
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takashi Oda | + 2057 word(s) | 2057 | 2021-02-07 10:23:33 | | | |
| 2 | Vicky Zhou | Meta information modification | 2057 | 2021-02-16 16:29:29 | | |
Video Upload Options
Acute glomerulonephritis (AGN) triggered by infection is still one of the major causes of acute kidney injury. During the previous two decades, there has been a major paradigm shift in the epidemiology of AGN. The incidence of poststreptococcal acute glomerulonephritis (PSAGN), which develops after the cure of group A Streptococcus infection in children has decreased, whereas adult AGN cases have been increasing, and those associated with nonstreptococcal infections, particularly infections by Staphylococcus, are now as common as PSAGN.
1. Introduction
Acute kidney injury (AKI) has been increasing in the previous few decades and has recently been recognized as an important cause of chronic kidney disease (CKD), which may progress to end stage renal disease (ESRD) [1]. However, the precise mechanism of the transition from AKI to CKD remains obscure and is a matter of great concern.
Acute glomerulonephritis (AGN) triggered by infection is still one of the major causes of AKI. During the past century, poststreptococcal acute glomerulonephritis (PSAGN) that develops after the cure of group A Streptococcus (GAS) infection after a distinct latent period in children comprised the majority of AGN cases [2][3]. However, during the previous two decades, there has been a major paradigm shift in the epidemiology of AGN. Probably owing to the improvement in living environments and the adequate use of antibiotics, the incidence of PSAGN has decreased, particularly in developed countries. On the other hand, adult AGN cases have been increasing, and those associated with nonstreptococcal infections, particularly infection of Staphylococcus, are now as common as PSAGN. Furthermore, in adult AGN patients, particularly older patients with comorbidities, infections are usually ongoing at the time when glomerulonephritis is diagnosed. This is why the term “infection-related glomerulonephritis (IRGN)” has recently been more commonly used instead of “post-infectious AGN” [3]. Notably, whereas most PSAGN in children resolve without any specific treatment, the prognosis of adult IRGN is poor, and older patients, particularly those with immunocompromised backgrounds, such as diabetes mellitus, malignancies, or alcoholism, are reported to be at high risk [4].
Thus, typical PSAGN in children is considered as a benign disease with a favorable prognosis that completely resolves without progression, in contrast to IRGN in adults, which often progresses into chronicity with an unfavorable renal prognosis. However, a long-term epidemiological study demonstrated that an episode of PSAGN in childhood is a strong risk factor for CKD and ESRD in adulthood, even after the complete remission of PSAGN [5][6][7][8][9][10]. Although the precise mechanism of the transition from AGN to CKD remains unknown, understanding it is important as it is expected to lead to the prevention of CKD and ESRD.
In this review, we therefore focus primarily on the possible factors that may contribute to the progression of IRGN, which is a major cause of AKI, into CKD. As summarized in Table 1 and Figure 1, the following four factors are listed and discussed: 1. persistent infection, 2. genetic background of the host’s complement system, 3. tubulointerstitial changes, and 4. pre-existing histological damage due to old age and comorbidities. Among these factors, 2 of them (1 and 2) are associated with the pathogenic mechanism of IRGN, whereas the other 2 factors (3 and 4) are independent of IRGN itself.
