
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fabiana Arduini | + 3276 word(s) | 3276 | 2021-02-04 03:37:30 | | | |
| 2 | Camila Xu | Meta information modification | 3276 | 2021-02-23 10:50:36 | | |
Video Upload Options
The electrochemical biosensor is defined as a chemical sensor constituted of a recognition element and an electrochemical transducer, in which the device is able to transform the biochemical information related to the interaction of the analyte with the recognition element into an electrochemically detectable signal.
1. Introduction
The electrochemical biosensors are classified by the function of the biocomponent used (e.g., enzyme, antibody, DNA sequence) or the transducer (e.g., potentiometric, amperometric, voltammetric transducers). The customization of biosensors with suitable biocomponents and transducers has opened the possibility to develop diverse types of biosensors for the detection of the target analyte, belonging to several sectors, including the biomedical, environmental, and defence ones. The first biosensor applied in the defence field was developed by Guilbault et al. in 1962 [1], in which two platinum electrodes were selected as the transducer and the cholinesterase enzyme as a biocomponent for nerve agent detection. This biosensor was designed and merely inspired by nature, because the principle is based on how the nerve agents act on the human beings. Indeed, the toxicity of nerve agents relies on their ability to irreversibly inhibit a key enzyme of nervous transmission, namely acetylcholinesterase, and thus this enzyme was selected as a biocomponent and immobilized on the electrochemical transducer. By measuring the enzymatic activity before and after the exposure, the decrease in the response points out the presence of cholinesterase inhibitors, including nerve agents, and the percentage of decrease is proportional to the amount of nerve agent detected. This biosensor allowed for the detection of Sarin at a concentration of 3 ng/mL using 10 min as incubation time, which is the time of reaction between enzyme and nerve agent, followed by the measurement of residual enzymatic activity. For the detection of nerve agents, two types of biosensors are mainly reported in the literature (Figure 1). The one most used relies on the use of cholinesterase as biocomponent, exploiting the capability of nerve agents to inhibit acetyl- and butyrylcholinesterase for a highly sensitive measurement. However, these biosensors are not specific because they generally detect organophosphorus compounds, working like a “family doctor” [2]. The cholinesterase biosensors are characterized by different degrees of sensitivity towards organophosphorus pesticides and nerve agents, in the function of inhibition properties. Furthermore, the inhibitive biosensors require an incubation step needed for a sensitive measure. This analytical tool can only be used once, unless a step with a reactivator compound is carried out after the nerve agent is measured [3]. The other approach is based on the use of organophosphate hydrolase enzyme able to hydrolyzed the organophosphorus compounds, thus detecting a nerve agent as the enzymatic substrate. This type of biosensor does not require a time of incubation, allowing for a faster measure than the one using a cholinesterase biosensor, but it is characterized by a lower sensitivity. For mustard agent detection, the most recent biosensors developed rely on the monitoring of inhibition using choline oxidase as the enzyme.
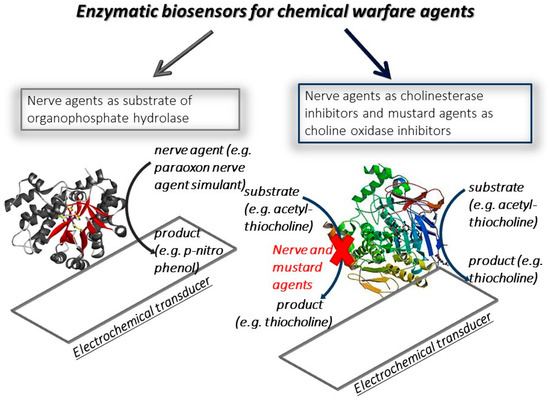
Figure 1. Scheme of enzymatic biosensor types for the detection of chemical warfare agents.
