Video Upload Options
The fluorescent carbon dot is a novel type of carbon nanomaterial. In comparison with semiconductor quantum dots and fluorescence organic agents, it possesses significant advantages such as excellent photostability and biocompatibility, low cytotoxicity and easy surface functionalization, which endow it a wide application prospect in fields of bioimaging, chemical sensing, environmental monitoring, disease diagnosis and photocatalysis as well. Biomass waste is a good choice for the production of carbon dots owing to its abundance, wide availability, eco-friendly nature and a source of low cost renewable raw materials such as cellulose, hemicellulose, lignin, carbohydrates and proteins, etc.
1. Introduction
First discovered by Xu et al. in 2004 [1] and subsequently named by Sun et al. in 2006 [2], carbon dots (C-dots) are novel fluorescent carbon nanomaterials and among the most important members of the carbon nanomaterials family. In general, C-dots are well-dispersed spherical particles with particle size less than 10 nm. Besides the high quantum yield and adjustable emission wavelength, which are also possessed by traditional semiconductor quantum dots, C-dots have many other excellent characteristics, including good photostability, low cytotoxicity, good biocompatibility, easy surface modification and high chemical inertness, and therefore have attracted considerable scholarly attention in recent years. Hence far, C-dots have been widely used in many fields such as cell imaging [3][4][5], in vivo imaging [6][7], drug delivery [8][9][10], fluorescence sensing [11][12][13], photocatalysis [14][15][16], multicolor light-emitting diode (LED) production [17][18], energy conversion and storage [19][20][21], etc. C-dots are gradually become one of the research hotspots in the above-mentioned fields and considered as a potential substitute for semiconductor quantum dots.
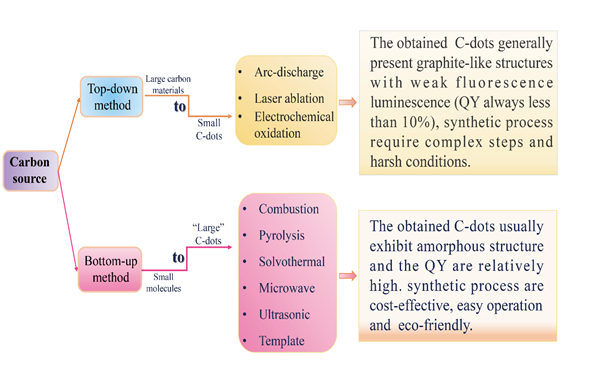
The controllable synthesis of carbon dots is currently still in the early stage of its development. The synthesis methods can be divided into two main categories, top-down and bottom-up approaches (Figure 1). The top-down approaches include electrochemical oxidation [22][23][24], arc discharge [25] and laser ablation [26][27][28], which involve the exfoliation process of larger carbonaceous materials (e.g., large-size graphene, carbon nanotube, graphite, commercial activated carbon) into nanoscale C-dots. During the synthesis of C-dots using top-down approaches, harsh experimental conditions (e.g., strong acid and arc discharge), tedious operation steps and expensive equipment are usually employed, which greatly limit their practical application. The bottom-up approaches, such as the microwave-assisted method [29][30][31], pyrolysis [32][33][34], solvothermal method [35][36][37], ultrasonic method [38][39][40], on the contrary, convert small molecules into C-dots via carbonization and passivation. They have the advantages of cost-effectiveness, easy operation and simple equipment requirements, and hence have been widely used in the synthesis of C-dots.
Figure 1. The top-down and bottom-up approaches for synthesizing of C-dots (QY refers to fluorescence quantum yield).
The raw materials for the synthesis of C-dots are very abundant and can be classified into two types, i.e., organic and inorganic carbon sources. The fluorescent quantum yields of C-dots produced from inorganic carbon sources are relatively low [41]. Further surface passivation of bare carbon dots is necessary to enhance their luminescent efficiency [42]. In practice, organic carbon source such as organic compounds, organic natural products and biomass waste is more popularly used in the preparation of C-dots [43][44][45].
2. Synthesis of C-dots from Biomass Wastes
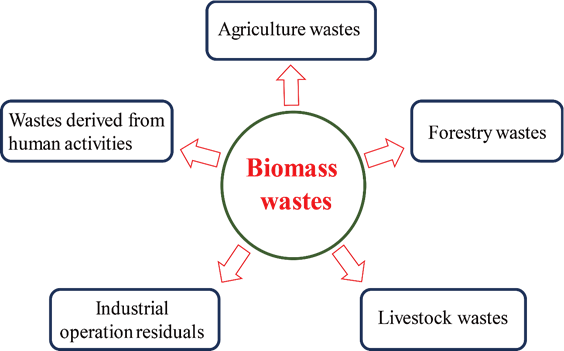
Biomass is a complex, abundant, heterogeneous,biodegradable and bio-organic substance that may be obtained from diverse sources such as perennial grass, organic domestic garbage, residues of agriculture, fishery, poultry, animal husbandry, forestry and related industries [46][47][48] (Figure 2). Biomass waste is a natural organic carbon source, mainly composed of cellulose, hemicellulose, lignin, ash, proteins and some other ingredients. Taking plant biomass waste as an example, among which, cellulose accounts for 30–60%, hemicellulose 20–40%, lignin 15–25% [49]. Biomass waste is a renewable, environmentally friendly, abundantly available and innocuous carbon source for C-dots production. However, most of the biomass waste is currently discarded, landfilled or openly burned, which not only lead to a waste of resource but also cause some environmental problems threatening human health [50]. Recently, there have been some attempts to utilize biomass wastes as raw materials in the production of C-dots. In the following sections, the methods for the synthesis of carbon dots from biomass waste are discussed.
