
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shoko Kure | + 2475 word(s) | 2475 | 2021-01-29 12:14:24 | | | |
| 2 | Peter Tang | Meta information modification | 2475 | 2021-02-22 12:32:56 | | | | |
| 3 | Peter Tang | Meta information modification | 2475 | 2021-02-23 07:03:27 | | |
Video Upload Options
Hürthle cells are characterized cytologically as large cells with abundant eosinophilic, granular cytoplasms, and large hyperchromatic nuclei with prominent nucleoli. The cytoplasm of a Hürthle cell is swollen due mainly to the presence of numerous mitochondria. The mitochondrial protein has affinity to bind with eosin. Therefore, Hürthle cells are also called oxyphilic cells. Hürthle cell lesions in the thyroid are composed of cells with this classic histology, but not all oncocytic cells in the thyroid are true Hürthle cells. Cells with less or incomplete eosinophilic, granular appearance can observed, at least focally, in any thyroid lesions, such as autoimmune thyroiditis, nodular goiter, aging, and irradiated thyroids. These oncocytic, non-Hürthle cells are called “oncocytic metaplasia”.
1. Introduction
Oncocytic tumors are found in various organs, including salivary glands, lacrimal glands, pancreas, liver, kidney, thyroid, parathyroid and pituitary glands. Thyroid neoplasms composed of oncocytic cells are called Hürthle cell tumors. These are also known as oncocytic/oxyphilic follicular cell tumors and used to be classified as a variant of follicular thyroid tumors. In the latest WHO classification [1], Hürthle cell tumors are identified as a special type of tumor derived from thyroid follicles, and distinguished from thyroid follicular tumors. Hürthle cell tumors can be benign or malignant. Hürthle cell tumors with capsular and/or vascular invasion, lymph nodes metastasis, or distant metastasis are Hürthle cell carcinoma (HCC). HCC represents 3–4% of thyroid carcinoma cases [2][3]. HCC has been considered as a more aggressive form of carcinoma compared to non-oncocytic thyroid carcinomas. Due to its rarity and conflicting information from previous studies, the pathological characteristics and biological behavior of HCC remain to be elucidated. In fact, most studies reporting on the biological features of thyroid differentiated tumors have included HCC as a part of follicular thyroid cancer or have failed to adequately separate malignant Hürthle cell tumor from benign tumor. Thus, studies especially focusing only on pure HCC are few [4]. There is still no consensus on the optimal treatment method for HCC, and the postoperative effect of radioactive iodine treatment is unclear.
2. Clinical Features
2.1. Demographic Features
HCC is more often observed in females, and the reported female to male ratio of HCC is 1.6–4.8:1 [4][5][6][7]. The affected age range is 54–62 years old [4][6][8][9][10][11]. In a retrospective study, tumor size, frequencies of extrathyroidal extension, lymph node metastasis, and distant metastasis, were similar between HCC and non-oncocytic follicular thyroid carcinomas [5]. To date, no correlation with radiation exposure has been reported [12].
2.2. Laboratory Tests
Thyroglobulin production varies by case. Both high thyroglobulin production (>500 ng/dL) [13][14][15], and limited production [16], which reflects less active follicular cells, have been reported. An increased thyroglobulin level is a biomarker for recurrence after thyroidectomy; however, some recurrent HCC cases have undetectable thyroglobulin levels. Clinical follow-up by thyroglobulin levels should be decided depending on the case.
2.3. Ultrasounds
Preoperative diagnosis by ultrasound is challenging. In ultrasounds, HCC shows a range of sonographic appearances from predominantly hypoechoic to hyperechoic lesions, and no preoperative features can differentiate HCC from adenoma [7][17]. Several attempts have been made to predict HCC before the operation. Ito et al. classified thyroid nodules into classes 1 to 5 with intermediate steps of 0.5 for classes 2 to 5, called ultrasound classes (USC) [18]. USC2 is defined as having a round and cystic nodule/isoechoic solid nodule—an adenomatous nodule or follicular adenoma. USC3 is defined as a round, hypoechoic solid nodule—a follicular adenoma, adenomatous nodule, or possibly carcinoma. USC4 is a solid nodule with an irregular border or the presence of psammoma calcifications—a carcinoma. Although USC is not related to the incidence of HCC, among patients diagnosed with oncocytic cell tumors by fine-needle aspiration cytology (FNAC), such patients are more likely to show malignancy when USC is 3 or greater.
