
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Quincy Rosemarie | + 2162 word(s) | 2162 | 2020-12-03 11:27:56 | | | |
| 2 | Quincy Rosemarie | + 10 word(s) | 2172 | 2021-02-11 08:11:40 | | | | |
| 3 | Catherine Yang | -73 word(s) | 2099 | 2021-02-14 15:02:45 | | |
Video Upload Options
Epstein–Barr Virus (EBV) contributes to the development of lymphoid and epithelial malignancies. While EBV’s latent phase is more commonly associated with EBV-associated malignancies, there is increasing evidence that EBV’s lytic phase plays a role in EBV-mediated oncogenesis. The lytic phase contributes to oncogenesis primarily in two ways: (1) the production of infectious particles to infect more cells, and (2) the regulation of cellular oncogenic pathways, both cell autonomously and non-cell autonomously.
1. Introduction
EBV-positive malignancies have been associated with the latent phase of EBV’s life cycle; a non-productive phase in which no progeny virus is formed. However, there is now increasing evidence that EBV’s lytic phase contributes to EBV oncogenesis (reviewed in [1][2][3][4][5]). Epstein–Barr Virus (EBV) is a human γ-herpesvirus that infects a variety of cells in vivo and naïve B-lymphocytes efficiently in vitro. EBV induces and maintains proliferation of the infected B cells. In these cells, EBV remains latent, with few viral genes being expressed reminiscent of the lysogenic state of some bacteriophage (reviewed in [6]). On rare occasions, these infected cells alter their transcription to support EBV’s productive cycle, in which progeny virus particles are produced. It has become increasingly clear that EBV’s lytic phase contributes to tumor progression and maintenance. This contribution occurs through both the production of infectious particles to infect more cells and the regulation of cellular oncogenic pathways.
2. EBV’s Lytic Phase Contributes to Tumorigenesis by Production of Infectious Viral Particles
No EBV-positive tumor forms without a precursor cell being infected by this virus. Multiple findings are consistent with viral load being proportional to the risk of EBV-associated malignancy. These include: 1. A large, prospective serological survey conducted in Uganda between 1972 and 1979 to test for an association between infection with EBV and the development of BL [7][8]; 2. The finding that there is an increase in EBV-transformed B cells in vivo during the acute phases of malaria, and that this increase arises in part from the production of new viral particles from infected cells leading to new infections of naïve B cells [9]; and 3. It seems likely that variants of EBV that support their lytic phase more efficiently would be more likely to yield higher viral loads and accordingly have an increased risk of being oncogenic [10].
3. EBV’s Lytic Gene Expression in EBV-Associated Tumor Samples
Examination of EBV’s gene expression in samples of EBV-associated tumors has identified the expression of EBV lytic genes in tumor cells. The most common lytic gene assayed, the immediate early gene BZLF1, has been identified in Burkitt Lymphoma (BL)[11], nasopharyngeal carcinoma (NPC)[12][13], and gastric carcinoma (GC)[14] samples. EBV-associated tumors also often harbor cells undergoing an incomplete or abortive lytic phase, in which some early lytic genes are expressed, but no viral particles are produced [13].
Despite evidence for an incomplete lytic phase, several studies have shown that late lytic genes are expressed in tumor samples[15][16]. One explanation for this conundrum is that some late lytic genes are “leaky”, having low-level expression during early lytic phase and further upregulated following lytic DNA replication[17]. However, leaky late genes do not explain the expression of true late genes in tumor samples, which may result from the inefficient detection of the genes required for lytic DNA replication or in their being expressed in only a small subset of the tumor cells. Clearly, EBV-associated tumors often express an assortment of lytic genes spanning the early and late lytic gene sets.
4. Contributions of EBV’s Lytic Genes to EBV’s Oncogenesis
Several studies have used animal models to uncover the importance of EBV’s lytic phase to lymphomagenesis. These studies used a variant of EBV that has been engineered to have its BZLF1 gene knocked out, thereby making it defective for lytic phase entry. BZLF1-KO or wildtype EBV were used to infect B cells and generate lymphoblastoid cells lines (LCLs), which were then injected into immunocompromised mice[18][19][20]. In these studies, wildtype LCLs were consistently more tumorigenic than BZLF1-KO LCLs. The findings of this analysis provide evidence for the contribution of EBV’s lytic phase to tumor development in vivo.
While EBV’s lytic phase has been shown to contribute to tumor development, evidence indicates that it is not necessary to have a complete lytic phase. For example, one study in NOD mice infected with an engineered variant of EBV lacking the lytic DNA polymerase gene, BALF5, showed that the complete lytic phase is not required for its contribution to tumor development[21]. Combined with findings from the BZLF1-KO studies and given that a complete lytic phase would lead to host cell lysis, these analyses confirm that EBV’s early lytic phase is important in tumorigenesis, whereas the late lytic phase is dispensable and even likely to inhibit tumor progression.