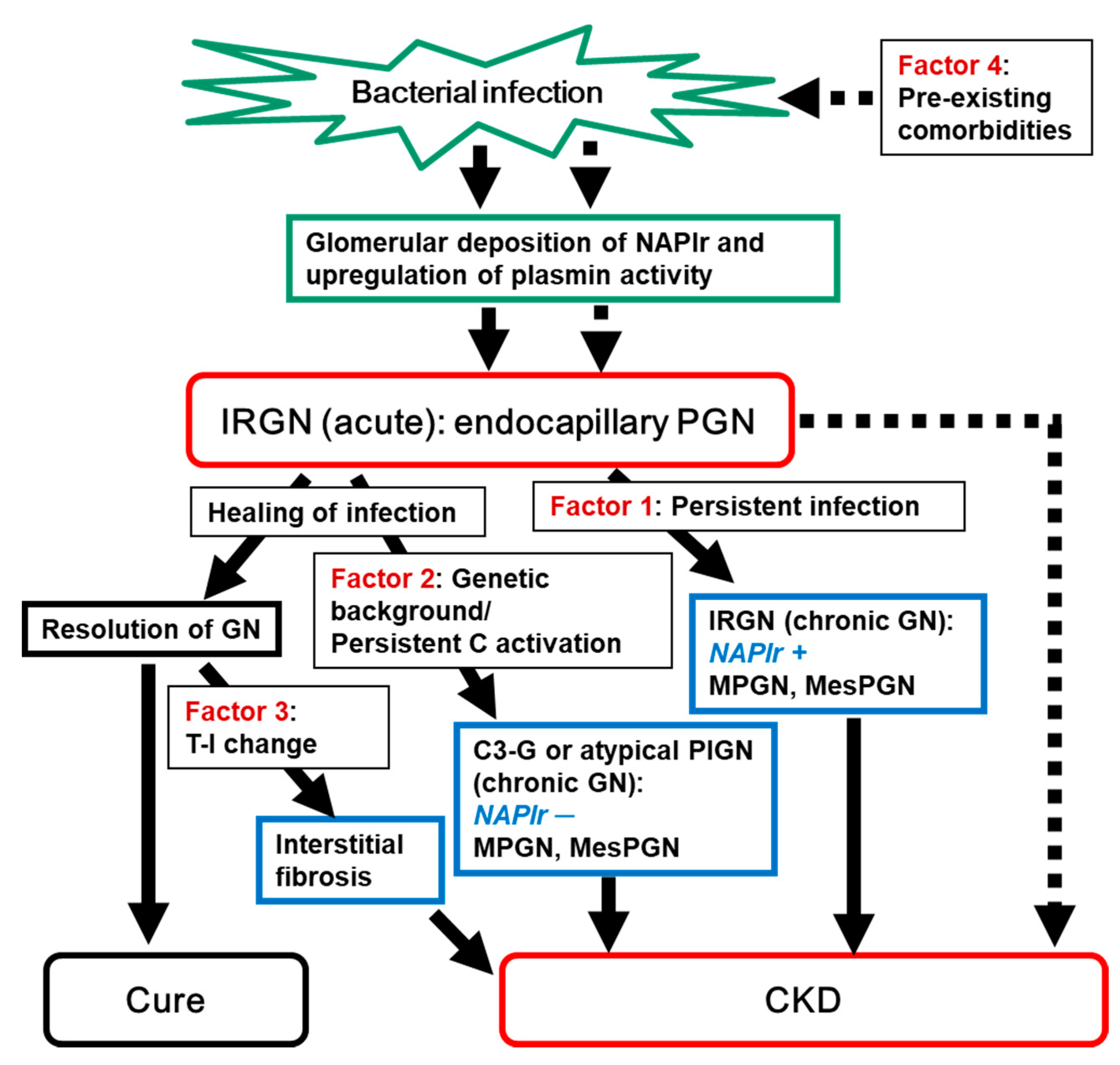
Table 1. Factors affecting the progression of infection-related glomerulonephritis to chronic kidney disease.
| Factor | Evaluation of the Involvement of Each Factor (Biomarkers) | Potential Intervention |
|---|---|---|
| 1. Persistent infection | Histological staining for NAPlr and plasmin activity [11] | Use of antimicrobial agents Removal of indwelling device |
| 2. Genetic background of the host’s complement system [12] | Serum complement levels, histological deposition of complement components, genetic testing | Use of complement—regulating medications (in the future) |
| 3. Tubulointerstitial changes | Interstitial staining for α-SMA | Not yet determined |
| 4. Pre-existing renal histological damage due to comorbidities [4][13] | Histopathological evaluation | Adequate treatment for comorbidities, such as hypertension and DM |
Some autoantibodies, such as the anti-neutrophil cytoplasmic antibody (ANCA), anti-nuclear antibody (ANA), anti-dsDNA antibody, and anti-factor B antibody have been reported to be detected in patients with IRGN [14][15][16][17][18][19][20][21]. Although these antibodies may contribute to the progression of IRGN, at present there is little data regarding their significance on the prognosis of IRGN, and as their involvement remains controversial, we touched on this point but did not list the antibodies as possible factors associated with IRGN.