2.4. Clinical Indicators
Indicators of malignancy among Hürthle cell tumors include male [19], tumor size >4 cm [7][12][15][19], US class ≥ 3 [15], older age (HCC 51.8 years old vs. Hürthle cell adenoma 43.1) [12]. Subsequently, surgical indications of Hürthle cell tumors are reported as USC ≥ 3, tumor size >4 cm, and thyroglobulin >500 ng/dL (with negative anti-thyroglobulin-antibody) [15].
3. Treatment
Either near total or total thyroidectomy or unilateral thyroidectomy is initially recommended for patients with HCC > 1 cm and <4 cm without extrathyroidal extension, and without lymph node metastasis [20]. Thyroid lobectomy alone is sufficient treatment for small, unifocal, intrathyroidal carcinomas in the absence of prior head and neck radiation, familial thyroid carcinoma, or clinically detectable cervical nodal metastases [20]. Total thyroidectomy is recommended for Hürthle cell neoplasms larger than 4 cm [21]. Whether total thyroidectomy as the primary procedure should be applied is controversial. While several studies showed more favorable outcome [6][19][21][22][23][24], the Surveillance, Epidemiology and End Results (SEER) in its large scale database, no significance difference in survival was observed between patients treated with total thyroidectomy and those treated with partial thyroidectomy [11]. Extensive surgery, external beam radiation, or chemotherapy did not confer a survival benefit [12].
The role of radioactive iodine (RAI) is still debated [5]. Guidelines regarding indications to use RAI for HCC are inconsistent, and RAI is not prevailing for HCC patients [25]. Jillard et al. suggested that RAI should be advocated for HCC patients with tumors > 2 cm and those with nodal and distant metastatic disease, as it improves survival [25]. Radioactive iodine therapy may prompt a survival benefit as an adjuvant ablation therapy, but only for those who do not have a residual disease [12]. Some teams may choose total thyroidectomy and RAI therapy. However, functionally, HCC shows decreased iodine uptake [14], resulting in lower responsiveness to RAI treatment [14].
4. Pathological Features
4.1. Cytological Features and Differential Diagnosis
Using fine needle aspiration cytology (FNAC), clusters of the monotonous follicular cells with large eosinophilic, granular cytoplasm are observed, and usually, hypercellularity is noted (Figure 1). The cells often show irregular nuclear outlines, prominent nucleoli, and bland chromatin. The tumor cells appear scarcely accompanied by colloid, and/or inflammation.
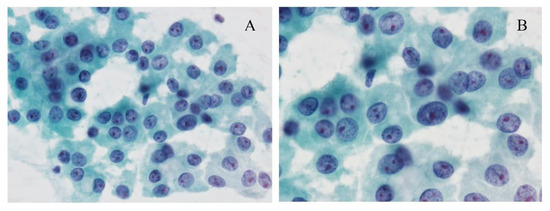
Figure 1. Cytological images of the Hürthle cell carcinoma (HCC). (A) The Papanicolaou-stained smear shows a monotonous cells oncocytic cells with prominent nucleoli arranged in loosely cohesive clusters. Papanicolaou stain, original magnification ×200. (B) The cells have prominent nucleoli, bland chromatin, and granular cytoplasm. Papanicolaou stain, original magnification ×600.
Oncocytic cells are also observed in nodular goiter, chronic thyroiditis, and oncocytic variants of papillary thyroid carcinoma and oncocytic variant of medullary thyroid carcinoma (OV-MTC). Therefore, the presence of these cells should not engender a diagnosis of Hürthle cell lesion [16]. In chronic thyroiditis and nodular goiter, isolated or small cohesive clusters of oncocytic cells are common, representing follicular cells with oncocytic metaplasia. In addition, in nodular goiter, follicular cells with abundant colloids are observed, and the follicles are not uniform. In chronic thyroiditis, lymphoplasmacytic infiltrations are frequently observed. However, when a large irregular sheet of oncocytic cells dominantly appear, differentiating between chronic thyroiditis and Hürthle cell tumor may be difficult. Indicators of true Hürthle cell tumors are (1) microfollicular architecture, (2) absence of colloid, (3) absence of inflammation, and (4) presence of transgressing blood vessels [26][27].
