| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michal Kielbik | + 3363 word(s) | 3363 | 2021-02-08 07:49:41 | | | |
| 2 | Rita Xu | -1177 word(s) | 2186 | 2021-02-18 09:52:47 | | |
Video Upload Options
Immunogenic cell death (ICD) is a type of death, which has the hallmarks of necroptosis and apoptosis, and is best characterized in malignant diseases.
1. Introduction
Cell death is an important process that plays a great role in the development and maintaining of the homeostasis of every living organism. There are many different types of eukaryotic cell death, classified depending on morphological changes inside the cell (i.e., shrinkage of the cytoplasm, chromatin condensation, nuclear fragmentation, degradation of intracellular organelles, rupture of cell membrane); enzymological criteria (i.e., participation or not of nucleases or proteases); functional aspects (i.e., programed, accidental, physiological, pathological); immunological character (immunogenic or non-immunogenic) . Liu et al. [1][2][3] performed literature research and counted that there are over 34 different cell death modes, among which best-known and most studied are apoptosis, necrosis, autophagy, necroptosis, anoikis and pyroptosis. The traditional process of apoptosis is a non-immunogenic one, even termed as tolerogenic cell death, that actively inhibits immune reaction [4][5]. In contrast, necrosis is uncontrolled, immunologically harmful process in which increased secretion of pro-inflammatory cytokines as well as recruitment of different immune cells leading to the development of immune responses in the tissue are observed [1][6][7][8]. Necroptosis (programed necrosis) is a regulated cell death type that mimics features of both apoptosis and necrosis and is also known to induce immune-related processes [9][10]. Apoptosis, on the other hand, under certain conditions, may occur in the way that the immune system is alerted, triggering immunity against the dying cell, which releases its cellular content into the microenvironment, that in turn leads to the recruitment and activation of immune cells. Such a form of apoptosis, which may also occur in the context of necroptosis, is called immunogenic cell death (ICD) [11]. ICD is characterized by the emission of particular molecules that induce the immune response. This kind of cell death is especially important and best characterized in malignant diseases. One of the best-known/most important molecules implicated in ICD is chaperone called calreticulin [12][13].
Calreticulin (also termed as CRT or CALR) is an endoplasmic reticulum (ER)-associated chaperone with numerous biological activities. Its functions strongly depend on cellular localization. In ER it is a main regulator of Ca2+ homeostasis and is also responsible for loading of cellular antigens into major histocompatibility complex (MHC) class I molecules. In cytoplasm, calreticulin is considered to be integrin activator and mediator of integrin-dependent cell adhesion [14][15][16]. Very important biological role of CRT is connected with cell death. In stressed or dying cells this chaperone is expressed on the surface of cell membrane or even release into extracellular milieu [17]. Plasma membrane CRT (also called ecto-CRT) serves as a co-stimulatory and pre-mortem (“eat-me”) signals to variety of immune cells but mainly to antigen presenting cells (APCs). Moreover, ecto-CRT is one of the main hallmarks of ICD in malignant diseases. Calreticulin present on outer membrane of tumor cells not only stimulates their phagocytosis by APCs, but also activates the adaptive immune response, thus it is believed to be crucial for surveillance against tumors [18][19][20]. The role of CRT in ICD in malignant diseases have been recently widely studied on both in vitro (cell lines) and in vivo (animals, patient’s tumor specimens) models. However, up to this date, available data is still scarce and highly controversial. As it has been shown in published research, ecto-CRT may be either positive or negative marker of patients/tumor cells survival, depending on type of tumor [21][22].
One of the most dangerous malignant diseases is ovarian cancer. The mortality of patients suffering from this kind of tumor is still very high in both the United States and Europe. The 5-year-survival of women with advanced ovarian cancer is very low and does not exceed 25%. There are a number of reasons for such a poor outcome. Among the most important are: late diagnosis of the disease (an overwhelming number of patients are diagnosed in the advanced stage of disease), high resistance of cancer cells to chemotherapeutics, unique metastatic properties and poor immunogenicity of ovarian cancer cells [23][24][25]. It is considered that better identification and evaluation of immune-modulating therapeutic approaches can be one of the pathways to overcome poor prognosis of ovarian cancer patients. In this context ICD might be a promising way [24].