Generally, clear evidence in this field is very scarce because no large prospective clinical studies have been performed owing to the rarity of IRGN, and, furthermore, a reliable animal model has not been established to date, partly owing to the differences in the infectiveness of pathogens among different species.
2. Persistent Infection as a Possible Cause of the Progression of IRGN into CKD
The simplest reason for the persistence and progression of IRGN into CKD is the persistence of the causative infection, resulting in the continuation of the pathogenic mechanism. In this setting, abnormalities in urinalysis, serum complement levels, and inflammation (ESR and CRP levels) continue, leading to disease progression into chronic glomerulonephritis. Many factors, such as the strain of pathogen (various bacteria and viruses), focus of infection, and conditions of the host (immune competence, comorbidities, use of indwelling devices, etc.) may affect the persistence of pathogens. In terms of bacterial strains, as described in the introduction, GAS infection tends to occur more frequently in children and is usually completely cured before the onset of glomerulonephritis. Glomerular histological analysis in such a condition usually shows the so-called acute change, i.e., prominent endocapillary proliferation mainly by the accumulation of infiltrating cells [22][23]. On the other hand, Staphylococcal IRGN mainly affects older adults who often have comorbidities, and the infection is ongoing when the glomerulonephritis develops [4]. Glomerular histological changes in such patients with ongoing infection may also show endocapillary proliferative glomerulonephritis in the early phase of the disease course. However, as the disease duration after the onset of glomerulonephritis becomes longer, glomerular changes appear to make a gradual transition from endocapillary proliferative glomerulonephritis to membranoproliferative glomerulonephritis (MPGN) or mesangial proliferative glomerulonephritis (MesPGN), probably through chronic glomerular endothelial damage and gradual transition from the accumulation of infiltrating cells to the proliferation of mesangial cells. Staphylococcal infections in older patients, particularly deep-seated infections, are frequently occult in nature and are quite difficult to detect. Therefore, complicating glomerulonephritis tends to be detected in its chronic stages, and histological analysis often shows a MPGN or MesPGN pattern with or without crescent formation and IgA deposition. Typical examples of this condition have been reported in IRGN caused by infective endocarditis due to Staphylococcus aureus and caused by ventriculoatrial shunt infections due to coagulase-negative Staphylococcus epidermidis.
For assessment of the continuation of the pathogenic mechanism due to persistent infection, the identification of histological biomarkers is desired. In this respect, nephritis-associated plasmin receptor (NAPlr) and associated plasmin activity may be useful [11][24][25]. NAPlr was originally isolated from the cytoplasmic fraction of GAS as a candidate nephritogenic protein of PSAGN, and was found to be the same molecule as streptococcal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [24][25]. Glomerular NAPlr deposition is frequently observed by immunofluorescence staining in early stage PSAGN patients (Figure 2); all patients within two weeks of disease onset are reported to show NAPlr deposition [25]. The deposited NAPlr binds with plasmin and maintains its activity by protecting it from physiological inhibitors, and is considered to cause glomerular damage directly by degrading extracellular matrix proteins and indirectly by activating pro-matrix metalloproteases. Additionally, glomerular plasmin activity can exert proinflammatory functions by activating and accumulating inflammatory cells [11].
