tolerated |
|
Ointment 30 g |
2–4 times/day |
||
|
Tablets |
2 every 8 h |
Uncommon nausea, vomiting, and gastrointestinal effects |
|
|
Clotrimazole |
Gel 1% |
3 times/day |
Occasionally skin irritation and burning sensation. |
|
Tablets 10 mg |
5 times/day |
||
|
Miconazole |
Gel |
100 mg/6 h |
Uncommon burning, irritation, nausea, and diarrhea. |
|
Ketoconazole |
Gel 2% |
3 times/day |
Nausea, vomiting |
|
Tablets |
200 mg, 1–2/day |
abdominal pain. |
|
|
Suspension 30 mL |
|||
|
Fluconazole |
Tablets |
50–100 mg/day |
Nausea, vomiting, diarrhea, and abdominal pain. |
|
Suspension |
10 mg/mL |
||
|
Itraconazole |
Capsule |
100–200 mg/day |
Nausea, vomiting, diarrhea, and abdominal pain. |
Table was adapted with permission of CEDRO, Centro Espanol de Derechos Reprograficos, from “Current treatment of oral candidiasis: A literature review”, 6, 5, 2014 [52]); permission conveyed through Copyright Clearance Center, Inc. (Danvers, MA, USA).
Based on the histopathological information via microscopic examination and fungal culture, clinicians should choose the most appropriate antifungal medication. Polyene was the first broad spectrum antifungal agent discovered in the 1940s and 1950s [56]. Polyenes, such as nystatin and amphotericin B, bind to and weaken ergosterols in fungal cell membranes that can initiate the leakage of K+ and Na+ ions, thus contributing to fungal cell death. Polyenes are considered fungicidal and have broad activity against most fungal organisms. Amphotericin B is an antifungal drug used for serious fungal infections and nystatin is used to treat Candida infections of the skin, vagina, mouth, and esophagus [56][57]. Although resistance to polyene medication is rare, some fungal species exhibit intrinsic resistance to polyenes [58][59]. Nystatin is only effective topically, and amphotericin B, which is effective orally and intravenously, is well-known for its severe and potentially lethal side effects such as high fever, kidney damage, and multiple-organ damage. The search for antifungal agents with an acceptable toxicity profile first led to the discovery of azole. Therefore the first azoles were discovered in 1944, but were not approved for use in humans until the early 1960s [56]. Azoles inhibit 14-α-sterol demethylase, a cytochrome P-450 enzyme involved in ergosterol synthesis [60], resulting in the accumulation of toxic sterol intermediaries and loss of membrane integrity. Most azoles are fungistatic and have a broad spectrum against filamentous fungi and yeasts [61][62]. The search for azole antifungal agents with an acceptable toxicity profile led to the discovery of the first ketoconazole. Later, the triazoles fluconazole and itraconazole were developed with an improved safety profile and comparatively broader range of antifungal activity. Analogs have been developed to overcome limitations, such as a suboptimal spectra of activity, need to develop resistance, unfavorable pharmacokinetics, drug–drug interactions, and toxicity [63]. Candida species resistance to the azole medications (e.g., itraconazole, clotrimazole, and fluconazole), including Candida glabrata, Candida tropicalis, or Candida parapsilosis are susceptible to polyene medication. Polyene medications are not well absorbed from the gastrointestinal tract but are effective for topical application [43]. Topical antifungal therapy is recommended as the primary treatment option for mild cases of Candida infection. If the lesion is refractory to topical treatment or recurs frequently, systemic antifungal therapy is suggested. However, systemic antifungal therapy must be considered as the primary treatment for patients with immunocompromised conditions due to the risk of candidemia [57].