2. Immunogenic Cell Death—ER Stress Connection
ICD has been defined as an unique class of regulated cell death that is able to elicit complete antigen-specific adaptive immune response through the emission of particular molecules that belong to a class of the damage-associated molecular patterns (DAMPs) family [12][26]. ICD is a stressor-dependent cell death, since it is induced as an effect of ER stress combined with the generation of reactive oxygen species (ROS). In response to stress conditions, when there is accumulation of misfolded proteins, ER initiates the activation of a complex signaling pathways network, called the unfolded protein response (UPR). There are three different ER membrane-associated sensors that initiate UPR signaling pathway: protein kinase R-like endoplasmic reticulum kinase (PERK), activating transcription factor-6 (ATF6) and inositol-requiring transmembrane kinase/endoribonuclease 1 (IRE1) [27][28]. PERK is one of the major signaling pathways responsible for attenuation of mRNA translation under ER stress, preventing the influx of newly synthesized proteins into ER compartments. This type I transmembrane protein, when activated, triggers the phosphorylation of α-subunit of eukaryotic initiation factor 2 (eIF2α), leading to the inhibition of polypeptide chain synthesis. Generally, the activity of PERK pathway may result in cell cycle arrest, and under prolonged or severe stress conditions activation of this kinase enhances the apoptosis-signaling pathway [27][29]. ATF6, a type II transmembrane protein located on the ER surface, is a basic leucine zipper transcription factor which, after exposure to stressful conditions, translocates to the Golgi complex. Over there it is cleaved by site-1 and site-2 proteases and generated fragment then translocates to the nucleus where it can up-regulate the expression of genes encoding proteins involved in the UPR, including ER chaperones and transcription factor X-box-binding protein 1 (XBP1). IRE1, a type I transmembrane protein, after oligomerization and autophosphorylation, activates XBP1, which further induces the expression of UPR stress genes [27][28][30]. During prolonged stress exposure, IRE1 was also considered to be a key initiator of ER stress-induced cell death, as it interacts with tumor necrosis factor receptor associated factor 2 (TRAF2). This leads to the activation of apoptotic-signaling kinase-1 (ASK1), which induces downstream kinases: Jun-N-terminal kinase (JNK) and p38 mitogen activated protein kinase (MAPK), and in consequence promote apoptosis [29][31].
ICD has been well characterized in the context of tumor therapy, as its induction is one of the mechanisms, by which conventional chemotherapeutics can kill tumor cells. However, there is a range of specific stimuli that can initiate ICD. Among them are: (a) obligatory intracellular pathogens, including numerous bacterial and viral species; (b) conventional chemotherapeutics, such as anthracyclines, some DNA-damaging agents, proteasomal inhibitors, poly-A-ribose polymerase inhibitors or mitotic poisons; (c) anti-cancer agents that target different types of cell components or processes inducing cell death pathways; (d) various oncolytic potential molecules, i.e., viruses; (e) some chemicals; (f) physical stressors, such as ionizing radiation, hypericin-based photodynamic therapy, high hydrostatic pressure, nanopulse stimulation or severe cytotoxic heat shock [19][32][33].
Generally, based on their different mechanisms of action involved in ER stress, ICD inducers are divided either into type I or type II. Type I inducers initiate ER stress indirectly, as a downstream effect, while type II inducers can directly launch the ER stress response, provoking cell death [26][34]. Among the type I inducers are the best-known anti-cancer drugs, such as anthracyclines (doxorubicin, mitoxantrone), taxanes (paclitaxel, docetaxel), gemcitabine, cyclophosphamide, fluorouracil (5FU), and alkylating agent melphalan. Platinum derivatives such as cisplatin and carboplatin are believed to be controversial and their effectiveness to resolve ICD depends on the type of tumor cells, concentration used and treatment duration. In contrast, oxaliplatin, a third-generation platinum analogue, seems to be indisputable type I ICD inducer. On the other hand, physical methods, such as photodynamic therapy, high hydrostatic pressure or molecules as oncolytic viruses are the example of type II inducers [30][35][36][37]. Both types of inducers, by the combined action of ER stress and ROS generation which activates danger signaling pathways, are able to trigger the timely release or membrane exposure of a series of DAMPs. These endogenous danger molecules interact with a range of scavenger receptors (i.e., LDL receptor-related protein, LRP1/CD91), purinergic receptors (i.e., P2RX7/P2RY2) and pattern recognition receptors (PRR, i.e., Toll-like receptor 4—TLR4) on the innate immune cells, such as monocytes, neutrophils, macrophages or dendritic cells (DCs) [38].