Figure 2. Main sources of biomass waste.
2.1. Pyrolysis
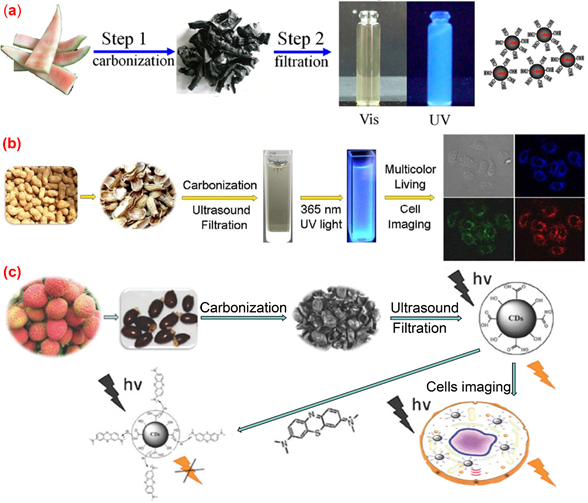
Pyrolysis is a widely used approach for preparing C-dots. The organic substance in the carbon source is gradually converted into carbon dots via heating, dehydration, degradation, carbonization under high temperature in either vacuum or inert atmospheres. High-concentration acid or alkali is generally used in the pyrolysis method to cleave carbon precursors into nanoparticles. All kinds of biomass materials, including watermelon peel, sago waste, coffee grounds and plant leaves, could be used as carbon sources for producing C-dots by pyrolysis method. The properties of the obtained C-dots can be regulated by changing the conditions of pyrolysis, such as pyrolysis temperature, pyrolysis duration, and the pH value of the reaction systems [51]. Zhou et al. [52] achieved large-scale production of C-dots by pyrolysis of waste watermelon peels under low-temperature followed by filtration. The obtained C-dots possess strong blue luminescence, excellent water solubility, good stability in solutions with a wide range of pH and high salinity. The as-prepared carbon dots were successfully used in HeLa cell imaging (Figure 3a). Fluorescence C-dots with a quantum yield of 10.6% and low inherent cytotoxicity was obtained by pyrolysis from lychee seeds [53] and used for fluorescence imaging of living HepG2 cells (Figure 3b). Xue et al. [54] proposed a simple and economical pyrolysis method for the synthesis of fluorescent C-dots from peanut shell waste. The C-dots obtained were N-doped carbon nanomaterials with a quantum yield of 9.91% and possessed good stability, good photo-bleaching resistance, high tolerance to large fluctuation in pH and ionic strength. The surface of the N-doped carbon dots contained a variety of functional groups such as hydroxyl, carboxyl and amino groups. The authors claimed that the emission spectrum of the C-dots prepared by their method was excitation-wavelength-dependent, which was attributed to a wide range of particle sizes and emissive sites. This kind of C-dot was successfully applied to live-cell multicolor imaging (Figure 3c). Praneerad and coworkers [55] prepared C-dots from durian peel waste by pyrolysis method and used them as an effective dopant to construct a composite electrode with a specific capacitance of 60 F·g-1, much higher than that of the pure activated carbon electrode. Sago industrial waste was also used as a carbon source for the synthesis of C-dots by a simple thermal pyrolysis method [56]. The effects of pyrolysis conditions on the physical appearance, particle sizes and fluorescence intensities of C-dots produced from sago industrial waste were investigated in detail [56]. In another article, bio-waste sago bark was also used as a starting precursor for the synthesis of C-dots through a simple one-step pyrolysis method [57], the results obtained by cell viability assay suggested that the C-dots produced from sago industrial waste have low cytotoxicity to cells, and hence can be potentially used for cell imaging. Furthermore, the porosity of the C-dots obtained by pyrolysis approaches endows them with potential applications in anticancer drug delivery.
Figure 3. Preparation of fluorescence C-dots from agriculture wastes by pyrolysis method. (a) Synthesis of water-soluble C-dots from watermelon peel [52]; (b) the preparation and application of fluorescent C-dots from lychee seeds [53]; (c) the preparation and application of C-dots from peanut shells [54].