The removal of Candida biofilm is necessary, in combination with appropriate medication. Successful treatment of candidiasis depends upon biofilm control, using daily oral hygiene and professional prophylaxis. The Candida biofilm is a thick extracellular polymeric substances layer with a dense network of yeasts, pseudohyphae, and hyphae [64]. The biofilm allows Candida to easily attach between cells and other surfaces, such as dentures. The biofilm provides barriers between Candida and the surrounding environment, thus protecting Candida from antifungal medications [44]. Therefore, the removal of Candida biofilm from the dentures, as well as from all sides of the oral cavity, contributes to lowering the failure rate of antifungal treatment; it is essential for the effective treatment of Candida infection.
4.3. Treatments of Salivary Gland Dysfunction
4.3.1. Symptomatic Management
Hyposalivation is symptomatically managed with methods such as lifestyle changes, synthetic saliva supplementation, salivary gland stimulants, and the use of sialagogues (e.g., muscarinic receptor agonists, pilocarpine, and sevimeline) to elevate the flow rate of saliva [65][66]. Among the sialagogue treatment options, pilocarpine is the most commonly selected medication to increase saliva secretion by stimulating the salivary gland. However, pilocarpine’s effect is temporary and causes side effects, including excessive sweating and tearing, chills, dizziness, flushing, nasal congestion, vocal changes, nervousness, tremors, and diarrhea [67][68]. To improve the side effects of pilocarpine, consistent and controlled release of pilocarpine in the salivary glands was considered [69]. Controlled drug-release systems have been developed and are expected to deliver therapeutic agents directly to salivary glands using novel biomedical approaches, such as hydrogels [70], polymer-based microchips [71], nanoshells [72], and microfluidics technology [73]. For example, polymer hydrogels for controlling pilocarpine release have already been clinically tested in patients with Sjögren’s syndrome [69]. However, the polymer hydrogel and associated medication could not improve the discomfort of patients who had completely destroyed acinar cells. Since the severity of salivary dysfunction may vary from patient to patient [69], the literature suggests that the most effective therapy depends on the evaluation of salivary glands damage.
4.3.2. Gene Therapy and Cellular Stimulation
Gene delivery therapy could be applied to salivary gland cells to ameliorate salivary gland function. Loss of functional water channels in salivary gland epithelia is often considered one of the hallmarks of salivary gland dysfunction, and recent advances are aimed at restoring the permeability in an attempt to increase saliva production. Gene therapy was attempted to deliver the human aquaporin 1 (AQP1) gene to the salivary gland via recombinant adenovirus delivery (AdhAQP1) in rats [74]. The result suggests that AQP1 gene transfer may have potential as an approach for the treatment of salivary hypofunction. In the human study, five of 11 patients experienced elevated salivary flow 3–4.7 years after the AdhAQP1 vector delivery treatment [75]. Clinically, gene delivery to salivary glands offers the accessibility of transfer vectors in a less invasive manner [76]. The administration of bioactive components, cells, and genes directly into the salivary gland is a promising therapeutic option, when salivary-gland cells are intact. Systemic and local delivery was performed to administer a multitude of reagents, including adenoviral vectors, primary cells, growth factors, antioxidant compounds, and cytokines [76][77][78][79][80][81][82][83]. In addition, bone-marrow-derived cell (BMC) recruitment by cytokine stimulation has been reported for recovery of salivary gland cells in vivo [84][85]. Studies demonstrate that subcutaneous injection of granulocyte colony stimulating factor mobilized BMC into the bloodstream and induced migration of BMC to the damaged salivary gland, resulting in improvement of morphology and function of the submandibular salivary gland [84].