The tumor cells of oncocytic variants of papillary thyroid carcinomas show fine granular cytoplasms and elongated nuclei, and a nuclear groove. Intracytoplasmic inclusion can be sparse in some cases. The cytological features of papillary fronds and monolayered sheets suggest an oncocytic variant of papillary thyroid carcinoma rather than Hürthle cell tumors [28][29]. In the FNAC sample of the OV-MTC, amyloid deposit supports the diagnosis, but is not always observed [30]. OV-MTC cells show polygonal, plasmacytoid characteristics with eccentrically placed round nuclei with “salt and pepper” chromatin [31]. Multi-vacuolization resembling histiocytes and loose granularity is observed in OV-MTC. In contrast, granularity in HCC is dense and firm [32]. For accurate diagnosis, immunohistochemistry using calcitonin and thyroglobulin in combination [33], as well as consideration of family history of the patient and laboratory data are needed.
Cytologic features such as small cell dysplasia (bland nuclei, cell diameter less than twice the nuclear diameter), large cell dysplasia (cell diameter at least twice the nuclear diameter, with prominent nucleoli and irregular nuclear outlines), nuclear crowding, and dyshesion have been proposed as a diagnostic clue for HCC [34]. These criteria were later shown by others to be significant features in favor of HCC over adenoma and other benign lesions [35]. However, distinction of HCC from Hürthle cell adenoma by FNAC still remains to be challenging due to the lack of other supporting data [36]. In the daily practice using Bethesda reporting system for thyroid cytology (TBSRTC) [37], a Hürthle cell neoplasm is typically classified as atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS, Bethesda III) or benign (Bethesda II). The appearance of Hürthle cell is encouraged to comment. The presence of Hürthle cells itself does not increase the risk of malignancy [38], but > 75% Hürthle cells in a benign/Bethesda II aspirate may pose an increased risk of malignancy [39].
4.2. Macroscopic Features
Macroscopically, HCC shows a mahogany brown appearance (Figure 2), which is due to air contact [14]. Necrosis and hemorrhaging may be observed due to its degenerative nature. Sometimes calcification is observed and is more often found in HCC than in Hürthle cell adenoma. Calcification is a result of a physiochemical reaction to altered thyroglobulin within the colloid of the tumor follicles.
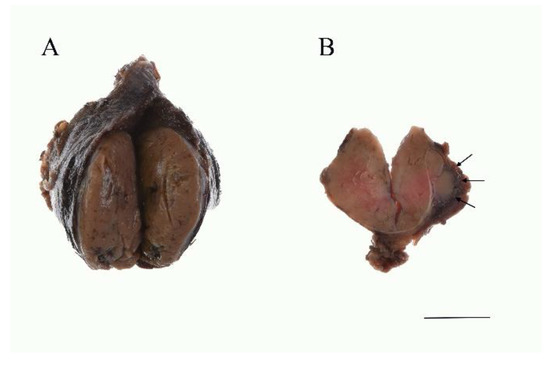
Figure 2. Gross findings of the HCC. (A) The tumor is mahogany brown color. Several foci of hemorrhage are noted. (B) Widely invasive HCC. Macroscopically, capsular invasion is evident (arrows). Scale bar indicates 2 cm.
4.3. Histological Features and Differential Diagnosis at Histology
In cases where the Hürthle cell tumor showed capsular invasion and/or vascular invasion, HCC is diagnosed (Figure 2B and Figure 3A,B). Based on its histology, the tumor is subcategorized as either minimally invasive or widely invasive. Minimally invasive HCC refers to encapsulated tumors with microscopically identifiable foci with capsular or vascular invasion less than four foci. Widely invasive HCC shows extensive capsular invasion and/or vascular invasion of four foci or greater.