For a reliable, cost-effective, easy, and on-site measurement, the electrochemical transducers are among the most suitable candidates, being characterized by high sensitivity and capability to work in complex matrices. Electrochemical detection in the last years has undergone a growing period due to the exploitation of cross-cutting technologies, as reported by Escarpa “With the appearance of advanced approaches such as screen-printed technology, biosensors, microchips and nanotechnology, among others, electroanalysis is undergoing a true Renaissance”[4]. Indeed, printed technologies, also on paper, combined with nanomaterials and flexible electronics have boosted the development of devices with unprecedented features, namely high sensitivity, wireless connection, reagent-free measurement, and foldable sensors aiming to develop smart devices in different fields, including the defence one. The defence sector requires devices that are able to detect the chemical warfare agents fast, on site, and by easy procedures, to take the correct countermeasures in a timely fashion; thus, the electrochemical biosensors are useful and highly attractive analytical tools, with all features requiring the defence field.
2. Nanomaterials as Electrode Modifier to Boost the Biosensor Analytical Features
The exploitation of nanomaterials in the different disciplines has allowed for the development of different products with improved features, rending the nanodimension one of the key features in the research and industrial sectors [5].
Searching in Google Scholar, the articles with the word “nanomaterial” and “electrochemical biosensor” in December 2020, more than 11,000 documents have been reported, demonstrating that the use of nanomaterials in the development of electrochemical biosensors is one of the determining research topics in the sensing field at the worldwide level.
In the sector of electrochemical biosensors for chemical warfare agents, the presence of nanomaterials has augmented the smart configurations of the devices with the development of analytical tools characterized by improved analytical features in terms of sensitivity, selectivity, and stability.
One of the first biosensors based on the use of nanomaterials for the detection of nerve agents was reported by Joshi et al. in 2005 [6], in which carbon nanotubes were used as a working electrode nanomodifier for the sensitive detection of acetylcholinesterase by-product. This biosensor was constructed by modifying the working electrode surface via drop casting with a dispersion of multiwalled carbon nanotubes at a concentration of 2 mg/mL in N,N-dimethylformamide. In detail, 15 µL of this solution were dropped onto the electrode surface and then the modified electrode was kept in an oven at 80 °C for 30 min under vacuum to allow for the evaporation of the solvent. After that, the acetylcholinesterase was dropped on the carbon nanotubes-modified electrode to immobilize the enzyme by physical adsorption. Rinsing twice with phosphate buffer allowed for the removal of the loosely adsorbed acetylcholinesterase molecules, leaving only the acetylcholinesterase molecules strictly bonded to carbon nanotubes. Being an amperometric monoenzymatic biosensor, acetylthiocholine was used as a substrate with the production of thiocholine as the enzymatic by-product. The amperometric detection of thiols is characterized by high voltage and the fouling problem, and indeed the detection of thiocholine at bare electrode happened at +600 mV vs. Ag pseudoreference, while the presence of carbon nanotubes allowed for detection at +200 mV with a stable signal, thus avoiding the fouling problem (Figure 2A). This biosensor was tested with paraoxon, used as model organophosphate nerve agents, with a detection limit of 0.5 nM (0.145 ppb). The presence of carbon nanotubes demonstrated that this nanomaterial was used to immobilize the enzyme as well as to detect the enzymatic product at a low applied potential, thus avoiding the fouling issue.
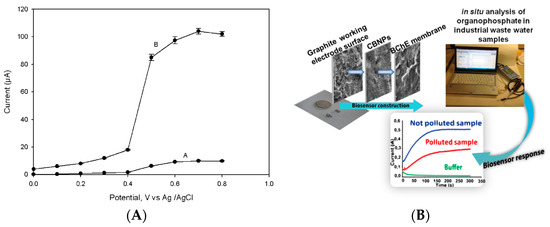
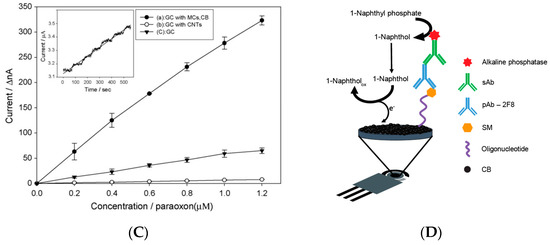
Figure 2. Biosensors based on nanomaterials for chemical warfare agent detection. (A) Study of applied potential using bare (A) and carbon nanotubes (B) modified electrode for the enzymatic by-product thiocholine [6]. (B) Miniaturised biosensor based on carbon black for the detection of model organophosphate nerve agent paraoxon [7]. (C) Paraoxon detection using a mesoporous-carbon black biosensor and organophosphorus hydrolase as the biocomponent [8]. (D) The scheme of carbon black-based heterogeneous oligonucleotide-antibody assay for sulphur mustard detection [9].