4.3.3. Stem Cell Therapy
Among the stem cell approaches, a majority of research relies on mesenchymal stem cells (MSCs) [86][87]. In the MSC salivary transplant in vivo studies, MSCs have been acquired from bone marrow, adipose tissue, or umbilical cord blood [86][88][89]. MSCs are able to be harvested in a non-invasive manner, with relative abundance. Although the differentiation of MSCs into salivary gland acinar cells has been observed in vitro, the actual contribution to differentiation in vivo is unclear and controversial. Their beneficial action can occur primarily through paracrine pro-survival/proliferative effects on the remaining local stem/progenitor cells and cells of the surrounding environment [90][91]. However, MSCs have primary safety concerns, including unknown long-term report, tumorigenic, and metastatic potential. In addition, donor-dependent efficacy and heterogeneous properties of MSCs pose critical obstacles [92]. Therefore, autologous stem cells are preferred to repair salivary gland function. Transplantation of pluripotent salivary gland-specific epithelial stem/progenitor cells has been shown to morphologically and functionally repair salivary gland tissues. Multi-level of potency from the salivary gland cells could be applied to repair compartments of the salivary gland, recover the secretory compartment conditions, and maintain the secretory compartment [93][94]. Permanently differentiated and post-mitotic acinar cells may be able to self-duplicate after damage in post-chronic sialadenitis [95], post-duct ligation [96], partial salivary gland excision [97], and post-radiation therapy. In fact, most patients requiring autologous cell therapy are elderly and do not have enough stem/progenitor cells [98][99]. Although the number of stem/progenitor cells was increased by using heparin sulfate–stimulated growth factors [100] or Aldehyde dehydrogenase-3 activator [101], the absolute number of stem/progenitor cells required for functional regeneration of the human gland has not been clearly defined.
4.3.4. Tissue Engineering
In cases of full destruction of salivary glands, it is insufficient to restore part of the damaged salivary glands and their function. To achieve a complete functional replacement of lost or damaged tissue, tissue-engineered organoids to reconstruct fully functional organs has been proposed [102][103]. In vitro tissue-engineered organoids, using three-dimensional biomaterials loaded with salivary gland cells and/or bioactive cues, can be embedded in extracellular matrix to connect with remaining tissue residues. This approach, called the “organ germ method”, has been evaluated for the regeneration of fully functional salivary glands in mice, which has been induced by mutual epithelial and mesenchymal interactions [103]. The bioengineered salivary gland responded to pilocarpine administration and taste stimulation by producing saliva. To be utilized in clinical practice, an appropriate cell source needs to be clearly identified. Recently, induced pluripotent stem cells or embryonic stem cells have been studied in salivary-gland tissue engineering [104].
Although notable progress in the treatment of hypofunctional salivary gland has been attempted over the last decades, no definitive treatment has been confirmed. Limitations of in vivo studies for translation to human trial are present due to the biological differences between human and rodent salivary glands and require further study [94]. In addition, potential differences in the development and/or regeneration strategies between different glands (e.g., parotid, submandibular, and sublingual) should be considered for future translation.
References
- Hellquist, H.; Skalova, A. Histopathology of the Salivary Glands; Springer: Berlin/Heidelberg, Germany, 2014.
- Aframian, D.J.; Keshet, N.; Nadler, C.; Zadik, Y.; Vered, M. Minor salivary glands: Clinical, histological and immunohistochemical features of common and less common pathologies. Acta Histochem. 2019, 121, 151451.
- Amano, O.; Mizobe, K.; Bando, Y.; Sakiyama, K. Anatomy and histology of rodent and human major salivary glands: -Overview of the Japan salivary gland society-sponsored workshop-. Acta Histochem. Cytochem. 2012, 45, 241–250.
- Hand, A.R.; Pathmanathan, D.; Field, R.B. Morphological features of the minor salivary glands. Arch. Oral Biol. 1999, 44 (Suppl. S1), S3–S10.
- Won, S.; Kho, H.; Kim, Y.; Chung, S.; Lee, S. Analysis of residual saliva and minor salivary gland secretions. Arch. Oral Biol. 2001, 46, 619–624.
- Rayment, S.A.; Liu, B.; Offner, G.D.; Oppenheim, F.G.; Troxler, R.F. Immunoquantification of human salivary mucins MG1 and MG2 in stimulated whole saliva: Factors influencing mucin levels. J. Dent. Res. 2000, 79, 1765–1772.
- Smith, D.J.; Joshipura, K.; Kent, R.; Taubman, M.A. Effect of age on immunoglobulin content and volume of human labial gland saliva. J. Dent. Res. 1992, 71, 1891–1894.