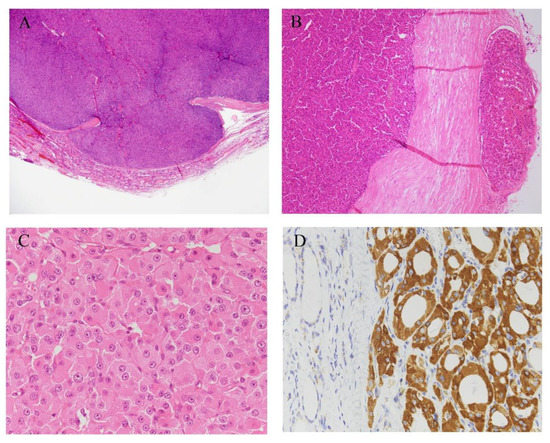
Figure 3. Histological images of HCCs. (A) HCC is diagnosed in cases where a Hürthle cell tumor shows capsular invasion, and/or (B) vascular invasion. (C) HCC is composed of polygonal large cells with abundant granular and acidophilic cytoplasm. HCC cells have large nuclei with prominent nucleoli. (D) Tumor cells are diffuse and strongly positive for anti-mitochondria antibodies. Hematoxylin and eosin stain (A–C), immunohistochemistry of anti-mitochondrial antibody (D). Original magnification, ×1.25 (A), ×40 (B), ×100 (D), and ×400 (C).
HCC often causes infarction after FNAC. This may make it difficult to observe the capsular invasions. Mitochondrial DNA common deletion is reported to be less frequent in infarcted Hürthle cell tumors than in non-infarcted Hürthle cell tumors. Still, the mechanism leading to infarction is unknown [40]. For clear identification of capsular invasion, clinical information such as where the tumor cells were aspirated by FNAC is considered important. Widely invasive HCC often shows capsular invasion in the form of compacted nodules, lacking stromal desmoplastic reaction. In some cases, determining whether there is a capsule is difficult.
HCC is composed of pleomorphic or polygonal large cells with abundant, granular, and acidophilic cytoplasm and large nuclei with prominent nucleoli (Figure 3C) [41]. It often shows follicular, trabecular, and/or solid architecture, and rarely shows a predominantly papillary pattern [14]. A pure follicular pattern is less common in HCC [1], but when such a follicular pattern is seen, the tumor accompanies fibrous bands between nests.
Cytoplasmic granularity in Hürthle cells is due to the presence of numerous mitochondria (Figure 3D). The amount and morphological characteristics of the mitochondria vary greatly from case to case [42]. However, according to the diagnostic criteria, more than 75% of oxyphilic cells must be observed for diagnosis. The high prevalence of Hürthle cell changing into thyroid lesions may reflect high oxidative stress and reactive oxygen species production in thyroid cells during normal iodine and thyroid hormone metabolism [43][44]. These high reactive oxygen species levels can result in mutagenic genetic events in mitochondrial DNA, leading to mitochondrial dysfunction [45][46]. Abnormality of mitochondria leads to defects in the energy production process, and a compensatory mechanism for these defects is considered to come into play [45]. This results in increased proliferation of numerous mitochondria, which is observed as oncocytic cytoplasm. Over time, as the number of the mitochondria increases, Hürthle cell appearance is expressed [41], and this is a continuous process rather than a “black-and white phenomenon” [45]. In cases of HCC, necrosis frequently appears either spontaneously or after fine-needle aspiration. This observation is reported to be as a consequence of mitochondrial abnormalities [47].
Thyroid lesions containing oncocytic cells are nodular goiter, oncocytic variants of papillary carcinoma and medullary carcinoma. Oncocytic variants of medullary thyroid carcinoma (MTC) are rare, showing extensive mitochondrial hyperplasia, prominent nucleoli, well-defined cytoplasm, and polygonal cells, which is in contrast with the spindle-shaped cells of conventional MTC. Although MTC is derived from C cells (parafollicular cells), not follicular cells, oncocytic variant MTC may be difficult to histologically differentiate. MTC is often accompanied by amyloid deposits, and this aids in diagnosis. However, amyloid deposits sometimes occur as a result of systemic or secondary amyloidosis [48]. HCC with neuroendocrine differentiation [49] or thyroid tumors in patients with basal high calcitonin levels should be diagnosed with caution. For the appropriate diagnosis, consideration of molecular testing, as well as family history and serum calcitonin levels, is essential [50]. Oncocytic parathyroid adenoma and adenocarcinoma also require differential diagnosis. Neoplastic lesions showing extensive invasion without hormone production are especially difficult to differentiate. Immunohistochemically, parathyroid tissue tests positive for chromogranin A, GATA3, while thyroid tissue tests positive for TTF-1, PAX8. However, in the hormone non-producing tumors, neither calcitonin nor PTH is clearly expressed. Tumor location with images and invasive patterns can be diagnostic clues in these cases.