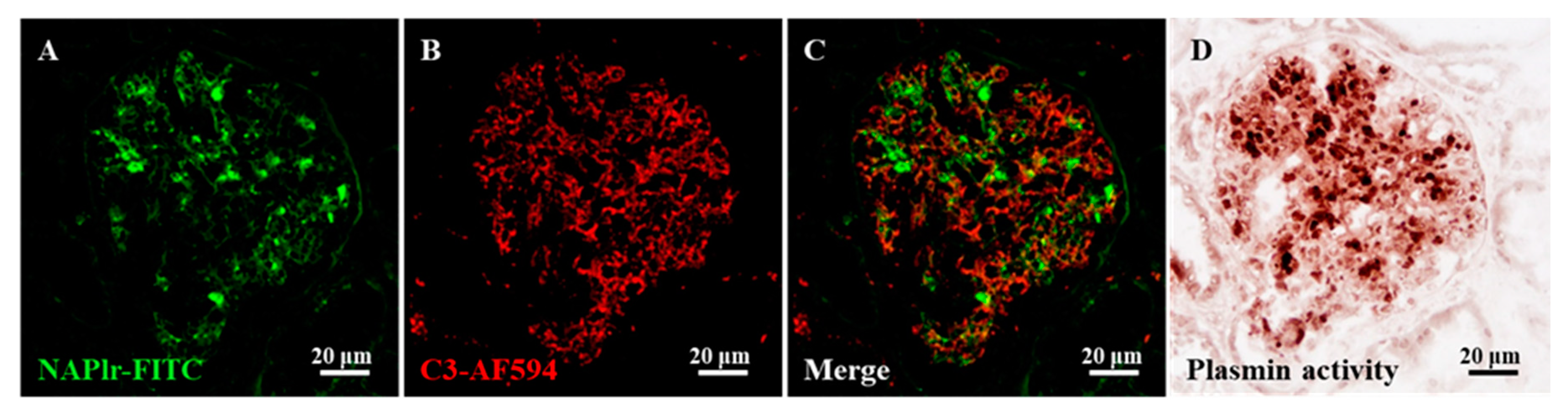
Recently, glomerular NAPlr deposition and associated plasmin activity were reported to be observed not only in patients with PSAGN but also in those with other glomerular diseases, in whom preceding streptococcal infection had been suggested [26][27][28][29][30][31]. In fact, the preceding infection might be an infection other than GAS, because the GAPDH of various bacteria show cross-immunoreactivity to the anti-NAPlr antibody, and simultaneously show plasmin-binding function [32][33][34]. From these results, NAPlr and associated plasmin activity are presently considered as general biomarkers of IRGN [35]. Positive glomerular staining of these markers usually disappears within 30 days after the onset of PSAGN [25]. However, the prolonged positive glomerular staining of these markers (for more than half a year) has been observed in some IRGN patients, suggesting persistent infection and its pathogenic significance in these patients [26][27].
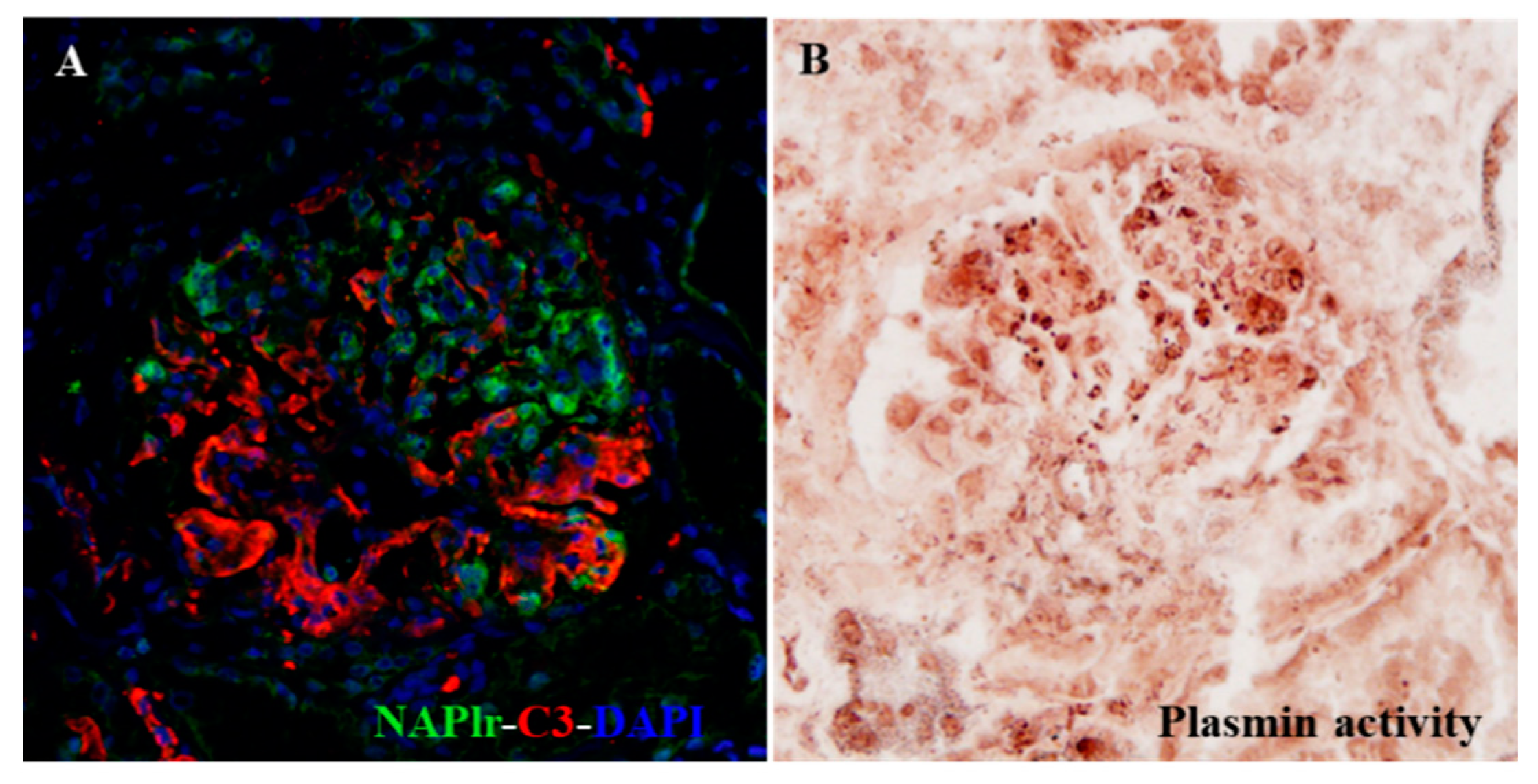
It is very important to shed light on the possible involvement of persistent infection in the pathogenic condition of glomerulonephritis, because this factor is potentially modifiable. Using NAPlr and plasmin activity as biomarkers, the pathogenic involvement of persistent infection can be detected. If these biomarkers are persistently positive, the most important therapeutic strategy would be to eradicate the persistent and pathogenic infection, which may result in blocking the transition of IRGN to CKD. Indeed, Noda et al. recently reported an interesting IRGN case caused by asymptomatic