The mechanisms by which EBV’s lytic phase affects tumorigenesis in part involves its role in the modulation of cellular pathways that influence tumor cells and tumor microenvironment. EBV’s lytic phase regulates various cellular oncogenic pathways, including those promoting angiogenesis, immunomodulation and immune evasion, genomic instability, as well as cell cycle and survival. These regulated pathways contribute to tumor formation and progression via cell-autonomous and non-cell-autonomous functions. Cell-autonomous functions that contribute to tumorigenesis occur in cells that undergo an incomplete (abortive) lytic phase; those cells that complete the lytic phase would die and can no longer contribute to the tumor. These functions include increased proliferation, immune evasion, and genomic instability, along with decreased apoptosis. On the other hand, non-cell-autonomous events can influence surrounding cells and foster a pro-tumorigenic microenvironment through angiogenesis, modifications of the extracellular matrix, and cytokine productions. Collectively, these cell-autonomous and non-cell-autonomous outcomes contribute to EBV-mediated oncogenesis.
Table 1. EBV’s lytic genes and their roles in oncogenesis.
|
Table 1. EBV’s lytic genes and their roles in oncogenesis. |
|||||
|
EBV Lytic Gene |
IE/E/L 1 |
Lytic Function |
Role in Oncogenesis |
Oncogenic Mechanism of Action |
References |
|
BZLF1 |
IE |
Transactivator |
Induction of pro-inflammatory cytokine expression and secretion (IL-8, IL-10, IL-13) |
Binding and activating target gene promoters |
|
|
BGLF5 |
E |
Alkaline exonuclease |
Downregulation of MHCs |
Host shut off; degradation of cellular mRNAs |
[25] |
|
BILF1 |
E |
gp64, vGPCR |
Inhibition of MHC trafficking |
|
[26] |
|
BLLF3 |
E |
dUTPase |
Induction of pro-inflammatory cytokine expression and secretion (IL-1β, IL-6, IL-8, IL-10) |
|
[27] |
|
BNLF2a |
E |
Inhibitor of TAP 2 |
Inhibition of CD8 T cell recognition of infected cells |
|
[28] |
|
BCRF1 |
L |
vIL-10 |
Inhibition of NK cell-mediated elimination of infected cells; inhibition of CD4 T cells |
|
[28] |
|
BDLF3 |
L |
gp150 |
Downregulation of MHCs |
Ubiquitination and degradation of MHCs |
[29] |
|
BZLF2 |
L |
gp42 |
Inhibition of MHC II-mediated antigen presentation |
|
[30] |
|
Angiogenesis and Invasion |
|||||
|
EBV Lytic Gene |
IE/E/L |
Lytic Function |
Role in Oncogenesis |
Oncogenic Mechanism of Action |
References |
|
BZLF1 |
IE |
Transactivator |
Upregulation of MMP1, MMP3, MMP9 |
Binding and activating target gene promoters |
|
|
BRLF1 |
IE |
Transactivator |
Upregulation of MMP9 |
Binding and activating target gene promoters |
[34] |
|
Genomic Instability |
|||||
|
EBV Lytic Gene |
IE/E/L |
Lytic Function |
Role in Oncogenesis |
Oncogenic Mechanism of Action |
References |
|
BALF3 |
E |
Terminase |
Induction of genomic aberration |
Induction of DNA damage |
[35] |
|
BGLF4 |
E |
S/T protein kinase |
Induction of genomic aberration |
Induction of DNA damage pathways and premature chromosome condensation |
|
|
BGLF5 |
E |
Alkaline exonuclease |
Induction of genomic aberration |
Induction of DNA damage |
[38] |
|
BALF4 |
L |
gp110 |
Induction of genomic aberration |
|
[39] |
|
BNRF1 |
L |
Major tegument protein |
Induction of genomic aberration |
|
[39] |
|
Cell Cycle Progression and Apoptosis |
|||||
|
EBV Lytic Gene |
IE/E/L |
Lytic Function |
Role in Oncogenesis |
Oncogenic Mechanism of Action |
References |
|
BALF1 |
E |
vBcl-2 |
Pro-survival, anti-apoptotic |
|
[40] |
|
BHRF1 |
E |
vBcl-2 |
Pro-survival, anti-apoptotic |
Inhibition of BIM, PUMA, BAK |
|
5. EBV’s Lytic miRNAs in Tumorigenesis
In addition to its lytic proteins, EBV also regulates tumorigenesis through its miRNAs. EBV encodes two clusters of miRNAs, one in the BHRF1 locus and one in the BART locus. The BART miRNAs are detected at all phases of EBV’s life cycle, while the BHRF1 miRNAs are not detected in some cells in culture and are when the same cells are induced to enter their lytic phase[28].
For example, one BHRF1miRNA, miR-BHRF1-2, inhibits the tumor suppressors, PTEN and PRDM1, which would likely foster EBV’s tumorigenesis[29][44]. This same miRNA also inhibits expression of the IL-1 receptor 1 to limit signaling via receptor engagement and, potentially, any resulting inflammatory response[45]. The BART miRNAs contribute to transformation by regulating expression of multiple cellular genes as well as inhibiting immune recognition of the infected cell[46][47][48]. Their continued presence in cells in EBV’s lytic phase is likely to contribute also to the success of this portion of the viral life cycle.