Immunohistochemistry is not mandatory for the diagnosis of HCC, except in cases to distinguish MTC or parathyroid tumors, and a specific diagnostic marker for HCC has not yet been obtained. HCC cells test positive for thyroid transcription factor-1 (TTF-1) and thyroglobulin, and negative for calcitonin and parathyroid hormone (PTH). Thyroglobulin may show perinuclear positivity. According to the literature, genes associated with Retinoid-interferon-induced Mortality-19 (GRIM-19) [51], p53 [47], and Cyclin D [52][53] can be immunohistochemically detected.
4.4. Ultrastructure
Using transmission electron microscopy, HCC cells are observed to have large, irregular nuclei with a deep notch [54]. Nuclei contain one to several nucleoli, and the cytoplasm is packed with several thousand mitochondria (Figure 4). A full-brown oncocytic cell is estimated to contain 4000 to 5000 mitochondria [55]. These mitochondria display two types of morphology. One is larger mitochondria with stacking “shelf-like” cristae [54][56]. The other is smaller and oval, and short cristae are peripherally arranged [54]. Other structural abnormalities are bundles of dense filaments, filamentous inclusions, and round electron-dense bodies in the matrix [16][41][57]. The amount and morphological characteristics of the mitochondria vary greatly from case to case [41][42]. The surface of HCC cells is irregular.
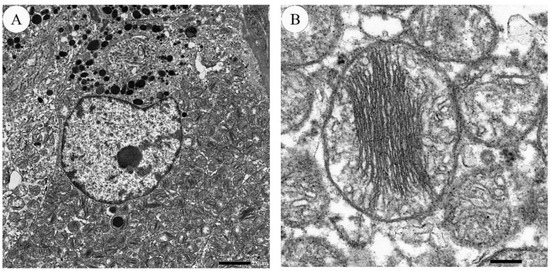
Figure 4. Electron microscopic images of HCC. (A) An HCC cell contains an irregular and notched nucleus. Cytoplasm is filled with numerous mitochondria. The bar indicates 2.0 micrometers. (B) Mitochondria display packed stacking cristae, arranged in the center. The bar indicates 200.0 nanometers.
References
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC: Lyon, France, 2017.
- Wei, S.; LiVolsi, V.A.; Montone, K.T.; Morrissette, J.J.D.; Baloch, Z.W. PTEN and TP53 Mutations in Oncocytic Follicular Carcinoma. Endocr. Pathol. 2015, 26, 365–369.
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2009, 19, 1167–1214.
- Chindris, A.M.; Casler, J.D.; Bernet, V.J.; Rivera, M.; Thomas, C.; Kachergus, J.M.; Necela, B.M.; Hay, I.D.; Westphal, S.A.; Grant, C.S.; et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J. Clin. Endocrinol. Metab. 2015, 100, 55–62.
- Haigh, P.I.; Urbach, D.R. The treatment and prognosis of Hurthle cell follicular thyroid carcinoma compared with its non-Hurthle cell counterpart. Surgery 2005, 138, 1152–1157.
- Oluic, B.; Paunovic, I.; Loncar, Z.; Djukic, V.; Diklic, A.; Jovanovic, M.; Garabinovic, Z.; Slijepcevic, N.; Rovcanin, B.; Micic, D.; et al. Survival and prognostic factors for survival, cancer specific survival and disease free interval in 239 patients with Hurthle cell carcinoma: A single center experience. BMC Cancer 2017, 17, 371.