2.2. Solvothermal Method
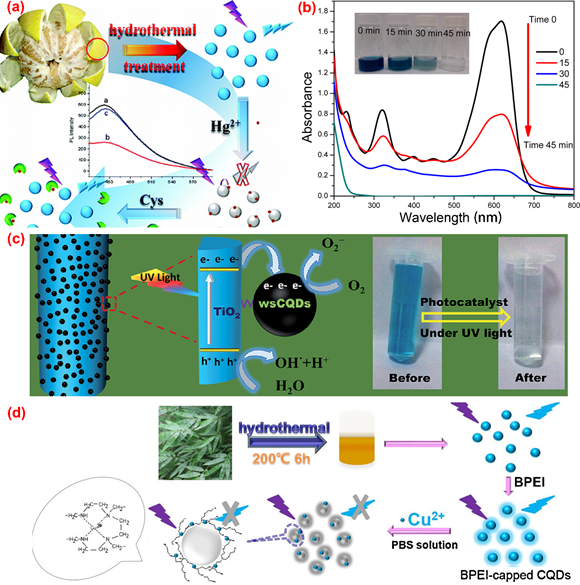
The solvothermal method is quite popular in the synthesis of C-dots. The organic or inorganic carbon source is mixed with one or several solvents in a stainless steel autoclave with a Teflon liner. After a certain period time of continuous heating, the raw carbon source will be converted to C-dots in the air or inert atmosphere under high temperature and pressure conditions. The solvothermal method has the advantages of environmental friendliness, low cost, easy operation and requirement of simple equipment. A variety of carbon precursors can be used for the synthesis of C-dots by the solvothermal method. Table 1 summarized the properties and applications of C-dots synthesized from various biomass waste by solvothermal method. For example, Lu et al. [58] utilized pomelo peel to synthesize water-soluble C-dots with a quantum yield of 6.9% for the selective and sensitive determination of Hg2+ by hydrothermal method (Figure 4a). Prasannan et al. [59] proposed a facile one-pot hydrothermal carbonization method for the synthesis of fluorescent C-dots from orange waste peels under mild conditions and composited the C-dots with ZnO for photo-catalyzing degradation of naphthol blue-black azo dye under UV irradiation (Figure 4b). Tyagi et al. [60] obtained C-dots with spherical morphology and oxygen-rich surface functionalities from lemon peel waste through a facile hydrothermal process and applied them to the determination of Cr6+ and the preparation of TiO2–C-dots for the photocatalytic degradation of methylene blue dye under UV light irradiation (Figure 4c). Liu et al. [61] synthesized C-dots with a quantum yield of 7.1% from waste bamboo leaves via the hydrothermal method and coated the C-dots with branched polyethylenimine through electrostatic adsorption for the selective and sensitive detection of Cu2+ in river water (Figure 4d). Moreover, Sarswat et al. [62] utilized food, beverage and combustion wastes as resources to produce multicolor and highly luminescent C-dots for the fabrication of light-emitting diodes. In Table 1, carbon dots obtained from various biomass waste by the solvothermal method were demonstrated. Obviously, due to the high efficiency and easy operation, the solvothermal method is one of the most frequently used methods for carbon dots preparation.
Figure 4. The preparation of fluorescence C-dots from biomass wastes through the hydrothermal method. (a) The preparation and application of C-dots from pomelo peel [58]; (b) absorption spectra of naphthol blue-black (NBB) azo dye over C-dots/ZnO at different irradiation intervals [59]; (c) the photocatalytic degradation of methylene blue (MB) on TiO2–C-dots composite under UV light irradiation [60]; (d) the synthesis and application of C-dots from bamboo leaves [61].
Table 1. The properties and applications of C-dots produced from different biomass waste by solvothermal method.
|
Biomass Waste |
Hydrothermal Condition |
Fluorescence Quantum Yield |
Application |
Ref.1 |
|
Wheat bran |
180 °C, 3 h |
- |
drug delivery |
[63] |
|
Sugarcane Bagasse char |
190 °C, 24 h |
- |
drug delivery |
[64] |
|
Waste food |
200 °C, 1.5 h |
- |
light emitting diodes |
[62] |
|
Orange peels |
180 °C, 12 h |
- |
photocatalysis |
[59] |
|
Onion waste |
120 °C, 2 h |
28% |
Fe3+ detection and multicolor imaging |
[65] |
|
Waste food |
195 °C, 225 °C, 255 °C, 12 h |
28%, 18%, 10%, 6% for blue, green, yellow and red C-dots, respectively |
Fe3+ detection |
[66] |
|
Tobacco leaves |
200 °C, 3 h |
27.9% |
three kinds of tetracyclines detection |
[67] |
|
Coffee grounds |
200 °C, 6–10 h |
24% |
Fe3+, Cu2+detection |
[68] |
|
Rice residue |
200 °C, 12 h |
23.48% |
Fe3+ ions and tetracyclines detection |
[69] |
|
Bael leaves |
170 °C, 5 h |
22% |
Fe3+ detection |
[70] |
|
Wheat straw |
250 °C, 10 h |
20% |
labeling, imaging and sensing |
[71] |
|
Lemon peels |
200 °C, 12 h. |
14% |
sensing and photocatalysis |
[60] |
|
Wheat straw and bamboo residues |
180 °C, 4 h |
13% |
cell imaging and in vivo bioimaging |
[72] |
|
Dried lemon peels |
200 °C, 6 h |
11% |
carmine detection |
[73] |
|
Tulsi leaves |
180 °C, 4 h |
9.3% |
Pb2+ detection |
[74] |
|
Magnolia flower |
200 °C, 8 h |
8.13% |
Fe3+ detection |
[75] |
|
Bamboo leaves |
200 °C, 6 h |
7.1% |
Cu2+detection |
[61] |
|
Pomelo peels |
200 °C, 3 h. |
6.9% |
Hg2+detection |
[58] |
|
Coconut husks |
200 °C, 3 h |
- |
pH sensor |
[76] |
|
Prawn shells |
180 °C, 12 h |
- |
nitrite detection |
[77] |
1 refers to the reference.