6. Inhibitor Studies: A Test for a Role for EBV’s Lytic Phase in Oncogenesis?
One essential role for EBV’s lytic phase in EBV’s oncogenesis is the production of infectious virus, which supports the infection and transformation of cells that subsequently can evolve into tumors. But what facets of EBV’s lytic phase contribute to oncogenesis after infection and transformation? A possible experimental route to address this question is to test small-molecule inhibitors of distinct steps within EBV’s lytic phase[49][50][51]. These tests have led to the conclusion that events downstream of EBV’s DNA synthesis during its lytic phase do not contribute detectably to its oncogenesis once cells have been infected and transformed. A second insight is that its lytic DNA synthesis does not contribute to the cancer phenotypes of these cells either.
We lack small-molecule inhibitors of steps earlier than DNA synthesis for EBV’s lytic phase. Another window, though, on the potential contributions of these steps to EBV’s oncogenesis comes from detailed studies of treating tumor patients with T cells educated against EBV-encoded antigens[52][53][54]. These epitope-specific, anti-EBV T cells are, however, tools that should allow testing for contributions of the viral genes expressed early in EBV’s lytic phase to EBV’s oncogenesis, particularly in tractable animal models. Such tools, therefore, can be instrumental in learning how these early lytic genes foster EBV’s oncogenesis.
7. Concluding Remarks
EBV’s lytic phase contributes to tumorigenesis primarily in two ways (see Figure 1): (1) the production of infectious particles to infect more cells, and (2) the regulation of cellular oncogenic pathways, mediated by lytic proteins and miRNAs. The production of infectious virus is a requisite precursor to the infection and transformation of cells that can subsequently evolve into tumors. Following infection, reactivation of the lytic phase supports the expression of miRNAs and early lytic genes which can regulate cellular pathways that promote tumorigenesis. Some of these tumorigenic effects are cell autonomous, affecting only the cells in which the relevant lytic genes are expressed. Others are non-cell autonomous, exerting influence over neighboring tumor cells through the production of secreted molecules and/or the modification of the tumor microenvironment. As the completion of a lytic phase results in cell death, we speculate that the contribution of lytic phase to tumorigenesis is in part mediated by an incomplete lytic phase (also termed abortive lytic phase), highlighting the importance of the early lytic phase. Given the relevance of the lytic phase to tumor progression and maintenance, it will be important to understand the mechanisms by which EBV’s lytic phase contributes to tumorigenesis in order to target it as an alternative means to treat EBV-associated malignancies.
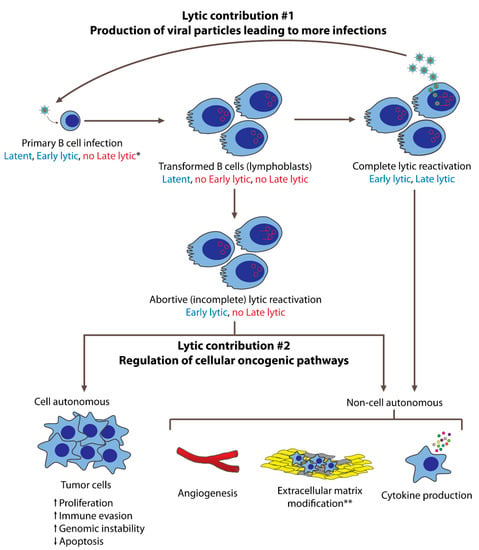
Figure 1. EBV’s lytic phase contributes to oncogenesis in both B cells and epithelial cells. In this figure, B cell lymphomagenesis is used to represent these contributions. Following primary infection and transformation, EBV is maintained latently in infected cells. On rare occasions, some cells undergo lytic reactivation, either completely or incompletely. Cells that undergo the complete lytic reactivation express both early and late lytic genes and produce new viral particles that can infect more cells. These newly infected cells are transformed and may subsequently evolve into tumor cells. Cells that complete the lytic phase eventually die, such that they do not contribute as proliferating tumor cells. However, they can contribute to oncogenesis via non-cell-autonomous mechanisms mediated by early lytic gene products. These contributions include angiogenesis, pro-tumorigenic cytokine production, and, in the case of NPCs, extracellular matrix modifications. Some cells that enter the lytic phase do not complete it, undergoing incomplete (abortive) lytic reactivation. These cells express early lytic genes but not late lytic genes, and thus do not produce new viral particles. These cells may continue to live, and contribute to oncogenesis cell autonomously by becoming tumor cells with increased proliferation, immune evasion, and genomic instability, as well as decreased apoptosis. Having expressed early lytic genes, abortive lytic cells may also contribute to oncogenesis non-cell autonomously. * Expression of latent, early lytic, or late lytic genes are indicated in blue (expressed) and red (not expressed). ** Extracellular matrix modifications are primarily studied in NPCs.
References
- Manners, O.; Murphy, J.C.; Coleman, A.; Hughes, D.J.; Whitehouse, A. Contribution of the KSHV and EBV lytic cycles to tumourigenesis. Curr. Opin. Virol. 2018, 32, 60–70, doi:10.1016/j.coviro.2018.08.014.
- Morales-Sánchez, A.; Fuentes-Panana, E.M. The immunomodulatory capacity of an epstein-barr virus abortive lytic cycle: Potential contribution to viral tumorigenesis. Cancers (Basel) 2018, 10, 98, doi:10.3390/cancers10040098.