- Santana, N.O.; Freitas, R.M.C.; Marcos, V.N.; Chammas, M.C.; Camargo, R.Y.A.; Schmerling, C.K.; Vanderlei, F.A.B.; Hoff, A.O.; Marui, S.; Danilovic, D.L.S. Diagnostic performance of thyroid ultrasound in Hurthle cell carcinomas. Arch. Endocrinol. Metab. 2019, 63, 300–305.
- Goffredo, P.; Roman, S.A.; Sosa, J.A. Hurthle cell carcinoma: A population-level analysis of 3311 patients. Cancer 2013, 119, 504–511.
- Sugino, K.; Kameyama, K.; Ito, K.; Nagahama, M.; Kitagawa, W.; Shibuya, H.; Ohkuwa, K.; Uruno, T.; Akaishi, J.; Suzuki, A.; et al. Does Hurthle cell carcinoma of the thyroid have a poorer prognosis than ordinary follicular thyroid carcinoma? Ann. Surg. Oncol. 2013, 20, 2944–2950.
- Petric, R.; Gazic, B.; Besic, N. Prognostic factors for disease-specific survival in 108 patients with Hürthle cell thyroid carcinoma: A single-institution experience. BMC Cancer 2014, 14, 777.
- Zhou, X.; Zheng, Z.; Chen, C.; Zhao, B.; Cao, H.; Li, T.; Liu, X.; Wang, W.; Li, Y. Clinical characteristics and prognostic factors of Hurthle cell carcinoma: A population based study. BMC Cancer 2020, 20, 407.
- Lopez-Penabad, L.; Chiu, A.C.; Hoff, A.O.; Schultz, P.; Gaztambide, S.; Ordonez, N.G.; Sherman, S.I. Prognostic factors in patients with Hurthle cell neoplasms of the thyroid. Cancer 2003, 97, 1186–1194.
- Heimann, A.; Moll, U. Spinal metastasis of a thyroglobulin-rich Hürthle cell carcinoma detected by fine needle aspiration. Light and electron microscopic study of an unusual case. Acta Cytol. 1989, 33, 639–644.
- Musholt, P.B.; Musholt, T.J.; Morgenstern, S.C.; Worm, K.; Sheu, S.Y.; Schmid, K.W. Follicular histotypes of oncocytic thyroid carcinomas do not carry mutations of the BRAF hot-spot. World J. Surg. 2008, 32, 722–728.
- Ito, Y. Diagnosis and surgical indications of oxyphilic follicular tumors in Japan: Surgical specimens and cytology. Endocr. J. 2016, 63, 977–982.
- Montone, K.T.; Baloch, Z.W.; LiVolsi, V.A. The thyroid Hürthle (oncocytic) cell and its associated pathologic conditions: A surgical pathology and cytopathology review. Arch. Pathol. Lab. Med. 2008, 132, 1241–1250.
- Maizlin, Z.V.; Wiseman, S.M.; Vora, P.; Kirby, J.M.; Mason, A.C.; Filipenko, D.; Brown, J.A. Hurthle cell neoplasms of the thyroid: Sonographic appearance and histologic characteristics. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2008, 27, 751–757.
- Ito, Y.; Amino, N.; Yokozawa, T.; Ota, H.; Ohshita, M.; Murata, N.; Morita, S.; Kobayashi, K.; Miyauchi, A. Ultrasonographic Evaluation of Thyroid Nodules in 900 Patients: Comparison Among Ultrasonographic, Cytological, and Histological Findings. Thyroid Off. J. Am. Thyroid Assoc. 2007, 17, 1269–1276.
- Wasvary, H.; Czako, P.; Poulik, J.; Lucas, R. Unilateral lobectomy for Hurthle cell adenoma. Am. Surg. 1998, 64, 729–732.
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1–133.
- Chen, H.; Nicol, T.L.; Zeiger, M.A.; Dooley, W.C.; Ladenson, P.W.; Cooper, D.S.; Ringel, M.; Parkerson, S.; Allo, M.; Udelsman, R. Hürthle cell neoplasms of the thyroid: Are there factors predictive of malignancy? Ann. Surg. 1998, 227, 542–546.
- Mills, S.C.; Haq, M.; Smellie, W.J.; Harmer, C. Hurthle cell carcinoma of the thyroid: Retrospective review of 62 patients treated at the Royal Marsden Hospital between 1946 and 2003. Eur. J. Surg. Oncol. 2009, 35, 230–234.