Carbon black is a nanomaterial widely used in the detection of analyte in the liquid phase since 2020 [10], demonstrating its outstanding features, such as the improved electron transfer [11][12][13], resistance to the fouling [14][15], easiness to prepare a stable dispersion [16], compatibility with the biocomponent [17][18], and cost-effectiveness (ca. 1 euro/kg). Carbon black was used to develop a biosensor for the detection of the same model compound (i.e., paraoxon) reported above, using a printed sensor modified with carbon black by drop casting 6 µL of carbon black dispersion 1 mg/mL prepared in a solution of water/N,N-dimethylformamide in a ratio of 1:1 (v/v). The enzyme butyrylcholinesterase was selected to be immobilized onto the working electrode surface using a chemical immobilization through glutaraldehyde and Nafion© (Figure 2B). The choice of butyrylcholinesterase is due to improve storage stability of this biosensor in respect to the acetylcholinesterase-based one, being the biosensor able to detect the organophosphates even when stored at RT in a dry condition for several weeks [19]. In addition, the presence of carbon black is able to detect thiocholine at low applied potentials (i.e., +300 mV vs. Ag/AgCl pseudoreference) without the fouling problem [20]. This biosensor detected paraoxon with a linear range up to 30 μg L−1 and a detection limit of 5 μg L−1 [7].
Carbon black was also used, combined with mesoporous carbon, for nerve agents using organophosphorus hydrolase as a biocomponent. The biosensor was fabricated by modifying a glassy carbon electrode with the drop-casting approach with a dispersion of mesoporous carbon and carbon black. The organophosphorus hydrolase was immobilized onto the modified electrode with Nafion© and the biosensor was dried at room temperature and kept in a refrigerator at 4 °C. The presence of carbon black improved the sensitivity, as reported by the authors “by incorporating CB into the electrode, the sensitivity of the MC-modified electrode was heightened”, even when compared to the electrode modified with carbon nanotubes (Figure 2C). Using the optimized experimental conditions, this biosensor allowed for the measurement of paraoxon with a sensitivity of 198 nA/M and a detection limit of 0.12 M (36 ppb) [8].
Carbon black has recently been used to develop a heterogeneous oligonucleotide-antibody biosensor for the detection of sulphur mustard (bis(2-chloroethyl)sulphide) [9]. The principle of the detection relies on the monitoring of the formation of a sulfur mustard-oligonucleotide adduct on the surface of printed electrodes. After that, the immunoassay was carried out using a primary antibody for the selective recognition of the sulphur mustard-oligonucleotide adduct followed by an alkaline phosphatase-conjugated anti-IgG as the secondary antibody for the measurement of the immunological chain. In detail, 1-naphthyl phosphate was used as the substrate and the enzymatic product 1-naphthol was measured in differential pulse voltammetry using a carbon black-modified electrode with improved sensitivity with respect to the bare electrode. Under optimized conditions, this biosensor allowed for a linear range up to 80 mM and a detection limit of 12 μM of sulphur mustard.
In an old paper published in 1948 by Barron et al., the effect of mustard agents on enzymatic activity was investigated, revealing choline oxidase as the most sensitive enzyme by the inhibition of nitrogen mustards [21]. Inspired by this work, we developed a bioassay based on the inhibition property of mustard agents on choline oxidase. In detail, the enzymatic activity was monitored by measuring the hydrogen peroxide by-product. The detection of hydrogen peroxide was carried out using an electrode modified with Prussian Blue nanoparticles because this electrochemical mediator is able to electrocatalyze the reduction of hydrogen peroxide at a low applied potential, i.e., close to 0 V vs. Ag/AgCl pseudoreference. The outstanding electrocatalytic activity of Prussian Blue is well established in the literature, and for this reason it is also called “artificial peroxidase” [22]. The nanoparticles of Prussian Blue were chemically synthetized on the working electrode surface as follows: 5 μL of 0.1 M potassium ferricyanide in 10 mM HCl with 5 μL of 0.1 M ferric chloride in 10 mM HCl were drop-cast on the working electrode surface, the solution was left on the electrode for 10 min, and then rinsed with a few millilitres of 10 mM HCl. The electrodes were then left for 90 min in the oven at 100 °C, allowing for an electrode modification with Prussian Blue nanoparticles with the dimensions of 95 ± 15 nm [23]. The effectiveness of this novel bioassay was successfully verified with the nitrogen mustard simulant bis(2-chloroethyl)amine and the sulphur mustard simulants, i.e., 2-chloroethyl ethyl sulphide and 2-chloroethyl phenyl sulphide [24], demonstrating the suitability of a novel biosensor for the detection of sulphur mustard.