- Li, H.; Liu, S.; Hu, J.; Luo, X.; Li, N.; Bode, A.M.; Cao, Y. Epstein-Barr virus lytic reactivation regulation and its pathogenic role in carcinogenesis. Int. J. Biol. Sci. 2016, 12, 1309–1318, doi:10.7150/ijbs.16564.
- Münz, C. Latency and lytic replication in Epstein–Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 2019, 17, 691–700, doi:10.1038/s41579-019-0249-7.
- Münz, C. Tumor Microenvironment Conditioning by Abortive Lytic Replication of Oncogenic γ-Herpesviruses. Adv. Exp. Med. Biol. 2020, 1225, 127–135, doi:10.1007/978-3-030-35727-6_9.
- Lwoff, A. Lysogeny. Bacteriol. Rev. 1953, 17, 269–337, doi:10.1128/mmbr.17.4.269-337.1953.
- De-Thé, G.; Geser, A.; Day, N.E.; Tukei, P.M.; Williams, E.M.; Beri, D.P.; Smith, P.G.; Dean, A.G.; Bornkamm, G.W.; Feorino, P.; et al. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt’s lymphoma from Ugandan prospective study. Nature 1978, 274, 756–761, doi:10.1038/274756a0.
- Geser, A.; De Thé, G.; Lenoir, G.; Day, N.E.; Williams, E.H. Final case reporting from the ugandan prospective study of the relationship between ebv and burktit’s lymphoma. Int. J. Cancer 1982, 29, 397–400, doi:10.1002/ijc.2910290406.
- K.M.C. Lam; N. Syed; D.H. Crawford; H. Whittle; Circulating Epstein-Barr virus-carrying B cells in acute malaria. The Lancet 1991, 337, 876-878, 10.1016/0140-6736(91)90203-2.
- Jillian A. Bristol; Reza Djavadian; Emily R. Albright; Carrie B. Coleman; Makoto Ohashi; Mitchell Hayes; James C. Romero-Masters; Elizabeth A. Barlow; Paul J. Farrell; Rosemary Rochford; et al.Robert F. KalejtaEric C. JohannsenShannon C. Kenney A cancer-associated Epstein-Barr virus BZLF1 promoter variant enhances lytic infection. PLOS Pathogens 2018, 14, e1007179, 10.1371/journal.ppat.1007179.
- Shao-An Xue; Louise G. Labrecque; Qi-Long Lu; S. Kate Ong; Irvin A. Lampert; Peter Kazembe; Elizabeth Molyneux; Robin L. Broadhead; Eric Borgstein; Beverly E. Griffin; et al. Promiscuous expression of Epstein-Barr virus genes in Burkitt's lymphoma from the central African country Malawi. International Journal of Cancer 2002, 99, 635-643, 10.1002/ijc.10372.
- Cochet, C.; Martel-Renoir, D.; Grunewald, V.; Bosq, J.; Cochet, G.; Schwaab, G.; Bernaudin, J.-F.; Joab, I. Expression of the Epstein-Barr Virus Immediate Early Gen, BZLF1, in Nasopharyngeal Carcinoma Tumor Cells. Virology 1993, 197, 358–365.
- Ramayanti, O.; Juwana, H.; Verkuijlen, S.A.M.W.; Adham, M.; Pegtel, M.D.; Greijer, A.E.; Middeldorp, J.M. Epstein-Barr virus mRNA profiles and viral DNA methylation status in nasopharyngeal brushings from nasopharyngeal carcinoma patients reflect tumor origin. Int. J. Cancer 2017, 140, 149–162, doi:10.1002/ijc.30418.
- Ivan Borozan; Marc Zapatka; Lori Frappier; Vincent Ferretti; Analysis of Epstein-Barr Virus Genomes and Expression Profiles in Gastric Adenocarcinoma. Journal of Virology 2017, 92, 1-18, 10.1128/jvi.01239-17.
- Borozan, I.; Zapatka, M.; Frappier, L.; Ferretti, V. Analysis of Epstein-Barr Virus Genomes and Expression Profiles in Gastric Adenocarcinoma. J. Virol. 2017, 92, 1–18, doi:10.1128/jvi.01239-17.
- Martel-Renoir, D.; Grunewald, V.; Touitou, R.; Schwaab, G.; Joab, I. Qualitative analysis of the expression of Epstein-Barr virus lytic genes in nasopharyngeal carcinoma biopsies. J. Gen. Virol. 1995, 76, 1401–1408, doi:10.1099/0022-1317-76-6-1401.
- Reza Djavadian; Mitchell Hayes; Eric Johannsen; CAGE-seq analysis of Epstein-Barr virus lytic gene transcription: 3 kinetic classes from 2 mechanisms. PLOS Pathogens 2018, 14, e1007114, 10.1371/journal.ppat.1007114.
- Hong, G.K.; Gulley, M.L.; Feng, W.-H.; Delecluse, H.-J.; Holley-Guthrie, E.; Kenney, S.C. Epstein-Barr Virus Lytic Infection Contributes to Lymphoproliferative Disease in a SCID Mouse Model. J. Virol. 2005, 79, 13993–14003, doi:10.1128/jvi.79.22.13993-14003.2005.