- Grossman, R.F.; Clark, O.H. Hurthle Cell Carcinoma. Cancer Control J. Moffitt Cancer Cent. 1997, 4, 13–17.
- Khafif, A.; Khafif, R.A.; Attie, J.N. Hürthle cell carcinoma: A malignancy of low-grade potential. Head Neck 1999, 21, 506–511.
- Jillard, C.L. Radioactive Iodine Treatment Is Associated with Improved Survival for Patients with Hürthle Cell Carcinoma. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 959–964.
- Anila, K.R.; Nayak, N.; George, P.S.; Jayasree, K. Cytomorphologic Spectrum of Hurthle Cell Lesions of Thyroid: A Study of 54 Cases. Gulf J. Oncol. 2018, 1, 6–10.
- Elliott, D.D.; Pitman, M.B.; Bloom, L.; Faquin, W.C. Fine-needle aspiration biopsy of Hurthle cell lesions of the thyroid gland: A cytomorphologic study of 139 cases with statistical analysis. Cancer 2006, 108, 102–109.
- Doria, M.I., Jr.; Attal, H.; Wang, H.H.; Jensen, J.A.; DeMay, R.M. Fine needle aspiration cytology of the oxyphil variant of papillary carcinoma of the thyroid. A report of three cases. Acta Cytol. 1996, 40, 1007–1011.
- Renshaw, A.A. Fine-needle aspirations of papillary carcinoma with oncocytic features: An expanded cytologic and histologic profile. Cancer Cytopathol. 2011, 119, 247–253.
- Kaushal, S.; Iyer, V.K.; Mathur, S.R.; Ray, R. Fine needle aspiration cytology of medullary carcinoma of the thyroid with a focus on rare variants: A review of 78 cases. Cytopathology 2011, 22, 95–105.
- Sams, S.B.; Tompkins, K.D.; Mayson, S.; Raeburn, C.D.; Mehrotra, S. Oncocytic variant of medullary thyroid carcinoma; a rare tumor with numerous diagnostic mimics by fine needle aspiration. Diagn. Cytopathol. 2017, 45, 1148–1152.
- Canberk, S. Oncocytic Variant of Medullary Thyroid Carcinoma. Endocr. Pathol. 2015, 26, 320–323.
- HARACH, H.R.; BERGHOLM, U. Medullary (C cell) carcinoma of the thyroid with features of follicular oxyphilic cell tumours. Histopathology 1988, 13, 645–656.
- Renshaw, A.A. Hurthle cell carcinoma is a better gold standard than Hurthle cell neoplasm for fine-needle aspiration of the thyroid: Defining more consistent and specific cytologic criteria. Cancer 2002, 96, 261–266.
- Wu, H.H.; Clouse, J.; Ren, R. Fine-needle aspiration cytology of Hurthle cell carcinoma of the thyroid. Diagn. Cytopathol. 2008, 36, 149–154.
- Grani, G.; Lamartina, L.; Durante, C.; Filetti, S.; Cooper, D.S. Follicular thyroid cancer and Hürthle cell carcinoma: Challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018, 6, 500–514.
- Cibas, E.S.; Ali, S.Z. The Bethesda System for Reporting Thyroid Cytopathology, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018.
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 1341–1346.
- Ren, Y. The Presence of Hürthle Cells Does Not Increase the Risk of Malignancy in Most Bethesda Categories in Thyroid Fine-Needle Aspirates. Thyroid Off. J. Am. Thyroid Assoc. 2020, 30, 425–431.
- Conti, L.; Vatrano, S.; Bertero, L.; Masu, L.; Pacchioni, D.; Daniele, L.; De Rosa, G.; Cassoni, P.; Volante, M.; Papotti, M. Mitochondrial DNA “common deletion” in post–fine needle aspiration infarcted oncocytic thyroid tumors. Hum. Pathol. 2017, 69, 23–30.
- Máximo, V.; Sobrinho-Simões, M. Hürthle cell tumours of the thyroid. A review with emphasis on mitochondrial abnormalities with clinical relevance. Virchows Arch. Int. J. Pathol. 2000, 437, 107–115.