Since the introduction of the danger model in 1994 [39], numerous DAMPs have been identified, and still the new ones are characterized. DAMPs are molecules that can be released, both, from extracellular matrix and intracellular compartments (ER, nucleus, cytosol, plasma membrane, mitochondria) as the response of dying cell [40]. The overall role of ICD-associated DAMPs involves: facilitating the recruitment of APCs or their precursors to the site of ICD; guiding the interaction between dying cell and APCs; triggering the phagocytosis of dead cells or their leftovers; promoting the maturation of APCs and their ability for effective antigen cross-presentation or enabling the recruitment of T-cells [19]. Among DAMPs most frequently associated with ICD are CRT, adenosine-5′-triphosphate (ATP), non-histone chromatin-binding protein—high-mobility group box 1 (HMGB1), type I and II interferons (IFN), annexin A1 and heat shock proteins (HSPs) 70 and 90. The majority of these molecules have non-immunogenic functions inside cell, but when exposed on cell surface or released extracellularly they become immunogenic [22][35][36].
Calreticulin will be thoroughly described in the next chapter of this manuscript. ATP, under physiological conditions, is involved in various cellular metabolic processes and intracellular responses, but during apoptosis its releasement from dying cell occurs in autophagy-dependent manner. The presence of ATP in extracellular space acts as “find me” signal, being a chemoattractant for DCs precursors. ATP binds to the P2RX7 receptor on DCs, providing the inflammasome-mediated secretion of interleukin 1β (IL-1β), an important cytokine that plays crucial role in immune response development [41]. Another DAMP triggered by ICD is HMGB1, normally found in the nucleus (but with cytoplasmic localization due to shuttling), that serves various nuclear and cytosolic functions. HMGB1 is released from dying or stressed cells and when present in the extracellular space, can signal tissue injury. Through binding to a range of receptors, including TLR2, TLR4 and the receptor for advanced glycosylation end products (RAGE), this molecule can initiate the inflammatory response. However, it should be stressed that TLR4 seems to be the exclusive HMGB1 receptor and it is relevant in the context of considering cell death as immunogenic. HMGB1-TLR4 triggers the synthesis of pro-inflammatory cytokines, such as type I IFN. Furthermore, this signal is crucial for activating DCs and facilitating antigen presentation to T cells [7][32][42]. Next markers principally associated with ICD are HSP70 and HSP90. These chaperones, present in the cytoplasmic compartment, are involved in protein folding and can be upregulated to express protective response to stress conditions, such as heat shock. Moreover, both HSPs may be exposed on the cell membrane and act as “eat me” particles, attracting phagocytes and natural killer (NK) cells [30]. Moreover, HSP70 and HSP90 play important role in the cross-presentation of tumor-derived antigenic peptides on MHC class I molecules, providing the cytotoxic T lymphocytes (CTLs, CD8+) response [7].