- Ma, S.-D.; Hegde, S.; Young, K.H.; Sullivan, R.; Rajesh, D.; Zhou, Y.; Jankowska-Gan, E.; Burlingham, W.J.; Sun, X.; Gulley, M.L.; et al. A New Model of Epstein-Barr Virus Infection Reveals an Important Role for Early Lytic Viral Protein Expression in the Development of Lymphomas. J. Virol. 2011, 85, 165–177, doi:10.1128/jvi.01512-10.
- McHugh, D.; Caduff, N.; Barros, M.H.M.; Rämer, P.C.; Raykova, A.; Murer, A.; Landtwing, V.; Quast, I.; Styles, C.T.; Spohn, M.; et al. Persistent KSHV Infection Increases EBV-Associated Tumor Formation In Vivo via Enhanced EBV Lytic Gene Expression. Cell Host Microbe 2017, 22, 61–73.e7, doi:10.1016/j.chom.2017.06.009.
- Yusuke Okuno; Takayuki Murata; Yoshitaka Sato; Hideki Muramatsu; Yoshinori Ito; Takahiro Watanabe; Tatsuya Okuno; Norihiro Murakami; Kenichi Yoshida; Akihisa Sawada; et al.Masami InoueKeisei KawaMasao SetoKoichi OhshimaYuichi ShiraishiKenichi ChibaHiroko TanakaSatoru MiyanoYohei NaritaMasahiro YoshidaFumi GoshimaJun-Ichi KawadaTetsuya NishidaHitoshi KiyoiSeiichi KatoShigeo NakamuraSatoko MorishimaTetsushi YoshikawaShigeyoshi FujiwaraNorio ShimizuYasushi IsobeMasaaki NoguchiAtsushi KikutaKeiji IwatsukiYoshiyuki TakahashiSeiji KojimaSeishi OgawaHiroshi Kimura Defective Epstein–Barr virus in chronic active infection and haematological malignancy. Nature Microbiology 2019, 4, 404-413, 10.1038/s41564-018-0334-0.
- Hsu, M.; Wu, S.-Y.; Chang, S.-S.; Su, I.-J.; Tsai, C.-H.; Lai, S.-J.; Shiau, A.-L.; Takada, K.; Chang, Y. Epstein-Barr Virus Lytic Transactivator Zta Enhances Chemotactic Activity through Induction of Interleukin-8 in Nasopharyngeal Carcinoma Cells. J. Virol. 2008, 82, 3679–3688, doi:10.1128/jvi.02301-07.
- Lee, C.-H.; Yeh, T.-H.; Lai, H.-C.; Wu, S.-Y.; Su, I.-J.; Takada, K.; Chang, Y. Epstein-Barr Virus Zta-Induced Immunomodulators from Nasopharyngeal Carcinoma Cells Upregulate Interleukin-10 Production from Monocytes. J. Virol. 2011, 85, 7333–7342, doi:10.1128/jvi.00182-11.
- Tsai, S.C.; Lin, S.J.; Chen, P.W.; Luo, W.Y.; Yeh, T.H.; Wang, H.W.; Chen, C.J.; Tsai, C.H. EBV Zta protein induces the expression of interleukin-13, promoting the proliferation of EBV-infected B cells and lymphoblastoid cell lines. Blood 2009, 114, 109–118, doi:10.1182/blood-2008-12-193375.
- Martin Rowe; Britt Glaunsinger; Daphne Van Leeuwen; Jianmin Zuo; David Sweetman; Don Ganem; Jaap Middeldorp; Emmanuel J. H. J. Wiertz; Maaike E. Ressing; Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proceedings of the National Academy of Sciences 2007, 104, 3366-3371, 10.1073/pnas.0611128104.
- Jianmin Zuo; Laura L. Quinn; Jennifer Tamblyn; Wendy A. Thomas; Regina Feederle; Henri-Jacques Delecluse; Andrew D. Hislop; Martin Rowe; The Epstein-Barr Virus-Encoded BILF1 Protein Modulates Immune Recognition of Endogenously Processed Antigen by Targeting Major Histocompatibility Complex Class I Molecules Trafficking on both the Exocytic and Endocytic Pathways. Journal of Virology 2010, 85, 1604-1614, 10.1128/jvi.01608-10.
- Ronald Glaser; Monica L. Litsky; David A. Padgett; Robert A. Baiocchi; Eric V. Yang; Min Chen; Peir-En Yeh; Kari B. Green-Church; Michael A. Caligiuri; Marshall V. Williams; et al. EBV-encoded dUTPase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology 2006, 346, 205-218, 10.1016/j.virol.2005.10.034.
- Simon Jochum; Andreas Moosmann; Stephan Lang; Wolfgang Hammerschmidt; Reinhard Zeidler; The EBV Immunoevasins vIL-10 and BNLF2a Protect Newly Infected B Cells from Immune Recognition and Elimination. PLOS Pathogens 2012, 8, e1002704, 10.1371/journal.ppat.1002704.