- Nesland, J.M.; Sobrinho-simões, M.A.; Holm, R.; Sambade, M.C.; Johannessen, J.V. Hürthle-Cell Lesions of the Thyroid: A Combined Study Using Transmission Electron Microscopy, Scanning Electron Microscopy, and Immunocytochemistry. Ultrastruct. Pathol. 1985, 8, 269–290.
- Wang, D.; Feng, J.F.; Zeng, P.; Yang, Y.H.; Luo, J.; Yang, Y.W. Total oxidant/antioxidant status in sera of patients with thyroid cancers. Endocr. Relat. Cancer 2011, 18, 773–782.
- Xing, M. Oxidative stress: A new risk factor for thyroid cancer. Endocr. Relat. Cancer 2012, 19, C7–C11.
- Maximo, V.; Lima, J.; Prazeres, H.; Soares, P.; Sobrinho-Simoes, M. The biology and the genetics of Hurthle cell tumors of the thyroid. Endocr. Relat. Cancer 2012, 19, R131–R147.
- Wallace, D.C. Mitochondrial diseases in man and mouse. Science (N. Y.) 1999, 283, 1482–1488.
- Müller-Höcker, J. Immunoreactivity of p53, Ki-67, and Bcl-2 in oncocytic adenomas and carcinomas of the thyroid gland. Hum. Pathol. 1999, 30, 926–933.
- Coca-Pelaz, A.; Vivanco-Allende, B.; Alvarez-Marcos, C.; Suarez-Nieto, C. Multifocal papillary thyroid carcinoma associated with primary amyloid goiter. Auris Nasus Larynx 2012, 39, 549–551.
- Munitiz, V.; Martinez-Barba, E.; Riquelme, J.; Rodriguez, J.M.; Pinero, A.; Parrilla, P. Elevated basal calcitonin levels in a patient with a hurthle-cell carcinoma of the thyroid and neuroendocrine differentiation: Report of a case. Surg. Today 2005, 35, 404–406.
- Spaulding, S.L.; Ho, R.; Everest, S.; Chai, R.L. The role of molecular testing in the diagnosis of medullary thyroid cancer: A case report of oncocytic medullary thyroid carcinoma and review of the literature. Am. J. Otolaryngol. 2020, 41, 102312.
- Donatini, G.; Beaulieu, A.; Castagnet, M.; Kraimps, J.L.; Levillain, P.; Fromont, G. Thyroid Hürthle cell tumors: Research of potential markers of malignancy. J. Endocrinol. Investig. 2016, 39, 153–158.
- Ding, L.; Jiang, Y.; Yang, W. Approach the Invasive Potential with Hurthle Cell Tumors of Thyroid. Pathol. Oncol. Res. 2019, 25, 697–701.
- Erickson, L.A.; Jin, L.; Goellner, J.R.; Lohse, C.; Pankratz, V.S.; Zukerberg, L.R.; Thompson, G.B.; van Heerden, J.A.; Grant, C.S.; Lloyd, R.V. Pathologic Features, Proliferative Activity, and Cyclin D1 Expression in Hurthle Cell Neoplasms of the Thyroid. Mod. Pathol. 2000, 13, 186–192.
- Ambu, R.; Riva, A.; Lai, M.L.; Loffredo, F.; Riva, F.T.; Tandler, B. Scanning electron microscopy of the interior of cells in Hurthle cell tumors. Ultrastruct. Pathol. 2000, 24, 211–219.
- Sobrinho-Simões, M.; Máximo, V.; Castro, I.V.; Fonseca, E.; Soares, P.; Garcia-Rostan, G.; Oliveira, M.C. Hürthle (oncocytic) cell tumors of thyroid: Etiopathogenesis, diagnosis and clinical significance. Int. J. Surg. Pathol. 2005, 13, 29–35.
- Tallini, G. Oncocytic tumours. Virchows Arch. Int. J. Pathol. 1998, 433, 5–12.
- Matias, C.; Nunes, J.F.; Sobrinho, L.G.; Soares, J. Giant mitochondria and intramitochondrial inclusions in benign thyroid lesions. Ultrastruct. Pathol. 1991, 15, 221–229.