- Rudney, J.D.; Krig, M.A.; Neuvar, E.K.; Soberay, A.H.; Iverson, L. Antimicrobial proteins in human unstimulated whole saliva in relation to each other, and to measures of health status, dental plaque accumulation and composition. Arch. Oral Biol. 1991, 36, 497–506.
- Skinner, C.E.; Fletcher, D.W. A review of the genus candida. Bacteriol. Rev. 1960, 24, 397–416.
- Enache-Angoulvant, A.; Torti, F.; Tassart, M.; Poirot, J.-L.; Jafari, A.; Roux, P.; Hennequin, C. Candidal abscess of the parotid gland due to Candida glabrata: Report of a case and literature review. Med. Mycol. 2010, 48, 402–405.
- Edgerton, M.; Koshlukova, S.E. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv. Dent Res. 2000, 14, 16–21.
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018.
- Reichart, P.A.; Samaranayake, L.P.; Philipsen, H.P. Pathology and clinical correlates in oral candidiasis and its variants: A review. Oral Dis. 2000, 6, 85–91.
- Muadcheingka, T.; Tantivitayakul, P. Distribution of Candida albicans and non-albicans Candida species in oral candidiasis patients: Correlation between cell surface hydrophobicity and biofilm forming activities. Arch. Oral Biol. 2015, 60, 894–901.
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459.
- Barnett, J.A. A history of research on yeasts 12: Medical yeasts part 1, Candida albicans. Yeast 2008, 25, 385–417.
- Scully, C. Oral and Maxillofacial Medicine: The Basis of Diagnosis and Treatment, 2nd ed.; Elsevier: Philadelphia, PA, USA, 2008; pp. 191–200.
- Lalla, R.V.; Patton, L.L.; Dongari-Bagtzoglou, A. Oral candidiasis: Pathogenesis, clinical presentation, diagnosis and treatment strategies. J. Calif. Dent. Assoc. 2013, 41, 263–268.
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral candidiasis: A disease of opportunity. J. Fungi 2020, 6, 15.
- Nagao, Y.; Hashimoto, K.; Sata, M. Candidiasis and other oral mucosal lesions during and after interferon therapy for HCV-related chronic liver diseases. BMC Gastroenterol. 2012, 12, 155.
- Murtaugh, L.C.; Keefe, M.D. Regeneration and repair of the exocrine pancreas. Annu. Rev. Physiol. 2015, 77, 229–249.
- Mahalakshmi, S.; Kandula, S.; Shilpa, P.; Kokila, G. Chronic Recurrent Non-specific Parotitis: A Case Report and Review. Ethiop. J. Health Sci. 2017, 27, 95–100.
- Tanida, T.; Okamoto, T.; Okamoto, A.; Wang, H.; Hamada, T.; Ueta, E.; Osaki, T. Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis. J. Oral Pathol. Med. 2003, 32, 586–594.
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80 (Suppl. S1), S3–S12.
- Ciociola, T.; Giovati, L.; Conti, S.; Magliani, W.; Santinoli, C.; Polonelli, L. Natural and synthetic peptides with antifungal activity. Future Med. Chem. 2016, 8, 1413–1433.
- Edgerton, M.; Koshlukova, S.E.; Araujo, M.W.; Patel, R.C.; Dong, J.; Bruenn, J.A. Salivary histatin 5 and human neutrophil defensin 1 kill Candida albicans via shared pathways. Antimicrob. Agents Chemother. 2000, 44, 3310–3316.
- Sahasrabudhe, K.S.; Kimball, J.R.; Morton, T.H.; Weinberg, A.; Dale, B.A. Expression of the antimicrobial peptide, human beta-defensin 1, in duct cells of minor salivary glands and detection in saliva. J. Dent. Res. 2000, 79, 1669–1674.
- Nadig, S.D.; Ashwathappa, D.T.; Manjunath, M.; Krishna, S.; Annaji, A.G.; Shivaprakash, P.K. A relationship between salivary flow rates and Candida counts in patients with xerostomia. J. Oral Maxillofac. Pathol. 2017, 21, 316.