- Laura L. Quinn; Luke R. Williams; Claire White; Calum Forrest; Jianmin Zuo; Martin Rowe; The Missing Link in Epstein-Barr Virus Immune Evasion: the BDLF3 Gene Induces Ubiquitination and Downregulation of Major Histocompatibility Complex Class I (MHC-I) and MHC-II. Journal of Virology 2015, 90, 356-367, 10.1128/jvi.02183-15.
- Maaike E. Ressing; Daphne Van Leeuwen; Frank A. W. Verreck; Sinéad Keating; Raquel Gomez; Kees L. M. C. Franken; Tom H. M. Ottenhoff; Melanie Spriggs; Ton N. Schumacher; Lindsey M. Hutt-Fletcher; et al.Martin RoweEmmanuel J. H. J. Wiertz Epstein-Barr Virus gp42 Is Posttranslationally Modified To Produce Soluble gp42 That Mediates HLA Class II Immune Evasion. Journal of Virology 2005, 79, 841-852, 10.1128/jvi.79.2.841-852.2005.
- Yoshizaki, T.; Sato, H.; Murono, S.; Pagano, J.S.; Furukawa, M. Matrix metalloproteinase 9 is induced by the Epstein-Barr virus BZLF1 transactivator. Clin. Exp. Metastasis 1999, 17, 431–436, doi:10.1023/A:1006699003525.
- Lu, J.; Chua, H.H.; Chen, S.Y.; Chen, J.Y.; Tsai, C.H. Regulation of matrix metalloproteinase-1 by Epstein-Barr virus proteins. Cancer Res. 2003, 63, 256–262.
- Lan, Y.Y.; Yeh, T.H.; Lin, W.H.; Wu, S.Y.; Lai, H.C.; Chang, F.H.; Takada, K.; Chang, Y. Epstein-Barr Virus Zta Upregulates Matrix Metalloproteinases 3 and 9 that Synergistically Promote Cell Invasion In Vitro. PLoS ONE 2013, 8, e56121, doi:10.1371/journal.pone.0056121.
- Yu-Yan Lan; Fang-Hsin Chang; Jen-Hao Tsai; Yao Chang; Epstein-Barr virus Rta promotes invasion of bystander tumor cells through paracrine of matrix metalloproteinase 9. Biochemical and Biophysical Research Communications 2018, 503, 2160-2166, 10.1016/j.bbrc.2018.08.006.
- Shih-Hsin Chiu; Chung-Chun Wu; Chih-Yeu Fang; Shu-Ling Yu; Hui-Yu Hsu; Yen-Hung Chow; Jen-Yang Chen; Epstein-Barr virus BALF3 mediates genomic instability and progressive malignancy in nasopharyngeal carcinoma. Oncotarget 2014, 5, 8583-8601, 10.18632/oncotarget.2323.
- Lee, C.-P.; Chen, J.-Y.; Wang, J.-T.; Kimura, K.; Takemoto, A.; Lu, C.-C.; Chen, M.-R. Epstein-Barr virus BGLF4 kinase induces premature chromosome condensation through activation of condensin and topoisomerase II. J. Virol. 2007, 81, 5166–5180, doi:10.1128/JVI.00120-07.
- Chang, Y.H.; Lee, C.P.; Su, M.T.; Wang, J.T.; Chen, J.Y.; Lin, S.F.; Tsai, C.H.; Hsieh, M.J.; Takada, K.; Chen, M.R. Epstein-barr virus BGLF4 kinase retards cellular S-phase progression and induces chromosomal abnormality. PLoS ONE 2012, 7, e39217, doi:10.1371/journal.pone.0039217.
- Chung-Chun Wu; Ming-Tsan Liu; Yu-Ting Chang; Chih-Yeu Fang; Sheng-Ping Chou; Hsin-Wei Liao; Kuan-Lin Kuo; Shih-Lung Hsu; Yi-Ren Chen; Pei-Wen Wang; et al.Yu-Lian ChenHsin-Ying ChuangChia-Huei LeeMing ChenWun-Shaing Wayne ChangJen-Yang Chen Epstein–Barr Virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Research 2009, 38, 1932-1949, 10.1093/nar/gkp1169.
- Anatoliy Shumilov; Ming-Han Tsai; Yvonne T. Schlosser; Anne-Sophie Kratz; Katharina Bernhardt; Susanne Fink; Tuba Mizani; Xiaochen Lin; Anna Jauch; Josef Mautner; et al.Annette Kopp-SchneiderRegina FeederleIngrid HoffmannHenri-Jacques Delecluse Epstein–Barr virus particles induce centrosome amplification and chromosomal instability. Nature Communications 2017, 8, 14257, 10.1038/ncomms14257.
- Markus Altmann; Wolfgang Hammerschmidt; Epstein-Barr Virus Provides a New Paradigm: A Requirement for the Immediate Inhibition of Apoptosis. PLOS Biology 2005, 3, e404, 10.1371/journal.pbio.0030404.
- Altmann, M.; Hammerschmidt, W. Epstein-barr virus provides a new paradigm: A requirement for the immediate inhibition of apoptosis. PLoS Biol. 2005, 3, e404, doi:10.1371/journal.pbio.0030404.