3. Printing Technologies to Deliver Disposable and Miniaturised Devices
Printing techniques have revolutionized electrochemistry because the classical working, reference and counter electrodes with dimensions of few cm have been replaced with flexible printed working, reference and counter electrodes with dimensions of few mm, requiring only few µL of sample. In addition, the cost largely decreases from ca. 500 € to about 1 €. Without doubt, printed biosensors have improved the life of diabetic patients, thanks to the printed electrochemical biosensor analysing their glucose in the blood at home with a simple finger prick, instead of going to hospital for more invasive analyses [25].
A butyrylcholinesterase biosensor based on a screen-printed electrode was developed by our group for the detection of nerve agents in the liquid and gas phases using a portable potentiostat. In detail, screen-printed electrodes were modified with Prussian Blue to detect thiocholine, the enzymatic by-product of butyrylcholinesterase at +200 mV vs. Ag/AgCl pseudoreference. Being the butyrylthiocholine hygroscopic, the stability of the substrate was studied by investigating the effect of nitrogen flow in delivering a stable room temperature substrate. The optimized biosensor was tested using a portable potentiostat that is commercially available with Sarin and VX standard solutions, showing detection limits of 10 ppb and 18 ppb, respectively. Furthermore, this biosensor demonstrated the capability of measuring Sarin in the gas phase by exposing the wet biosensor with phosphate buffer to Sarin gas. The effect of incubation time was evaluated by studying the incubation time from 30 s up to 10 min at concentrations of 0.1 mg/m3 and 0.5 mg/m3 of Sarin gas, demonstrating that only 30 s of incubation time are needed to detect Sarin at the concentration of 0.1 mg/m3 [26]. This biosensor was also configured to be embedded with a sampling prototype constituted of an electrochemical cell, a little fan for sampling air, and an electronic circuit for applying the potential, registering the current, turning on-off the fan, and giving an alarm if the signal decreases by 20%, delivering a smart lab on a chip (Figure 3B). In detail, the biosensor was inserted in the cell by adding the substrate by a syringe. The detection of nerve agents in the gas phase was accomplished because the fan is able to sample 20–25 L of air/min by flowing the air into the electrochemical cell and enriching the working solution by nerve agents. This prototype encompasses an electronic circuit that is able to manage the fan and the miniaturized potentiostat by using (i) an analogic section for applying the potential (+200 mV) followed by an input I/V amplifier for converting the input current signal into the output voltage signal and a low-pass filter to exclude the transient frequency disturbs, (ii) a digital section with an A/D converter for an input stage of the microcontroller. Paraoxon was used as a simulant and was tested at a concentration of 1 ppm for 12 times, always giving the switch-on of the alarm. Furthermore, blank solution did not cause any alarm, thus demonstrating the suitability of the device [27]. Screen-printed electrodes were also used to evaluate the sensitivity of different biocomponents, namely recombinant, electric eel, and bovine erythrocytes origin acetylcholinesterase, observing that human recombinant acetylcholinesterase seemed to be the most sensitive toward the inhibition by Sarin with a limit detection of 0.45 × 10−8 mol/L [28]. The same group developed a biosensing system using a platinum screen-printed electrode and acetylcholinesterase from electric eel in a solution to test Sarin, Soman, Tabun, and VX with the limits of detection of 7.41 × 10−12 mol/L for Sarin, 6.31 × 10−12 mol/L for Soman, 6.17 × 10−11 mol/L for Tabun, and 2.19 × 10−11 mol/L for VX, respectively [29].