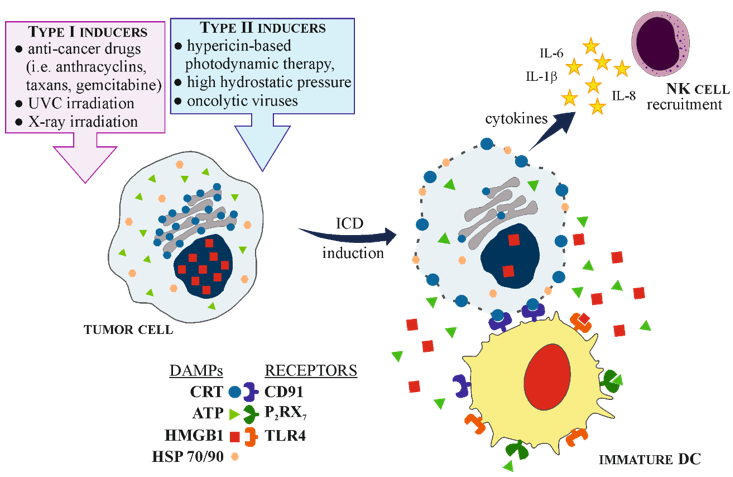
The development of anti-tumor immune response, initiated by ICD, involves a few important steps: (1) induction of ICD by the exposure of tumor cell to specific ICD inducers; (2) changes inside the tumor cell, leading to ER stress; (3) chronic release and membrane exposure of DAMPs, especially CRT, HMGB1, HSP70, HSP90 and ATP; (4) recognition of DAMPs by particular PRR on APCs, mainly on DCs; (5) activation and maturation of DCs; (6) promotion of dying tumor cell engulfment by mature DCs; (7) processing of tumor-derived antigen specific cargoes inside DCs; (8) triggering T-cell immune response by tumor antigen presentation along with MHC I and costimulatory molecules, especially to CTLs; (9) killing of tumor cells. Each step can be amplified by the action of specific DAMPs [22][35][43]. Moreover, during and after the induction of ICD in malignant cells, numerous cytokines and chemokines are identified, for example pro-inflammatory cytokines such as IL-6, IL-1β or tumor necrosis factor α (TNF-α), which can increase the MHC class I expression on APCs, promote T cell differentiation and NK cell activation. Furthermore, activated DCs, release IL-12 that enhances the functionality of NK cells. On the other hand, tumor cells treated with ICD inducers also secrete immunomodulatory cytokines as IL-8 or IL-6 [30] (Figure 1).
Figure 1. Schematic presentation of immunogenic cell death (ICD) mechanism. (a) ICD is induced after the exposure of tumor cell to type I or type II ICD inducers. Dying tumor cell releases and exposes on its plasma membrane different damage associated molecular patterns (DAPMs) molecules that are recognized by specific pattern recognition receptors (PRRs) on immature dendritic cells (DCs), leading to their maturation and activation. Additionally, tumor cells secrete cytokines that recruit natural killer (NK) cells. (b) Mature DC engulfs dying tumor cell, processes tumor antigens and presents it along with major histocompatibility complex (MHC) class I and costimulatory molecules (CD83/86) to CD8+ T-cells. Moreover, mature DCs secrete a range of cytokines that promote T-cells differentiation into CD8+ phenotype, as well as activate NK cells. Finally, activated NK cells and CD8+ T-cells are able to kill tumor cells.
References
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Classification of Cell Death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ 2005, 12 Suppl 2, 1463–1467, doi:10.1038/sj.cdd.4401724.
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009, 16, 3–11, doi:10.1038/cdd.2008.150.
- Liu, X.; Yang, W.; Guan, Z.; Yu, W.; Fan, B.; Xu, N.; Liao, D.J. There Are Only Four Basic Modes of Cell Death, Although There Are Many Ad-Hoc Variants Adapted to Different Situations. Cell Biosci 2018, 8, 6, doi:10.1186/s13578-018-0206-6.
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol 2007, 35, 495–516, doi:10.1080/01926230701320337.
- Bellamy, C.O.; Malcomson, R.D.; Harrison, D.J.; Wyllie, A.H. Cell Death in Health and Disease: The Biology and Regulation of Apoptosis. Semin Cancer Biol 1995, 6, 3–16, doi:10.1006/scbi.1995.0002.
- Raucci, A.; Palumbo, R.; Bianchi, M.E. HMGB1: A Signal of Necrosis. Autoimmunity 2007, 40, 285–289, doi:10.1080/08916930701356978.
- Tesniere, A.; Panaretakis, T.; Kepp, O.; Apetoh, L.; Ghiringhelli, F.; Zitvogel, L.; Kroemer, G. Molecular Characteristics of Immunogenic Cancer Cell Death. Cell Death Differ 2008, 15, 3–12, doi:10.1038/sj.cdd.4402269.
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol Int 2019, 43, 582–592, doi:10.1002/cbin.11137.
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical Inhibitor of Nonapoptotic Cell Death with Therapeutic Potential for Ischemic Brain Injury. Nat Chem Biol 2005, 1, 112–119, doi:10.1038/nchembio711.