- Kelly, G.L.; Long, H.M.; Stylianou, J.; Thomas, W.A.; Leese, A.; Bell, A.I.; Bornkamm, G.W.; Mautner, J.; Rickinson, A.B.; Rowe, M. An epstein-barr virus anti-apoptotic protein constitutively expressed in transformed cells and implicated in burkitt lymphomagenesis: The Wp/BHRF1 link. PLoS Pathog. 2009, 5, e1000341, doi:10.1371/journal.ppat.1000341.
- Fitzsimmons, L.; Cartlidge, R.; Chang, C.; Sejic, N.; Galbraith, L.C.A.; Suraweera, C.D.; Croom-Carter, D.; Dewson, G.; Tierney, R.J.; Bell, A.I.; et al. EBV BCL-2 homologue BHRF1 drives chemoresistance and lymphomagenesis by inhibiting multiple cellular pro-apoptotic proteins. Cell Death Differ. 2020, 27, 1554–1568, doi:10.1038/s41418-019-0435-1.
- Richard Amoroso; Leah Fitzsimmons; Wendy A. Thomas; Gemma L. Kelly; Martin Rowe; Andrew I. Bell; Quantitative Studies of Epstein-Barr Virus-Encoded MicroRNAs Provide Novel Insights into Their Regulation. Journal of Virology 2010, 85, 996-1010, 10.1128/jvi.01528-10.
- Ma, J.; Nie, K.; Redmond, D.; Liu, Y.; Elemento, O.; Knowles, D.M.; Tam, W. EBV-miR-BHRF1-2 targets PRDM1/Blimp1: Potential role in EBV lymphomagenesis. Leukemia 2016, 30, 594–604, doi:10.1038/leu.2015.285.
- Bernhardt, K.; Haar, J.; Tsai, M.H.; Poirey, R.; Feederle, R.; Delecluse, H.J. A Viral microRNA Cluster Regulates the Expression of PTEN, p27 and of a bcl-2 Homolog. PLoS Pathog. 2016, 12, e1005405, doi:10.1371/journal.ppat.1005405.
- Camille M. Skinner; Nikita S. Ivanov; Sarah A. Barr; Yan Chen; Rebecca L. Skalsky; An Epstein-Barr Virus MicroRNA Blocks Interleukin-1 (IL-1) Signaling by Targeting IL-1 Receptor 1. Journal of Virology 2017, 91, e00530-17, 10.1128/jvi.00530-17.
- Riley, K.J.; Rabinowitz, G.S.; Yario, T.A.; Luna, J.M.; Darnell, R.B.; Steitz, J.A. EBV and human microRNAs co-target oncogenic and apoptotic viral and human genes during latency. EMBO J. 2012, 31, 2207–2221, doi:10.1038/emboj.2012.63.
- Skalsky, R.L.; Corcoran, D.L.; Gottwein, E.; Frank, C.L.; Kang, D.; Hafner, M.; Nusbaum, J.D.; Feederle, R.; Delecluse, H.J.; Luftig, M.A.; et al. The viral and cellular microRNA targetome in lymphoblastoid cell lines. PLoS Pathog. 2012, 8, e1002484, doi:10.1371/journal.ppat.1002484.
- Albanese, M.; Tagawa, T.; Buschle, A.; Hammerschmidt, W. Innate and Adaptive Antiviral Immunity. J. Virol. 2017, 91, 1–6.
- Gutiérrez, M.I.; Judde, J.G.; Magrath, I.T.; Bhatia, K.G. Switching viral latency to viral lysis: A novel therapeutic approach for Epstein-Barr virus-associated neoplasia. Cancer Res. 1996, 56, 969–972.
- Feng, W.; Hong, G.; Delecluse, H.-J.; Kenney, S.C. Lytic Induction Therapy for Epstein-Barr Virus-Positive B-Cell Lymphomas. J. Virol. 2004, 78, 1893–1902, doi:10.1128/jvi.78.4.1893-1902.2004.
- Perrine, S.P.; Hermine, O.; Small, T.; Suarez, F.; O’Reilly, R.; Boulad, F.; Fingeroth, J.; Askin, M.; Levy, A.; Mentzer, S.J.; et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood 2007, 109, 2571–2578, doi:10.1182/blood-2006-01-024703.
- Heslop, H.E.; Ng, C.Y.C.; Li, C.; Smith, C.A.; Loftin, S.K.; Krance, R.; Brenner, M.K.; Rooney, C.M. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat. Med. 1996, 2, 551–555, doi:10.1038/nm0596-551.
- Maaike E. Ressing; Daphne Van Leeuwen; Frank A. W. Verreck; Sinéad Keating; Raquel Gomez; Kees L. M. C. Franken; Tom H. M. Ottenhoff; Melanie Spriggs; Ton N. Schumacher; Lindsey M. Hutt-Fletcher; et al.Martin RoweEmmanuel J. H. J. Wiertz Epstein-Barr Virus gp42 Is Posttranslationally Modified To Produce Soluble gp42 That Mediates HLA Class II Immune Evasion. Journal of Virology 2005, 79, 841-852, 10.1128/jvi.79.2.841-852.2005.