sinusitis, which suggests the importance of detecting the hidden infection by histological staining of NAPlr and plasmin activity. Eradication of the hidden but pathogenic infection in this patient resulted in clinical remission of the disease (Figure 3) [36].
The histological transition from endocapillary proliferative glomerulonephritis to MPGN is also observed in some cases of viral IRGN. Indeed, we encountered a patient in which the first renal biopsy showed endocapillary proliferative glomerulonephritis typical of AGN associated with parvovirus B19 (PVB19) infection, and in the second biopsy, which was performed 4 years subsequently because of persistent proteinuria and prolonged low serum complement C3 level with positivity for the IgM antibody for PVB19 (persistent PVB19 infection), showed MPGN with mesangial interposition and with thickening and double contours of the glomerular basement membrane (GBM) [37]. This case provides lines of evidence that the transition from acute endocapillary proliferative glomerulonephritis to MPGN can actually occur during prolonged infection.
3. Concluding Remarks
IRGN is still considered to be one of the major causes of AKI, and the transition from IRGN to CKD has constantly been a focus of attention. We therefore summarized the possible factors contributing to it.
Regarding the persistence of infection, positive glomerular staining for NAPlr and associated plasmin activity can be used as general histological markers. If they are persistently positive, eradication of the infection is the most important therapeutic strategy to stop the transition of IRGN to CKD.