- Karbach, J.; Walter, C.; Al-Nawas, B. Evaluation of saliva flow rates, Candida colonization and susceptibility of Candida strains after head and neck radiation. Clin. Oral Investig. 2012, 16, 1305–1312.
- Ohga, N.; Yamazaki, Y.; Sato, J.; Asaka, T.; Morimoto, M.; Hata, H.; Satoh, C.; Kitagawa, Y. Elimination of oral candidiasis may increase stimulated whole salivary flow rate. Arch. Oral Biol. 2016, 71, 129–133.
- Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life 2009, 2, 303–307.
- Porcheri, C.; Mitsiadis, T.A. Physiology, pathology and regeneration of salivary glands. Cells 2019, 8, 976.
- Ogle, O.E. Salivary Gland Diseases. Dent. Clin. N. Am. 2020, 64, 87–104.
- Raab-Traub, N.; Rajadurai, P.; Flynn, K.; Lanier, A.P. Epstein-Barr virus infection in carcinoma of the salivary gland. J. Virol. 1991, 65, 7032–7036.
- Deshmukh, U.S.; Nandula, S.R.; Thimmalapura, P.-R.; Scindia, Y.M.; Bagavant, H. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J. Oral Pathol. Med. 2009, 38, 42–47.
- Ferretti, S.; Bonneau, O.; Dubois, G.R.; Jones, C.E.; Trifilieff, A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003, 170, 2106–2112.
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008.
- Festa, A.; D’Agostino, R.; Howard, G.; Mykkänen, L.; Tracy, R.P.; Haffner, S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000, 102, 42–47.
- Ohyama, Y.; Carroll, V.A.; Deshmukh, U.; Gaskin, F.; Brown, M.G.; Fu, S.M. Severe focal sialadenitis and dacryoadenitis in NZM2328 mice induced by MCMV: A novel model for human Sjögren’s syndrome. J. Immunol. 2006, 177, 7391–7397.
- King, L.S.; Yasui, M. Aquaporins and disease: Lessons from mice to humans. Trends Endocrinol. Metab. 2002, 13, 355–360.
- Yamamura, Y.; Motegi, K.; Kani, K.; Takano, H.; Momota, Y.; Aota, K.; Yamanoi, T.; Azuma, M. TNF-α inhibits aquaporin 5 expression in human salivary gland acinar cells via suppression of histone H4 acetylation. J. Cell Mol. Med. 2012, 16, 1766–1775.
- Williams, D.W.; Lewis, M.A.O. Oral Microbiology: Isolation and identification of candida from the oral cavity. Oral Dis. 2008, 6, 3–11.
- Hellstein, J.W.; Marek, C.L. Candidiasis: Red and white manifestations in the oral cavity. Head Neck Pathol. 2019, 13, 25–32.
- Williams, D.; Lewis, M. Pathogenesis and treatment of oral candidosis. J. Oral Microbiol. 2011, 3, 3.
- Reamy, B.V.; Derby, R.; Bunt, C.W. Common tongue conditions in primary care. Am. Fam. Physician 2010, 81, 627–634.
- Khan, T.S.; Muddebihal, F.; Koshy, A. Chronic atrophic candidiasis: A case report and review of literature. Univ. Res. J. Dent. 2015, 5, 123.
- Aoun, G.; Berberi, A. Prevalence of Chronic Erythematous Candidiasis in Lebanese Denture Wearers: A Clinico-microbiological Study. Mater. Sociomed. 2017, 29, 26–29.
- Sitheeque, M.A.M.; Samaranayake, L.P. Chronic hyperplastic candidosis/candidiasis (candidal leukoplakia). Crit. Rev. Oral Biol. Med. 2003, 14, 253–267.
- Sharon, V.; Fazel, N. Oral candidiasis and angular cheilitis. Dermatol. Ther. 2010, 23, 230–242.