- Yoshizaki, T.; Sato, H.; Murono, S.; Pagano, J.S.; Furukawa, M. Matrix metalloproteinase 9 is induced by the Epstein-Barr virus BZLF1 transactivator. Clin. Exp. Metastasis 1999, 17, 431–436, doi:10.1023/A:1006699003525.
- Lu, J.; Chua, H.H.; Chen, S.Y.; Chen, J.Y.; Tsai, C.H. Regulation of matrix metalloproteinase-1 by Epstein-Barr virus proteins. Cancer Res. 2003, 63, 256–262.
- Lan, Y.Y.; Yeh, T.H.; Lin, W.H.; Wu, S.Y.; Lai, H.C.; Chang, F.H.; Takada, K.; Chang, Y. Epstein-Barr Virus Zta Upregulates Matrix Metalloproteinases 3 and 9 that Synergistically Promote Cell Invasion In Vitro. PLoS ONE 2013, 8, e56121, doi:10.1371/journal.pone.0056121.
- Yu-Yan Lan; Fang-Hsin Chang; Jen-Hao Tsai; Yao Chang; Epstein-Barr virus Rta promotes invasion of bystander tumor cells through paracrine of matrix metalloproteinase 9. Biochemical and Biophysical Research Communications 2018, 503, 2160-2166, 10.1016/j.bbrc.2018.08.006.
- Shih-Hsin Chiu; Chung-Chun Wu; Chih-Yeu Fang; Shu-Ling Yu; Hui-Yu Hsu; Yen-Hung Chow; Jen-Yang Chen; Epstein-Barr virus BALF3 mediates genomic instability and progressive malignancy in nasopharyngeal carcinoma. Oncotarget 2014, 5, 8583-8601, 10.18632/oncotarget.2323.
- Lee, C.-P.; Chen, J.-Y.; Wang, J.-T.; Kimura, K.; Takemoto, A.; Lu, C.-C.; Chen, M.-R. Epstein-Barr virus BGLF4 kinase induces premature chromosome condensation through activation of condensin and topoisomerase II. J. Virol. 2007, 81, 5166–5180, doi:10.1128/JVI.00120-07.
- Chang, Y.H.; Lee, C.P.; Su, M.T.; Wang, J.T.; Chen, J.Y.; Lin, S.F.; Tsai, C.H.; Hsieh, M.J.; Takada, K.; Chen, M.R. Epstein-barr virus BGLF4 kinase retards cellular S-phase progression and induces chromosomal abnormality. PLoS ONE 2012, 7, e39217, doi:10.1371/journal.pone.0039217.
- Chung-Chun Wu; Ming-Tsan Liu; Yu-Ting Chang; Chih-Yeu Fang; Sheng-Ping Chou; Hsin-Wei Liao; Kuan-Lin Kuo; Shih-Lung Hsu; Yi-Ren Chen; Pei-Wen Wang; et al.Yu-Lian ChenHsin-Ying ChuangChia-Huei LeeMing ChenWun-Shaing Wayne ChangJen-Yang Chen Epstein–Barr Virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Research 2009, 38, 1932-1949, 10.1093/nar/gkp1169.
- Anatoliy Shumilov; Ming-Han Tsai; Yvonne T. Schlosser; Anne-Sophie Kratz; Katharina Bernhardt; Susanne Fink; Tuba Mizani; Xiaochen Lin; Anna Jauch; Josef Mautner; et al.Annette Kopp-SchneiderRegina FeederleIngrid HoffmannHenri-Jacques Delecluse Epstein–Barr virus particles induce centrosome amplification and chromosomal instability. Nature Communications 2017, 8, 14257, 10.1038/ncomms14257.
- Markus Altmann; Wolfgang Hammerschmidt; Epstein-Barr Virus Provides a New Paradigm: A Requirement for the Immediate Inhibition of Apoptosis. PLOS Biology 2005, 3, e404, 10.1371/journal.pbio.0030404.
- Altmann, M.; Hammerschmidt, W. Epstein-barr virus provides a new paradigm: A requirement for the immediate inhibition of apoptosis. PLoS Biol. 2005, 3, e404, doi:10.1371/journal.pbio.0030404.
- Kelly, G.L.; Long, H.M.; Stylianou, J.; Thomas, W.A.; Leese, A.; Bell, A.I.; Bornkamm, G.W.; Mautner, J.; Rickinson, A.B.; Rowe, M. An epstein-barr virus anti-apoptotic protein constitutively expressed in transformed cells and implicated in burkitt lymphomagenesis: The Wp/BHRF1 link. PLoS Pathog. 2009, 5, e1000341, doi:10.1371/journal.ppat.1000341.
- Fitzsimmons, L.; Cartlidge, R.; Chang, C.; Sejic, N.; Galbraith, L.C.A.; Suraweera, C.D.; Croom-Carter, D.; Dewson, G.; Tierney, R.J.; Bell, A.I.; et al. EBV BCL-2 homologue BHRF1 drives chemoresistance and lymphomagenesis by inhibiting multiple cellular pro-apoptotic proteins. Cell Death Differ. 2020, 27, 1554–1568, doi:10.1038/s41418-019-0435-1.