Understanding the possible involvement of the genetic background of the host’s complement system and pre-existing comorbidities is also important, because both factors are potentially modifiable.
Regarding the tubulointerstitial changes, interstitial α-SMA staining was suggested to be useful for its assessment in IRGN patients. However, the precise mechanism underlying the association of glomerular damage and tubulointerstitial changes with α-SMA expression remain unknown. Furthermore, it is not known as to how tubulointerstitial change can be modified, and this is an important matter for future investigation.
References
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448.
- Satoskar, A.A.; Parikh, S.V.; Nadasdy, T. Epidemiology, pathogenesis, treatment and outcomes of infection-associated glomerulonephritis. Nat. Rev. Nephrol. 2020, 16, 32–50.
- Nasr, S.H.; Radhakrishnan, J.; D’Agati, V.D. Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013, 83, 792–803.
- Nasr, S.H.; Fidler, M.E.; Valeri, A.M.; Cornell, L.D.; Sethi, S.; Zoller, A.; Stokes, M.B.; Markowitz, G.S.; D’Agati, V.D. Postinfectious glomerulonephritis in the elderly. J. Am. Soc. Nephrol. 2011, 22, 187–195.
- Baldwin, D.S.; Gluck, M.C.; Schacht, R.G.; Gallo, G. The long-term course of poststreptococcal glomerulonephritis. Ann. Intern. Med. 1974, 80, 342–358.
- Schacht, R.G.; Gluck, M.C.; Gallo, G.R.; Baldwin, D.S. Progression to uremia after remission of acute poststreptococcal glomerulonephritis. N. Engl. J. Med. 1976, 295, 977–981.
- Gallo, G.R.; Feiner, H.D.; Steele, J.M., Jr.; Schacht, R.G.; Gluck, M.C.; Baldwin, D.S. Role of intrarenal vascular sclerosis in progression of poststreptococcal glomerulonephritis. Clin. Nephrol. 1980, 13, 49–57.
- Hoy, W.E.; White, A.V.; Dowling, A.; Sharma, S.K.; Bloomfield, H.; Tipiloura, B.T.; Swanson, C.E.; Mathews, J.D.; McCredie, D.A. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012, 81, 1026–1032.
- Hoy, W.E.; White, A.V.; Tipiloura, B.; Singh, G.; Sharma, S.K.; Bloomfield, H.; Swanson, C.E.; Dowling, A.; McCredie, D.A. The multideterminant model of renal disease in a remote Australian Aboriginal population in the context of early life risk factors: Lower birth weight, childhood post-streptococcal glomerulonephritis, and current body mass index influence levels of albuminuria in young Aboriginal adults. Clin. Nephrol. 2015, 83 (Suppl. 1), 75–81.
- Karmarkar, M.G.; Hule, G.P.; Hase, N.K.; Mehta, P.R.; Walter, S.R.; Sriprakash, K.S. Seroprevalence of Streptococcal Inhibitor of Complement (SIC) suggests association of streptococcal infection with chronic kidney disease. BMC Nephrol. 2013, 14, 101.
- Oda, T.; Yamakami, K.; Omasu, F.; Suzuki, S.; Miura, S.; Sugisaki, T.; Yoshizawa, N. Glomerular plasmin-like activity in relation to nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. J. Am. Soc. Nephrol. 2005, 16, 247–254.
- Sethi, S.; Fervenza, F.C.; Zhang, Y.; Zand, L.; Meyer, N.C.; Borsa, N.; Nasr, S.H.; Smith, R.J. Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2012, 83, 293–299.
- Ramanathan, G.; Abeyaratne, A.; Sundaram, M.; Fernandes, D.K.; Pawar, B.; Perry, G.J.; Sajiv, C.; Majoni, S.W. Analysis of clinical presentation, pathological spectra, treatment and outcomes of biopsy-proven acute postinfectious glomerulonephritis in adult indigenous people of the Northern Territory of Australia. Nephrology 2017, 22, 403–411.