2.3. Microwave-Assisted Method
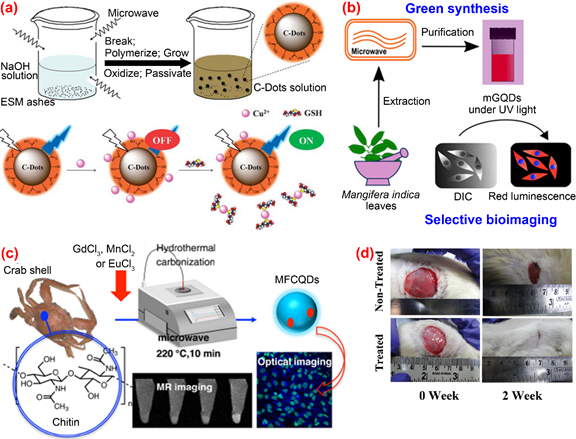
The microwave-assisted method is a widely employed method for directly carbonizing organic substances into C-dots under microwave radiation. Due to its efficiency, simplicity in terms of device and operation, the microwave-assisted method is a cost-effective approach with a strong competitive edge for producing large amounts of fluorescent C-dots. Wang et al. [78] proposed a waste-reused and eco-friendly microwave-assisted approach for preparing C-dots from protein-rich kitchen waste of eggshell membranes. The obtained C-dots possessed good water solubility and excellent fluorescent with a quantum yield of about 14% and were used for the simultaneous determination of Cu2+ and glutathione (Figure 5a). Kumawat et al. [79] developed a relatively simple one-pot microwave-assisted method for preparing C-dots from Mangifera indica leaves. Their C-dots exhibited excitation-independent NIR emission, superior cellular uptake, high photostability, good biocompatibility and intracellular temperature sensing capability. (Figure 5b). A one-pot microwave-assisted hydrothermal method was reported by Yao et al. [80] for the synthesis of novel magnetofluorescent carbon quantum dots (i.e., Gd@CQDs, Mn@CQDs and Eu@CQDs, respectively) from waste crab shell and transition-metal ions Gd3+, Mn2+ and Eu3+ (Figure 5c). The obtained magneto-fluorescent C-dots exhibited high stability in a wide range of pH and NaCl concentration. Among the three C-dots/transition-metal ions composites, Gd@CQDs can conjugate with folic acid through a covalent bond to form Gd@CQDs, which exhibits specific targeting property to cancer cells with overexpressing folate receptor. Therefore, FA-Gd@CQDs could be a promising candidate for drug delivery. Bankoti et al. [81] produced C-dots co-doped with nitrogen, sulfur and phosphorous through simple microwave treatment of culinary waste onion peel. The as-prepared C-dots displayed strong green luminescence, high stability against pH and UV exposure, excellent blood compatibility, good cytocompatibility, free radical scavenging activity and efficacy in accelerating wound healing (Figure 5d).
Figure 5. Preparation and application of fluorescence C-dots from biomass wastes by microwave-assisted approaches. (a) The preparation and application of C-dots from eggshell membranes [79] (b) the synthesis and application of C-dots from mango leaves [80]; (c) the preparation and application of C-dots from crab shell [81]; (d) application of C-dots synthesized from onion peel to test the efficacy in accelerating wound healing [82].
2.4. Ultrasonic-Assisted Method
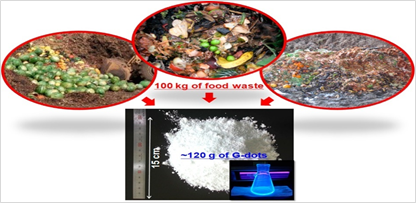
Ultrasonic-assisted method for the synthesis of C-dots has the advantages of low cost and easy operation. C-dots could be obtained through the ultrasonic treatment of mixtures of solvents and carbon sources. The properties of C-dots could be regulated by simply adjusting the experimental conditions such as the ultrasonic power, reaction time, the proportion of solvents and carbon sources, etc. Park et al. [82] proposed a simple method based on ultrasonic treatment for the large-scale synthesis of water-soluble C-dots from food waste-derived carbon source and achieved the production of 120 g C-dots produced from 100 kg of food waste mixtures (Figure 6). The obtained C-dots exhibited high water solubility, high photostability, good photoluminescence and low cytotoxicity for in vitro bioimaging. Furthermore, the byproducts produced in the synthesis of C-dots from the food-waste-derived source could promote seed germination and plant growth.
Figure 6. Large-scale synthesis of green C-dots from food wastes [83].
2.5. Other synthetic Methods
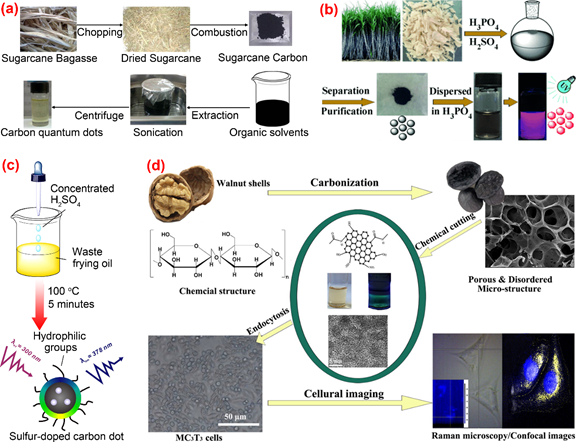
Thambiraj et al. [83] developed a top-down approach involving chemical oxidation and a simple exfoliation process for green synthesis of fluorescent C-dots from waste sugarcane bagasse pulp. The as-prepared C-dots had features of high fluorescent quantum yield (about 18.7%), highly crystallinity and excellent biocompatibility (Figure 7a). Sugarcane bagasse was also used as a carbon source by Jiang et al. [84] in the preparation of red-emitting C-dots for selective determination of gaseous ammonia via carbonization using concentrated sulfuric and phosphoric acid (Figure 7b). One-step synthesis of sulfur-doped C-dots from waste frying oil by the use of concentrated sulfuric acid as the carbonization agent was proposed by Hu et al. [85]. Due to its strong dehydrating and oxidizing properties, concentrated sulfuric acid could promote the conversion of saturated C-C single bonds into unsaturated C=C double bonds and the formation of hydrophilic C-O-H and O=C-O-H from hydrophobic C-H. The C-dots synthesized from frying oil displayed a pH-sensitive fluorescent behavior and therefore were used the fluorescence probe for sensing and imaging pH changes in living cells (Figure 7c). Cheng et al. [86] reported a chemical cutting approach for preparing fluorescent C-dots from walnut shells. The prepared C-dots were featured with good photostability, resistance to high ionic strength, stable up-conversion fluorescence and excellent cytocompatibility, which could be applied to the imaging of living cells and tissues (Figure 7d). C-dots with high fluorescence quantum yield of 36% were prepared from polystyrene foam waste by Zhang et al. [87] using a facile synthesis method based on the traditional organic solvent extraction. C-dots with different emission wavelengths were obtained under different solvent conditions. The authors studied the mechanisms of the formation process and the luminescence performance of the C-dots. The effectiveness of surface passivation was considered as the main factor causing the difference in fluorescence emission wavelengths of C-dots.