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A Regulated Inflammatory Mode of Cell Death. J Neuroinflammation 2018, 15, 199, doi:10.1186/s12974-018-1235-0.
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The Molecular Machinery of Regulated Cell Death. Cell Research 2019, 29, 347–364, doi:10.1038/s41422-019-0164-5.
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu Rev Immunol 2013, 31, 51–72, doi:10.1146/annurev-immunol-032712-100008.
- Gold, L.I.; Eggleton, P.; Sweetwyne, M.T.; Van Duyn, L.B.; Greives, M.R.; Naylor, S.-M.; Michalak, M.; Murphy-Ullrich, J.E. Calreticulin: Non-Endoplasmic Reticulum Functions in Physiology and Disease. FASEB J 2010, 24, 665–683, doi:10.1096/fj.09-145482.
- Nakamura, K.; Zuppini, A.; Arnaudeau, S.; Lynch, J.; Ahsan, I.; Krause, R.; Papp, S.; De Smedt, H.; Parys, J.B.; Muller-Esterl, W.; et al. Functional Specialization of Calreticulin Domains. J Cell Biol 2001, 154, 961–972, doi:10.1083/jcb.200102073.
- Michalak, M.; Groenendyk, J.; Szabo, E.; Gold, L.I.; Opas, M. Calreticulin, a Multi-Process Calcium-Buffering Chaperone of the Endoplasmic Reticulum. Biochem J 2009, 417, 651–666, doi:10.1042/BJ20081847.
- Kotsias, F.; Cebrian, I.; Alloatti, A. Antigen Processing and Presentation. Int Rev Cell Mol Biol 2019, 348, 69–121, doi:10.1016/bs.ircmb.2019.07.005.
- Asadzadeh, Z.; Safarzadeh, E.; Safaei, S.; Baradaran, A.; Mohammadi, A.; Hajiasgharzadeh, K.; Derakhshani, A.; Argentiero, A.; Silvestris, N.; Baradaran, B. Current Approaches for Combination Therapy of Cancer: The Role of Immunogenic Cell Death. Cancers (Basel) 2020, 12, doi:10.3390/cancers12041047.
- Radogna, F.; Diederich, M. Stress-Induced Cellular Responses in Immunogenic Cell Death: Implications for Cancer Immunotherapy. Biochem Pharmacol 2018, 153, 12–23, doi:10.1016/j.bcp.2018.02.006.
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus Guidelines for the Definition, Detection and Interpretation of Immunogenic Cell Death. J Immunother Cancer 2020, 8, doi:10.1136/jitc-2019-000337.
- Fucikova, J.; Spisek, R.; Kroemer, G.; Galluzzi, L. Calreticulin and Cancer. Cell Research 2020, 1–12, doi:10.1038/s41422-020-0383-9.
- Schcolnik-Cabrera, A.; Oldak, B.; Juárez, M.; Cruz-Rivera, M.; Flisser, A.; Mendlovic, F. Calreticulin in Phagocytosis and Cancer: Opposite Roles in Immune Response Outcomes. Apoptosis 2019, 24, 245–255, doi:10.1007/s10495-019-01532-0.
- Serrano-Del Valle, A.; Anel, A.; Naval, J.; Marzo, I. Immunogenic Cell Death and Immunotherapy of Multiple Myeloma. Front Cell Dev Biol 2019, 7, 50, doi:10.3389/fcell.2019.00050.
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of Ovarian Cancer: A Review. Cancer Biol Med 2017, 14, 9–32, doi:10.20892/j.issn.2095-3941.2016.0084.
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin Oncol Nurs 2019, 35, 151–156, doi:10.1016/j.soncn.2019.02.001.
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388, doi:10.1016/S0140-6736(13)62146-7.
- Krysko, D.; Garg, A.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic Cell Death and DAMPs in Cancer Therapy Available online: https://pubmed.ncbi.nlm.nih.gov/23151605/ (accessed on 2 December 2020).
- Kadowaki, H.; Nishitoh, H. Signaling Pathways from the Endoplasmic Reticulum and Their Roles in Disease. Genes (Basel) 2013, 4, 306–333, doi:10.3390/genes4030306.