- Ying, C.M.; Yao, D.T.; Ding, H.H.; Yang, C.D. Infective endocarditis with antineutrophil cytoplasmic antibody: Report of 13 cases and literature review. PLoS ONE 2014, 9, e89777.
- Cervi, A.; Kelly, D.; Alexopoulou, I.; Khalidi, N. ANCA-associated pauci-immune glomerulonephritis in a patient with bacterial endocarditis: A challenging clinical dilemma. Clin. Nephrol. Case Stud. 2017, 5, 32–37.
- Bele, D.; Kojc, N.; Perše, M.; Černe Čerček, A.; Lindič, J.; Aleš Rigler, A.; Večerić-Haler, Ž. Diagnostic and treatment challenge of unrecognized subacute bacterial endocarditis associated with ANCA-PR3 positive immunocomplex glomerulonephritis: A case report and literature review. BMC Nephrol. 2020, 21, 40.
- Satoskar, A.A.; Suleiman, S.; Ayoub, I.; Hemminger, J.; Parikh, S.; Brodsky, S.V.; Bott, C.; Calomeni, E.; Nadasdy, G.M.; Rovin, B.; et al. Staphylococcus infection-associated GN–Spectrum of IgA staining and prevalence of ANCA in a single-center cohort. Clin. J. Am. Soc. Nephrol. 2017, 12, 39–49.
- Sève, P.; Ferry, T.; Koenig, M.; Cathebras, P.; Rousset, H.; Broussolle, C. Lupus-like presentation of parvovirus B19 infection. Semin. Arthritis Rheum. 2005, 34, 642–648.
- Cugler, T.; Carvalho, L.M.; Facincani, I.; Yamamoto, A.Y.; Silva, G.E.; Costa, R.S.; Ferriani, V.P. Severe glomerulonephritis and encephalopathy associated with parvovirus B19 infection mimicking systemic lupus erythematosus. Scand. J. Rheumatol. 2012, 41, 79–81.
- Amel, R.; Monia, K.; Anis, M.; Fatma, B.F.; Chadia, L. Systemic lupus erythematous revealed by cytomegalovirus infection. Pan Afr. Med. J. 2016, 24, 241.
- Chauvet, S.; Berthaud, R.; Devriese, M.; Mignotet, M.; Vieira Martins, P.; Robe-Rybkine, T.; Miteva, M.A.; Gyulkhandanyan, A.; Ryckewaert, A.; Louillet, F.; et al. Anti-Factor B Antibodies and Acute Postinfectious GN in Children. Anti-Factor B Antibodies and Acute Postinfectious GN in Children. J. Am. Soc. Nephrol. 2020, 31, 829–840.
- Yoshizawa, N.; Oda, T.; Oshikawa, Y.; Akashi, Y.; Suzuki, Y.; Shimizu, J.; Shimizu, E.; Niwa, H.; Treser, G. Cell-mediated immune response in acute poststreptococcal glomerulonephritis. Nihon Jinzo Gakkai Shi 1994, 36, 322–330.
- Oda, T.; Yoshizawa, N.; Takeuchi, A.; Nakabayashi, I.; Nishiyama, J.; Ishida, A.; Tazawa, K.; Murayama, M.; Hotta, O.; Taguma, Y. Glomerular proliferating cell kinetics in acute post-streptococcal glomerulonephritis (APSGN). J. Pathol. 1997, 183, 359–368.
- Yoshizawa, N.; Yamakami, K.; Fujino, M.; Oda, T.; Tamura, K.; Matsumoto, K.; Sugisaki, T.; Boyle, M.D. Nephritis-associated plasmin receptor and acute poststreptococcal glomerulonephritis: Characterization of the antigen and associated immune response. J. Am. Soc. Nephrol. 2004, 15, 1785–1793.
- Yamakami, K.; Yoshizawa, N.; Wakabayashi, K.; Takeuchi, A.; Tadakuma, T.; Boyle, M.D. The potential role for nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. Methods 2000, 21, 185–197.