Figure 7. The preparation and application of C-dots by other synthetic methods. (a) The synthetic process of C-dots from sugarcane bagasse pulp [84]; (b) the synthesis of C-dots from bagasse [85]; (c) sulfur-doped carbon dots obtained from waste frying oil [86]; (d) the synthesis and application of C-dots from walnut shells [87].
Among the above-mentioned synthetic approaches for the preparation of C-dots, the hydrothermal approach is the most widely used one for its advantages of convenience, cost-effectiveness, easy operation and eco-friendliness. However, the synthesis of C-dots by the hydrothermal approach is generally considered a time-consuming process. Although the microwave-assisted method is not used as frequently as hydrothermal and pyrolysis approaches, its features of time-saving, low cost and easy operation are extremely attractive for the green synthesis of C-dots from renewable biomass wastes.
References
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737.
- Sun, Y.P.; Bing, Z.; Yi, L.; Wei, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Xin, W.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757.
- Zhang, M.R.; Su, R.; Zhong, J.; Fei, L.; Cai, W.; Guan, Q.W.; Li, W.J.; Li, N.; Chen, Y.S.; Cai, L.L. Red/orange dual-emissive carbon dots for pH sensing and cell imaging. Nano Res. 2019, 12, 815–821.
- Zhang, Y.N.; Zhang, X.W.; Shi, Y.P.; Sun, C.; Zhou, N.; Wen, H.X. The synthesis and functional study of multicolor nitrogen-doped carbon dots for live cell nuclear imaging. Molecules 2020, 25, 306–316.
- Liu, J.J.; Dong, Y.Y.; Ma, Y.X.; Han, Y.X.; Ma, S.; Chen, H.L.; Chen, X.G. One-step synthesis of red/green dual-emissive carbon dots for ratiometric sensitive ONOO− probing and cell imaging. Nanoscale 2018, 10, 13589–13598.
- Qin, K.H.; Zhang, D.F.; Ding, Y.F.; Zheng, X.D.; Xiang, Y.Y.; Hua, J.H.; Zhang, Q.; Ji, X.L.; Li, B.; Wei, Y.L. Applications of hydrothermal synthesis of Escherichia coli derived carbon dots in in vitro and in vivo imaging and p-nitrophenol detection. Analyst 2020, 145, 177–183.
- Liu, J.J.; Li, D.W.; Zhang, K.; Yang, M.X.; Sun, H.C.; Yang, B. One step hydrothermal synthesis of nitrogen‐doped conjugated carbonized polymer dots with 31% efficient red mmission for In Vivo imaging. Small 2018, 14, 1703919–1703929.
- Shu, Y.; Lu, J.; Mao, Q.X.; Song, R.S.; Wang, X.Y.; Chen, X.W.; Wang, J.H. Ionic liquid mediated organophilic carbon dots for drug delivery and bioimaging. Carbon 2017, 114, 324–333.
- Kailasa, S.K.; Bhamore, J.R.; Koduru, J.R.; Park, T.J. Carbon dots as carriers for the development of controlled drug and gene delivery systems. In Biomedical Applications of Nanoparticles; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, Holland, 2019; pp. 295–317.
- Yang, T.; Huang, J.L.; Wang, Y.T.; Zheng, A.Q.; Shu, Y.; Wang, J.H. β‐cyclodextrin‐decorated carbon dots serve as nanocarriers for targeted drug delivery and controlled release. ChemNanoMat 2019, 5, 479–487.
- Jana, J.; Lee, H.J.; Chung, J.S.; Kim, M.H.; Hur, S.H. Blue emitting nitrogen-doped carbon dots as a fluorescent probe for nitrite ion sensing and cell-imaging. Anal. Chim. Acta 2019, 1079, 212–219.
- Wang, J.; Li, R.S.; Zhang, H.Z.; Wang, N.; Zhang, Z.; Huang, C.Z. Highly fluorescent carbon dots as selective and visual probes for sensing copper ions in living cells via an electron transfer process. Biosens. Bioelectron. 2017, 97, 157–163.
- Hu, J.; Tang, F.; Jiang, Y.; Liu, C. Rapid screening and quantitative detection of Salmonella using a quantum dot nanobead-based biosensor. Analyst 2020, 145, 2184–2190.
- Han, M.; Zhu, S.J.; Lu, S.; Song, Y.B.; Feng, T.L.; Tao, S.Y.; Liu, J.J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218.
- Yu, H.J.; Shi, R.; Zhao, Y.F.; Waterhouse, G.I.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Smart utilization of carbon dots in semiconductor photocatalysis. Adv. Mater. 2016, 28, 9454–9477.
- Zhou, Y.; Zahran, E.M.; Quiroga, B.A.; Perez, J.; Mintz, K.J.; Peng, Z.; Liyanage, P.Y.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Size-dependent photocatalytic activity of carbon dots with surface-state determined photoluminescence. Applied Catalysis B 2019, 248, 157–166.
- Yuan, F.L.; Yuan, T.; Sui, L.Z.; Wang, Z.B.; Xi, Z.; Li, Y.C.; Li, X.H.; Fan, L.Z.; Tan, Z.a.; Chen, A.; et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 2249–2260.