- Zhou, P.R.; Hua, H.; Liu, X.S. Quantity of candida colonies in saliva: A diagnostic evaluation for oral candidiasis. Chin. J. Dent. Res. 2017, 20, 27–32.
- Coronado-Castellote, L.; Jiménez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, e279–e286.
- Garcia-Cuesta, C.; Sarrion-Pérez, M.-G.; Bagán, J.V. Current treatment of oral candidiasis: A literature review. J. Clin. Exp. Dent. 2014, 6, e576–e582.
- Vigneswaran, N.; Muller, S. Pharmacologic management of oral mucosal inflammatory and ulcerative diseases. In Contemporary Dental Pharmacology: Evidence-Based Considerations; Jeske, A.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 91–108.
- Aguirre Urizar, J.M. Oral candidiasis. Rev. Iberoam Micol. 2002, 19, 17–21.
- Martínez-Beneyto, Y.; López-Jornet, P.; Velandrino-Nicolás, A.; Jornet-García, V. Use of antifungal agents for oral candidiasis: Results of a national survey. Int. J. Dent. Hyg. 2010, 8, 47–52.
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279.
- Lombardi, A.; Ouanounou, A. Fungal infections in dentistry: Clinical presentations, diagnosis, and treatment alternatives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 533–546.
- Parente-Rocha, J.A.; Bailão, A.M.; Amaral, A.C.; Taborda, C.P.; Paccez, J.D.; Borges, C.L.; Pereira, M. Antifungal Resistance, Metabolic Routes as Drug Targets, and New Antifungal Agents: An Overview about Endemic Dimorphic Fungi. Mediators Inflamm. 2017, 2017, 9870679.
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb. Perspect. Med. 2014, 5, a019752.
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2016, 7, 2173.
- Chang, Y.-L.; Yu, S.-J.; Heitman, J.; Wellington, M.; Chen, Y.-L. New facets of antifungal therapy. Virulence 2017, 8, 222–236.
- Pianalto, K.; Alspaugh, J. New horizons in antifungal therapy. J. Fungi 2016, 2, 26.
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517.
- Kumamoto, C.A. Candida biofilms. Curr. Opin. Microbiol. 2002, 5, 608–611.
- Vissink, A.; Mitchell, J.B.; Baum, B.J.; Limesand, K.H.; Jensen, S.B.; Fox, P.C.; Elting, L.S.; Langendijk, J.A.; Coppes, R.P.; Reyland, M.E. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: Successes and barriers. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 983–991.
- Silvestre, F.J.; Minguez, M.P.; Suñe-Negre, J.M. Clinical evaluation of a new artificial saliva in spray form for patients with dry mouth. Med. Oral Patol. Oral Cir. Bucal 2009, 14, E8–E11.
- Jansma, J.; Vissink, A.; Spijkervet, F.K.; Roodenburg, J.L.; Panders, A.K.; Vermey, A.; Szabó, B.G.; Gravenmade, E.J. Protocol for the prevention and treatment of oral sequelae resulting from head and neck radiation therapy. Cancer 1992, 70, 2171–2180.
- Jellema, A.P.; Slotman, B.J.; Doornaert, P.; Leemans, C.R.; Langendijk, J.A. Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 751–760.
- Gibson, J.; Halliday, J.A.; Ewert, K.; Robertson, S. A controlled release pilocarpine buccal insert in the treatment of Sjögren’s syndrome. Br. Dent. J. 2007, 202, E17.
- Tabata, Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003, 9 (Suppl. S1), S5–S15.
- Richards Grayson, A.C.; Choi, I.S.; Tyler, B.M.; Wang, P.P.; Brem, H.; Cima, M.J.; Langer, R. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat. Mater. 2003, 2, 767–772.
- Hirsch, L.R.; Gobin, A.M.; Lowery, A.R.; Tam, F.; Drezek, R.A.; Halas, N.J.; West, J.L. Metal nanoshells. Ann. Biomed. Eng. 2006, 34, 15–22.