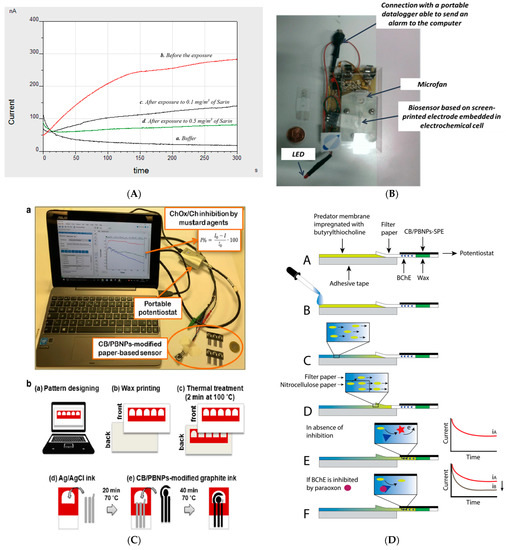
Figure 3. (A) Amperograms using the screen-printed electrodes modified with Prussian blue and butyrylcholinesterase before and after the exposure to Sarin gas [26]. (B) Screen-printed based butyrylcholinesterase biosensor integrated into a lab on a chip [27]. (C) Paper-based screen-printed electrodes as a platform to detect mustard agents using choline oxidase as a biocomponent. (a–e) procedural steps for the realisation of wax-printed office paper serigraphic printing of conductive inks [30]. (D) Paper-based screen-printed electrode loaded with butyrylcholinesterase to detect paraoxon as a nerve agent simulant using the following steps (A–F) [31].
Yoon et al. [32] used screen-printed electrodes to immobilize the acetylcholinesterase enzyme by cross-linking with glutaraldehyde and bovine serum albumin. This biosensor was then inserted in an enzymatic chamber on the microfluidic chip, fabricated using polymethylmethacrylate as the material to develop a lab on a chip for the detection of nerve agents. Using acetylthiocholine chloride as the substrate, thiocholine was monitored at a constant applied voltage of 0.1 V using a working electrode modified with cobalt phthalocyanine as the electrochemical mediator. The miniaturization of the device can be used for nerve agent detection as nerve agents are able to inhibit acetylcholinesterase enzymes.
Wang’s group reported the first solid-contact fluoride screen-printed electrode for the detection of G-Serie nerve agents using organophosphorus acid anhydrolase as a biocomponent [33]. The detection relies on the capability of organophosphorus acid anhydrolase to break the P-F bond of the G-type nerve agents releasing detectable fluoride ions. The potentiometric biosensor was tested with diisopropyl fluorophosphates as a nerve agent simulant, reaching a dynamic range of 250–3000 μM and any interference when methyl paraoxon, methyl parathion, phenol, and aniline were analysed.
Paper-Based Printed Devices
Paper-based electrochemical (bio)sensors have recently acquired huge interest by the scientific community due to their sustainable features in terms of plastic-free measure and low cost. In addition, the measurements using paper-based biosensors can be carried out by unskilled personnel and these devices overcome the major drawbacks of microfluidic devices, including the requirement for micropumps, the bubble issues, and high costs for microfluidic pattern fabrication. A further added value in the defence contest relies on the capability of being incinerated after the measure, reducing the management of the waste contaminated with chemical warfare agents. As is well known, when a drop of water wets the paper, the water flows in the cellulosic network, and it also wets the electrical contacts and hinders the correct electrochemical measure. To overcome this drawback, we exploited the wax printing technique to easily create the hydrophilic region surrounded by the hydrophobic one to contain the working solution within the electrochemical cell. As an example, I report a paper-based bioassay for the detection of mustard agents using a portable instrument (Figure 3C(a)) [30]. For the manufacturing of paper-based screen-printed electrodes, the hydrophobic zone was printed onto office paper sheets, using a ColorQube 8580 Xerox printer, and treated at 100 °C for 2 min to let the wax homogeneously permeate the paper, producing the hydrophobic pattern designed. Then, the three electrodes were printed by using the screen-printing technique (Figure 3C(b)). For mustard agent detection, choline oxidase enzyme was used as a biocomponent, measuring the degree of inhibition with an office paper-based electrochemical sensor bulk modified with Prussian Blue nanoparticles and carbon black with the overriding goal of measuring the enzymatic by-product H2O2 at a low applied potential by the chronoamperometric mode. This bioassay was conceived to work in a drop in the presence of choline oxidase and chemical warfare agent. It was successfully applied for the measurement of sulphur mustard Yprite with a detection limit of 0.9 mM.