- Zheng, J.X.; Liu, X.H.; Yang, Y.Z.; Liu, X.G.; Xu, B.S. Rapid and green synthesis of fluorescent carbon dots from starch for white light-emitting diodes. New Carbon Mater. 2018, 33, 276–288.
- Hu, C.; Li, M.Y.; Qiu, J.S.; Sun, Y.P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 2019, 48, 2315–2337.
- Fernando, K.S.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376.
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Erdem, E. High-capacitance hybrid supercapacitor based on multi-colored fluorescent carbon-dots. Sci. Rep. 2017, 7, 11222.
- Joseph, J.L.; Anappara, A.A. White light emitting carbon dots prepared by the electrochemical exfoliation of graphite. ChemPhysChem 2017, 18, 292–298.
- Liu, M.L.; Xu, Y.H.; Niu, F.S.; Gooding, J.J.; Liu, J.Q. Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst 2016, 141, 2657–2664.
- Hou, Y.X.; Lu, Q.J.; Deng, J.H.; Li, H.T.; Zhang, Y.Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta 2015, 866, 69–74.
- Dey, S.; Govindaraj, A.; Biswas, K.; Rao, C.N.R. Luminescence properties of boron and nitrogen doped graphene quantum dots prepared from arc-discharge-generated doped graphene samples. Chem. Phys. Lett. 2014, 595–596, 203–208.
- Si, J.H.; Yan, L.H.; Nguyen, V.; Hou, X. Femtosecond laser-assisted synthesis of highly photoluminescent carbon nanodots for Fe3+ detection with high sensitivity and selectivity. Opt. Mater. Express 2016, 6, 312–320.
- Nguyen, V.; Zhao, N.; Yan, L.; Zhong, P.; Le, P.H. Double-pulse femtosecond laser ablation for synthesis of ultrasmall carbon nanodots. Mater. Res. Express 2020, 7, 015606–015612.
- Xu, H.H.; Yan, L.; Nguyen, V.; Yu, Y.; Xu, Y.M. One-step synthesis of nitrogen-doped carbon nanodots for ratiometric pH sensing by femtosecond laser ablation method. Appl. Surf. Sci. 2017, 414, 238–243.
- Li, X.; Chai, C.; Zhang, Y.; Wang, Y.; Choi, M.M.F. Microwave synthesis of nitrogen and sulfur co-doped carbon dots for the selective detection of Hg2+ and glutathione. Opt. Mater. 2020, 99, 109559–109567.
- Tabaraki, R.; Abdi, O. Microwave assisted synthesis of N-doped carbon dots: Aneasy, fast and cheap sensor for determination of aspartic acid in sport supplements. J. Fluoresc. 2019, 29, 751–756.
- Deng, Z.Q.; Liu, C.; Jin, Y.Z.; Pu, J.L.; Wang, B.; Chen, J.C. High quantum yield blue- and orange-emitting carbon dots: One-step microwave synthesis and applications as fluorescent films and in fingerprint and cellular imaging. Analyst 2019, 144, 4569–4574.
- Qin, J.; Zhang, L.M.; Yang, R. Solid pyrolysis synthesis of excitation-independent emission carbon dots and its application to isoniazid detection. J. Nanopart. Res. 2019, 21, 59–70.
- Tian, M.; Wang, Y.T.; Zhang, Y. Synthesis of fluorescent nitrogen-doped carbon quantum dots for selective detection of picric acid in water samples. J. Nanosci. Nanotechnol. 2018, 18, 8111–8117.
- Hu, Y.W.; Wang, Y.X.; Wang, C.T.; Ye, Y.W.; Xue, Q.J. One-pot pyrolysis preparation of carbon dots as eco-friendly nanoadditives of water-based lubricants. Carbon 2019, 152, 511–520.
- Lu, J.Y.; Su, L.B.; Feng, G.; Jiang, J.T.; Hong, G.R.S.; Sun, Z.M.; Li, L.L. Potential application of nitrogen-doped carbon quantum dots synthesized by a solvothermal method for detecting silver Ions in food packaging. Int. J. Environ. Res. Public Health 2019, 16, 2518–2529.
- Lin, L.P.; Wang, Y.H.; Xiao, Y.L.; Liu, W. Hydrothermal synthesis of carbon dots codoped with nitrogen and phosphorus as a turn-on fluorescent probe for cadmium(II). Microchim. Acta 2019, 186, 147.
- Xu, D.D.; Lei, F.; Chen, H.H.; Yin, L.Q.; Shi, Y.; Xie, J.J. One step hydrothermal synthesis and optical properties of self-quenching-resistant carbon dots towards fluorescent ink and as nanosensors for Fe3+ detection. RSC Adv. 2019, 9, 8290–8299.
- Liu, H.; Zhang, H.W.; Li, J.Y.; Tang, Y.Y.; Cao, Y.; Jiang, Y. Organic-inorganic hybrid carbon dots for cell imaging. Mater. Res. Express 2018, 5, 045009–045015.
- Rong, M.C.; Yang, X.H.; Huang , L.Z.; Chi, S.T.; Zhou, Y.B. Hydrogen peroxide-assisted ultrasonic synthesis of BCNO QDs for the anthrax biomarker detection. ACS Appl. Mater. Interfaces 2018, 11, 2336–2343.
- Huang, H.Y.; Cui, Y.; Liu, M.Y.; Chen, J.Y.; Wan, Q.; Wen, Y.Q.; Deng, F.J.; Zhou, N.G.; Zhang, X.Y.; Wei, Y. A one-step ultrasonic irradiation assisted strategy for the preparation of polymer-functionalized carbon quantum dots and their biological imaging. J. Colloid Interface Sci. 2018, 532, 767–773.