- Sershen, S.R.; Mensing, G.A.; Ng, M.; Halas, N.J.; Beebe, D.J.; West, J.L. Independent Optical Control of Microfluidic Valves Formed from Optomechanically Responsive Nanocomposite Hydrogels. Adv. Mater. Weinheim 2005, 17, 1366–1368.
- Delporte, C.; O’Connell, B.C.; He, X.; Lancaster, H.E.; O’Connell, A.C.; Agre, P.; Baum, B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA 1997, 94, 3268–3273.
- Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Billings, M.E.; Goldsmith, C.M.; Tandon, M.; Helmerhorst, E.J.; Catalán, M.A.; et al. Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene Ther. 2017, 24, 176–186.
- Samuni, Y.; Baum, B.J. Gene delivery in salivary glands: From the bench to the clinic. Biochim. Biophys. Acta 2011, 1812, 1515–1521.
- Varghese, J.J.; Schmale, I.L.; Wang, Y.; Hansen, M.E.; Newlands, S.D.; Ovitt, C.E.; Benoit, D.S.W. Retroductal nanoparticle injection to the murine submandibular gland. J. Vis. Exp. 2018, 135, e57521.
- Shan, Z.; Li, J.; Zheng, C.; Liu, X.; Fan, Z.; Zhang, C.; Goldsmith, C.M.; Wellner, R.B.; Baum, B.J.; Wang, S. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol. Ther. 2005, 11, 444–451.
- Redman, R.S.; Ball, W.D.; Mezey, E.; Key, S. Dispersed donor salivary gland cells are widely distributed in the recipient gland when infused up the ductal tree. Biotech. Histochem. 2009, 84, 253–260.
- Grundmann, O.; Fillinger, J.L.; Victory, K.R.; Burd, R.; Limesand, K.H. Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IGF-1 administration. BMC Cancer 2010, 10, 417.
- Okazaki, Y.; Kagami, H.; Hattori, T.; Hishida, S.; Shigetomi, T.; Ueda, M. Acceleration of rat salivary gland tissue repair by basic fibroblast growth factor. Arch. Oral Biol. 2000, 45, 911–919.
- Zheng, C.; Cotrim, A.P.; Rowzee, A.; Swaim, W.; Sowers, A.; Mitchell, J.B.; Baum, B.J. Prevention of radiation-induced salivary hypofunction following hKGF gene delivery to murine submandibular glands. Clin. Cancer Res. 2011, 17, 2842–2851.
- Marmary, Y.; Adar, R.; Gaska, S.; Wygoda, A.; Maly, A.; Cohen, J.; Eliashar, R.; Mizrachi, L.; Orfaig-Geva, C.; Baum, B.J.; et al. Radiation-Induced Loss of Salivary Gland Function Is Driven by Cellular Senescence and Prevented by IL6 Modulation. Cancer Res. 2016, 76, 1170–1180.
- Lombaert, I.M.A.; Wierenga, P.K.; Kok, T.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin. Cancer Res. 2006, 12, 1804–1812.
- Lombaert, I.M.A.; Brunsting, J.F.; Wierenga, P.K.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Cytokine treatment improves parenchymal and vascular damage of salivary glands after irradiation. Clin. Cancer Res. 2008, 14, 7741–7750.
- Rocchi, C.; Emmerson, E. Mouth-Watering Results: Clinical Need, Current Approaches, and Future Directions for Salivary Gland Regeneration. Trends Mol. Med. 2020, 26, 649–669.
- Jensen, D.H.; Oliveri, R.S.; Trojahn Kølle, S.-F.; Fischer-Nielsen, A.; Specht, L.; Bardow, A.; Buchwald, C. Mesenchymal stem cell therapy for salivary gland dysfunction and xerostomia: A systematic review of preclinical studies. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 335–342.e1.
- Lim, J.-Y.; Yi, T.; Choi, J.-S.; Jang, Y.H.; Lee, S.; Kim, H.J.; Song, S.U.; Kim, Y.-M. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol. 2013, 49, 136–143.