- Liu, Z.; Lv, Y.; Zhao, N.; Guan, G.; Wang, J. Protein Kinase R-like ER Kinase and Its Role in Endoplasmic Reticulum Stress-Decided Cell Fate. Cell Death Dis 2015, 6, e1822, doi:10.1038/cddis.2015.183.
- Sano, R.; Reed, J.C. ER Stress-Induced Cell Death Mechanisms. Biochim Biophys Acta 2013, 1833, 3460–3470, doi:10.1016/j.bbamcr.2013.06.028.
- Showalter, A.; Limaye, A.; Oyer, J.L.; Igarashi, R.; Kittipatarin, C.; Copik, A.J.; Khaled, A.R. Cytokines in Immunogenic Cell Death: Applications for Cancer Immunotherapy. Cytokine 2017, 97, 123–132, doi:10.1016/j.cyto.2017.05.024.
- Nishitoh, H.; Matsuzawa, A.; Tobiume, K.; Saegusa, K.; Takeda, K.; Inoue, K.; Hori, S.; Kakizuka, A.; Ichijo, H. ASK1 Is Essential for Endoplasmic Reticulum Stress-Induced Neuronal Cell Death Triggered by Expanded Polyglutamine Repeats. Genes & Development 2002, 16, 1345, doi:10.1101/gad.992302.
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 2018, 25, 486–541, doi:10.1038/s41418-017-0012-4.
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic Cell Death in Cancer and Infectious Disease. Nat Rev Immunol 2017, 17, 97–111, doi:10.1038/nri.2016.107.
- Inoue, H.; Tani, K. Multimodal Immunogenic Cancer Cell Death as a Consequence of Anticancer Cytotoxic Treatments. Cell Death Differ 2014, 21, 39–49, doi:10.1038/cdd.2013.84.
- Zhou, J.; Wang, G.; Chen, Y.; Wang, H.; Hua, Y.; Cai, Z. Immunogenic Cell Death in Cancer Therapy: Present and Emerging Inducers. J Cell Mol Med 2019, 23, 4854–4865, doi:10.1111/jcmm.14356.
- Fumet, J.-D.; Limagne, E.; Thibaudin, M.; Ghiringhelli, F. Immunogenic Cell Death and Elimination of Immunosuppressive Cells: A Double-Edged Sword of Chemotherapy. Cancers (Basel) 2020, 12, doi:10.3390/cancers12092637.
- Rébé, C.; Demontoux, L.; Pilot, T.; Ghiringhelli, F. Platinum Derivatives Effects on Anticancer Immune Response. Biomolecules 2019, 10, doi:10.3390/biom10010013.
- Vandenabeele, P.; Vandecasteele, K.; Bachert, C.; Krysko, O.; Krysko, D.V. Immunogenic Apoptotic Cell Death and Anticancer Immunity. In Apoptosis in Cancer Pathogenesis and Anti-cancer Therapy: New Perspectives and Opportunities. Advances in Experimental Medicine and Biology 930; Gregory CD, ed.; Springer International Publishing: Cham, Switzerland, 2016, pp. 133-149, doi:10.1007/978-3-319-39406-0_6.
- Matzinger, P. Tolerance, Danger, and the Extended Family. Annu Rev Immunol 1994, 12, 991–1045, doi:10.1146/annurev.iy.12.040194.005015.
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw 2018, 18, e27, doi:10.4110/in.2018.18.e27.
- Terenzi, A.; Pirker, C.; Keppler, B.K.; Berger, W. Anticancer Metal Drugs and Immunogenic Cell Death. J Inorg Biochem 2016, 165, 71–79, doi:10.1016/j.jinorgbio.2016.06.021.
- Diederich, M. Natural Compound Inducers of Immunogenic Cell Death. Arch Pharm Res 2019, 42, 629–645, doi:10.1007/s12272-019-01150-z.
- Wang, Y.-J.; Fletcher, R.; Yu, J.; Zhang, L. Immunogenic Effects of Chemotherapy-Induced Tumor Cell Death. Genes Dis 2018, 5, 194–203, doi:10.1016/j.gendis.2018.05.003.