- Qiao, Z.A.; Wang, Y.F.; Gao, Y.; Li, H.W.; Dai, T.Y.; Liu, Y.L.; Huo, Q.S. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem. Commun. 2010, 46, 8812–8814.
- Zheng, H.; Wang, Q.; Long, Y.; Zhang, H.; Huang, X.; Zhu, R. Enhancing the luminescence of carbon dots with a reduction pathway. Chem. Commun. 2011, 47, 10650–10652.
- Liu, H.; Li, Z.; Sun, Y.; Geng, X.; Hu, Y.; Meng, H.; Ge, J.; Qu, L. Synthesis of luminescent carbon dots with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability. Sci. Rep. 2018, 8, 1086.
- Yarur, F.; Macairan, J.R.; Naccache, R. Ratiometric detection of heavy metal ions using fluorescent carbon dots. Environ. Sci. 2019, 6, 1121–1130.
- Xue, B.; Yang, Y.; Sun, Y.; Fan, J.; Li, X.; Zhang, Z. Photoluminescent lignin hybridized carbon quantum dots composites for bioimaging applications. Int. J. Biol. Macromol. 2019, 122, 954–961.
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481.
- Ghimire, A.; Frunzo, L.; Pontoni, L.; d’Antonio, G.; Lens, P.N.L.; Esposito, G.; Pirozzi, F. Dark fermentation of complex waste biomass for biohydrogen production by pretreated thermophilic anaerobic digestate. J. Environ. Manag. 2015, 152, 43–48.
- Queiroz, L.S.; de Souza, L.K.C.; Thomaz, K.T.C.; Leite Lima, E.T.; da Rocha Filho, G.N.; do Nascimento, L.A.S.; de Oliveira Pires, L.H.; Faial, K.d.C.F.; da Costa, C.E.F. Activated carbon obtained from amazonian biomass tailings (acai seed): Modification, characterization, and use for removal of metal ions from water. J. Environ. Manag. 2020, 270, 110868–110876.
- Koupaie, E.H.; Dahadha, S.; Lakeh, A.A.B.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J. Environ. Manag. 2019, 233, 774–784.
- Babbar, N.; Oberoi, H.S. Enzymes in value-addition of agricultural and agro-industrial residues. In Enzymes in Value-Addition of Wastes; Brar, S.K., Verma, M., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 29–50.
- Zuo, P.L.; Lu, X.H.; Sun, Z.G.; Guo, Y.H.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542.
- Zhou, J.J.; Sheng, Z.H.; Han, H.Y.; Zou, M.Q.; Li, C.X. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 2012, 66, 222–224.
- Xue, M.Y.; Zou, M.B.; Zhao, J.J.; Zhan, Z.H.; Zhao, S.L. Green preparation of fluorescent carbon dots from lychee seeds and their application for the selective detection of methylene blue and imaging in living cells. J. Mater. Chem. B 2015, 3, 6783–6789.
- Xue, M.Y.; Zhan, Z.H.; Zou, M.B.; Zhang, L.L.; Zhao, S.L. Green synthesis of stable and biocompatible fluorescent carbon dots from peanut shells for multicolor living cell imaging. New J. Chem. 2016, 40, 1698–1703.
- Praneerad, J.; Neungnoraj, K.; In, I.; Paoprasert, P. Environmentally friendly supercapacitor based on carbon dots from durian peel as an electrode. Key Eng. Mater. 2019, 803, 115–119.
- Tan, X.W.; Romainor, A.N.B.; Chin, S.F.; Ng, S.M. Carbon dots production via pyrolysis of sago waste as potential probe for metal ions sensing. J. Anal. Appl. Pyrolysis 2014, 105, 157–165.
- Abdul Manaf, S.A.; Hegde, G.; Mandal, U.K.; Wui, W.T.; Roy, P. Functionalized carbon nano-scale drug delivery systems from biowaste sago bark for cancer cell imaging. Curr. Drug Deliv. 2017, 14, 1071–1077.
- Lu, W.B.; Qin, X.Y.; Liu, S.; Chang, G.H.; Zhang, Y.W.; Luo, Y.L.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X.P. Economical, Green Synthesis of Fluorescent Carbon Nanoparticles and Their Use as Probes for Sensitive and Selective Detection of Mercury(II) Ions. Anal. Chem. 2012, 84, 5351–5357.
- Prasannan, A.; Imae, T. One-pot synthesis of fluorescent carbon dots from orange waste peels. Ind. Eng. Chem. Res. 2013, 52, 15673–15678.
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432.
- Liu, Y.; Zhao, Y.; Zhang, Y. One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper(II) ion detection. Sens. Actuators B Chem. 2014, 196, 647–652.
- Sarswat, P.K.; Free, M.L. Light emitting diodes based on carbon dots derived from food, beverage, and combustion wastes. PCCP 2015, 17, 27642–27652.
- John, T.S.; Yadav, P.k.; Kumar, D.; Singh, S.K.; Hasan, S.H. Highly fluorescent carbon dots from wheat bran as a novel drug delivery system for bacterial inhibition. Luminescence 2020, 3, doi:10.1002/bio.3801.
- Chung, H.K.; Wongso, V.; Sambudi, N.S. Biowaste-derived carbon dots/hydroxyapatite nanocomposite as drug delivery vehicle for acetaminophen. J. Sol-Gel Sci. Technol. 2020, 93, 214–223.
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from Onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639.
- Ying, Z.; Yao, L.; Li, Y.Q.; He, Z.Y.; Quan, X.; Chen, Y.S.; Street, J.S.; Hao, G.; Nelles, M. Multicolor carbon nanodots from food waste and their heavy metal ion detection application. RSC Adv. 2018, 8, 23657–23662.