- Kojima, T.; Kanemaru, S.-I.; Hirano, S.; Tateya, I.; Ohno, S.; Nakamura, T.; Ito, J. Regeneration of radiation damaged salivary glands with adipose-derived stromal cells. Laryngoscope 2011, 121, 1864–1869.
- Grønhøj, C.; Jensen, D.H.; Glovinski, P.V.; Jensen, S.B.; Bardow, A.; Oliveri, R.S.; Specht, L.; Thomsen, C.; Darkner, S.; Kiss, K.; et al. First-in-man mesenchymal stem cells for radiation-induced xerostomia (MESRIX): Study protocol for a randomized controlled trial. Trials 2017, 18, 108.
- Grønhøj, C.; Jensen, D.H.; Vester-Glowinski, P.; Jensen, S.B.; Bardow, A.; Oliveri, R.S.; Fog, L.M.; Specht, L.; Thomsen, C.; Darkner, S.; et al. Safety and Efficacy of Mesenchymal Stem Cells for Radiation-Induced Xerostomia: A Randomized, Placebo-Controlled Phase 1/2 Trial (MESRIX). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 581–592.
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759.
- Van Luijk, P.; Pringle, S.; Deasy, J.O.; Moiseenko, V.V.; Faber, H.; Hovan, A.; Baanstra, M.; van der Laan, H.P.; Kierkels, R.G.J.; van der Schaaf, A.; et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci. Transl. Med. 2015, 7, 305ra147.
- Pringle, S.; Maimets, M.; van der Zwaag, M.; Stokman, M.A.; van Gosliga, D.; Zwart, E.; Witjes, M.J.H.; de Haan, G.; van Os, R.; Coppes, R.P. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 2016, 34, 640–652.
- Ihrler, S.; Blasenbreu-Vogt, S.; Sendelhofert, A.; Rössle, M.; Harrison, J.D.; Löhrs, U. Regeneration in chronic sialadenitis: An analysis of proliferation and apoptosis based on double immunohistochemical labelling. Virchows Arch. 2004, 444, 356–361.
- Aure, M.H.; Konieczny, S.F.; Ovitt, C.E. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev. Cell 2015, 33, 231–237.
- Boshell, J.L.; Pennington, C. Histological observations on the effects of isoproterenol on regenerating submandibular glands of the rat. Cell Tissue Res. 1980, 213, 411–416.
- Maimets, M.; Bron, R.; de Haan, G.; van Os, R.; Coppes, R.P. Similar ex vivo expansion and post-irradiation regenerative potential of juvenile and aged salivary gland stem cells. Radiother. Oncol. 2015, 116, 443–448.
- Feng, J.; van der Zwaag, M.; Stokman, M.A.; van Os, R.; Coppes, R.P. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother. Oncol. 2009, 92, 466–471.
- Patel, V.N.; Lombaert, I.M.A.; Cowherd, S.N.; Shworak, N.W.; Xu, Y.; Liu, J.; Hoffman, M.P. Hs3st3-modified heparan sulfate controls KIT+ progenitor expansion by regulating 3-O-sulfotransferases. Dev. Cell 2014, 29, 662–673.
- Banh, A.; Xiao, N.; Cao, H.; Chen, C.-H.; Kuo, P.; Krakow, T.; Bavan, B.; Khong, B.; Yao, M.; Ha, C.; et al. A novel aldehyde dehydrogenase-3 activator leads to adult salivary stem cell enrichment in vivo. Clin. Cancer Res. 2011, 17, 7265–7272.
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230.
- Ogawa, M.; Oshima, M.; Imamura, A.; Sekine, Y.; Ishida, K.; Yamashita, K.; Nakajima, K.; Hirayama, M.; Tachikawa, T.; Tsuji, T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat. Commun. 2013, 4, 2498.
- Hirayama, M.; Oshima, M.; Tsuji, T. Development and prospects of organ replacement regenerative therapy. Cornea 2013, 32 (Suppl. S1), S13–S21.