- Sawanobori, E.; Umino, A.; Kanai, H.; Matsushita, K.; Iwasa, S.; Kitamura, H.; Oda, T.; Yoshizawa, N.; Sugita, K.; Higashida, K. A prolonged course of Group A streptococcus-associated nephritis: A mild case of dense deposit disease (DDD)? Clin. Nephrol. 2009, 71, 703–707.
- Suga, K.; Kondo, S.; Matsuura, S.; Kinoshita, Y.; Kitano, E.; Hatanaka, M.; Kitamura, H.; Hidaka, Y.; Oda, T.; Kagami, S. A case of dense deposit disease associated with a group A streptococcal infection without the involvement of C3NeF or complement factor H deficiency. Pediatr. Nephrol. 2010, 25, 1547–1550.
- Okabe, M.; Tsuboi, N.; Yokoo, T.; Miyazaki, Y.; Utsunomiya, Y.; Hosoya, T. A case of idiopathic membranoproliferative glomerulonephritis with a transient glomerular deposition of nephritis-associated plasmin receptor antigen. Clin. Exp. Nephrol. 2012, 16, 337–341.
- Iseri, K.; Iyoda, M.; Yamamoto, Y.; Kobayashi, N.; Oda, T.; Yamaguchi, Y.; Shibata, T. Streptococcal Infection-related Nephritis (SIRN) Manifesting Membranoproliferative Glomerulonephritis Type I. Intern. Med. 2016, 55, 647–650.
- Kikuchi, Y.; Yoshizawa, N.; Oda, T.; Imakiire, T.; Suzuki, S.; Miura, S. Streptococcal origin of a case of Henoch-Schoenlein purpura nephritis. Clin. Nephrol. 2006, 65, 124–128.
- Yano, K.; Suzuki, H.; Oda, T.; Ueda, Y.; Tsukamoto, T.; Muso, E. Crescentic poststreptococcal acute glomerulonephritis accompanied by small vessel vasculitis: Case report of an elderly male. BMC Nephrol. 2019, 20, 471.
- Odaka, J.; Kanai, T.; Ito, T.; Saito, T.; Aoyagi, J.; Betsui, H.; Oda, T.; Ueda, Y.; Yamagata, T. A case of post-pneumococcal acute glomerulonephritis with glomerular depositions of nephritis-associated plasmin receptor. CEN Case Rep. 2015, 4, 112–116.
- Komaru, Y.; Ishioka, K.; Oda, T.; Ohtake, T.; Kobayashi, S. Nephritis-associated plasmin receptor (NAPlr) positive glomerulonephritis caused by Aggregatibacter actinomycetemcomitans bacteremia: A case report. Clin. Nephrol. 2018, 90, 155–160.
- Hirano, D.; Oda, T.; Ito, A.; Yamada, A.; Kakegawa, D.; Miwa, S.; Umeda, C.; Takemasa, Y.; Tokunaga, A.; Wajima, T.; et al. Glyceraldehyde-3-phosphate dehydrogenase of Mycoplasma pneumoniae induces infection-related glomerulonephritis. Clin. Nephrol. 2019, 92, 263–272.
- Uchida, T.; Oda, T. Glomerular deposition of nephritis-associated plasmin receptor (NAPlr) and related plasmin activity: Key diagnostic biomarkers of bacterial infection-related glomerulonephritis. Int. J. Mol. Sci. 2020, 21, 2595.
- Noda, S.; Mandai, S.; Oda, T.; Shinoto, T.; Sato, H.; Sato, K.; Hirokawa, K.; Noda, Y.; Uchida, S. Asymptomatic sinusitis as an origin of infection-related glomerulonephritis manifesting steroid-resistant nephrotic syndrome: A case report. Medicine 2020, 99, e20572.
- Uchida, T.; Oda, T.; Watanabe, A.; Yamamoto, K.; Katsurada, Y.; Shimazaki, H.; Tamai, S.; Kumagai, H. Transition from endocapillary proliferative glomerulonephritis to membranoproliferative glomerulonephritis in a patient with a prolonged human parvovirus B19 infection. Clin. Nephrol. 2014, 82, 62–67.