- Miao, H.; Wang, Y.Y.; Yang, X.M. Carbon dots derived from tobacco for visually distinguishing and detecting three kinds of tetracyclines. Nanoscale 2018, 10, 8139–8145.
- Liang, W.; Li, W.T.; Wu, B.; Zhen, L.; Wang, S.L.; Yuan, L.; Pan, D.Y.; Wu, M.H. Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem. Eng. J. 2016, 300, 75–82.
- Qi, H.J.; Teng, M.; Liu, M.; Liu, S.X.; Li, J.; Yu, H.P.; Teng, C.; Huang, Z.H.; Liu, H.; Shao, Q.; et al. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341.
- Pramanik, A.; Biswas, S.; Kumbhakar, P. Solvatochromism in highly luminescent environmental friendly carbon quantum dots for sensing applications: Conversion of bio-waste into bio-asset. Spectrochim. Acta Part A 2017, 191, 498–512.
- Ming, Y.; Zhong, R.B.; Gao, H.Y.; Li, W.R.; Yun, X.L.; Liu, J.R.; Zhao, X.M.; Zhao, G.F.; Feng, Z. One-step, Green and Economic Synthesis of Water-Soluble Photoluminescent Carbon Dots by Hydrothermal Treatment of Wheat Straw and Their Bio-applications in Labeling, Imaging and Sensing. Appl. Surf. Sci. 2015, 355, 1136–1144.
- 72. Xing, H.C.; Ling, D.H.; Yan, S.; Yan, W.; Robert, N.; Qiang, Y. Synthesis of carbon quantum dot nanoparticles derived from byproducts in bio-refinery process for cell imaging and in vivo bioimaging. Nanomaterials 2019, 9, 387–398.
- Su, A.M.; Wang, D.; Xin, S.; Zhong, Q.M.; Chen, Y.R.; Liu, J.C.; Wang, Y.L. Synthesis of fluorescent carbon quantum dots from dried lemon peel for determination of carmine in drinks. Chem. Res. Chin. Univ. 2018, 34, 164–168.
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green synthesis of carbon dots from ocimum sanctum for effective fluorescent sensing of Pb2+ ions and live cell imaging. Sens. Actuators B Chem. 2017, 242, 679–686.
- Wang, C.J.; Shi, H.X.; Yang, M.; Yan, Y.J.; Liu, E.Z.; Ji, Z.; Fan, J. Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater. Res. Bull. 2020, 124, 110730–110738.
- Chunduri, L.A.A.; Kurdekar, A.; Patnaik, S.; Dev, B.V.; Rattan, T.M.; Kamisetti, V. Carbon quantum Dots from coconut husk: evaluation for antioxidant and cytotoxic activity. Mater. Focus 2016, 5, 55–61.
- Zhang, H.M.; Kang, S.H.; Wang, G.Z.; Zhang, Y.X.; Zhao, H.J. Fluorescence determination of nitrite in water using prawn-shell derived nitrogen-doped carbon nanodots as fluorophores. ACS Sensors 2016, 1, 875–881.
- Wang, Q.; Liu, X.; Zhang, L.C.; Lv, Y. Microwave-assisted synthesis of carbon nanodots through an eggshell membrane and their fluorescent application. Analyst 2012, 137, 5392–5397.
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene quantum dots from mangifera indica: application in near-infrared bioimaging and intracellular nanothermometry. ACS Sustain. Chem. Eng. 2017, 5, 1382–1391.
- Yao, Y.Y.; Gedda, G.; Girma, W.M.; Yen, C.L.; Ling, Y.C.; Chang, J.Y. Magnetofluorescent carbon dots derived from crab shell for targeted dual-modality bioimaging and drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 13887–13899.
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Das, B.; Mitra, A.; Dhara, S. Onion derived carbon nanodots for live cell imaging and accelerated skin wound healing. J. Mater. Chem. B 2017, 5, 6579–6592.
- Park, S.Y.; Lee, H.U.; Park, E.S.; Lee, S.C.; Lee, J.W.; Jeong, S.W.; Chi, H.K.; Lee, Y.C.; Yun, S.H.; Lee, J. Photoluminescent Green Carbon Nanodots from Food-Waste-Derived Sources: Large-Scale Synthesis, Properties, and Biomedical Applications. ACS Appl. Mater. Interfaces 2014, 6, 3365–3370.
- Thambiraj, S.; Shankaran, R. Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl. Surf. Sci. 2016, 390, 435–443.
- Jiang, B.P.; Zhou, B.; Shen, X.C.; Yu, Y.X.; Ji, S.C.; Wen, C.C.; Liang, H. Selective probing of gaseous ammonia using red‐emitting carbon dots based on an interfacial response mechanism. Chem. Eur. J. 2015, 21, 18993–18999.
- Hu, Y.P.; Yang, J.; Tian, J.W.; Jia, L.; Yu, J.-S. Waste frying oil as a precursor for one-step synthesis of sulfur-doped carbon dots with pH-sensitive photoluminescence. Carbon 2014, 77, 775–782.
- Cheng, C.G.; Shi, Y.N.; Li, M.; Xing, M.; Wu, Q.L. Carbon quantum dots from carbonized walnut shells: Structural evolution, fluorescence characteristics, and intracellular bioimaging. Mater. Sci. Eng. C 2017, 79, 473–480.
- Zhang, R.; Liu, Y.B.; Yu, L.; Li, Z.; Sun, S.Q. Preparation of high-quality biocompatible carbon dots by extraction, with new thoughts on the luminescence mechanisms. Nanotechnology 2013, 24, 225601–225610.