The first paper-based biosensor for reagent-free analysis of nerve agents was reported by our group, thanks to the combination of a strip of nitrocellulose membrane loaded with the substrate and a paper-based test screen-printed electrode functionalized with butyrylcholinesterase [31]. As the paper-based device loaded with the reagents was able to carry out a single measure, the measurement of nerve agent simulant was carried out by using two platforms that compared the non-inhibited chronoamperometric signal to the inhibited one. In detail, the blank signal was obtained by adding 10 µL of distilled water on the edge of the Predator® membrane where butyrylthiocholine was adsorbed (Figure 3D(B)). Then, the water flow pushed butyrylthiocholine toward the test area (Figure 3D(C)), wetting the electrochemical cell where the enzyme with the phosphate buffer salts were loaded (Figure 3D(D)). When the water reached the testing area (ca. 30 s), we waited for 2 min for the enzymatic reaction, producing the enzymatic by-product thiocholine (Figure 3D(E)). To measure paraoxon concentration, the testing area was firstly loaded with 5 µL of the nerve agent simulant. After that, the device response was measured by following the procedure reported above, observing the decrease of the signal due to the decrease of enzyme activity (Figure 3D(F)). This reagent-free device demonstrated the capability of detecting paraoxon, taken as nerve the agent simulant, down to 3 µg/L.
References
- Guilbault, G.G.; Kramer, D.N.; Cannon, P.L., Jr. Electrical Determination of Organophosphorous Compounds. Anal. Chem. 1962, 34, 1437–1439.
- Arduini, F.; Amine, A.; Moscone, D.; Palleschi, G. Biosensors based on cholinesterase inhibition for insecticides, nerve agents and aflatoxin B1 detection (review). Microchim. Acta 2010, 170, 193–214.
- Gogol, E.V.; A Evtugyn, G.; Marty, J.L.; Budnikov, H.C.; Winter, V.G. Amperometric biosensors based on nafion coated screen-printed electrodes for the determination of cholinesterase inhibitors. Talanta 2000, 53, 379–389.
- Escarpa, A. Food electroanalysis: Sense and simplicity. Chem. Rec. 2011, 12, 72–91.
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11.
- Joshi, K.A.; Tang, J.; Haddon, R.; Wang, J.; Chen, W.; Mulchandani, A. A Disposable Biosensor for Organophosphorus Nerve Agents Based on Carbon Nanotubes Modified Thick Film Strip Electrode. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Elec-troanal. 2005, 17, 54–58.
- Arduini, F.; Forchielli, M.; Amine, A.; Neagu, D.; Cacciotti, I.; Nanni, F.; Moscone, D.; Palleschi, G. Screen-printed biosensor modified with carbon black nanoparticles for the determination of paraoxon based on the inhibition of butyrylcholinesterase. Microchim. Acta 2015, 182, 643–651.
- Lee, J.H.; Park, J.Y.; Min, K.; Cha, H.J.; Choi, S.S.; Yoo, Y.J. A novel organophosphorus hydrolase-based biosensor using mesoporous carbons and carbon black for the detection of organophosphate nerve agents. Biosens. Bioelectron. 2010, 25, 1566–1570.
- Colozza, N.; Mazzaracchio, V.; Kehe, K.; Tsoutsoulopoulos, A.; Schioppa, S.; Fabiani, L.; Steinritz, D.; Moscone, D.; Arduini, F. Development of novel carbon black-based heterogeneous oligonucleotide-antibody assay for sulfur mustard detection. Sens. Actuators B Chem. 2021, 328, 129054.
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon black as an outstanding and af-fordable nanomaterial for electrochemical (bio)sensor design. Biosens. Bioelectron. 2020, 156, 112033.
- Della Pelle, F.; Angelini, C.; Sergi, M.; Del Carlo, M.; Pepe, A.; Compagnone, D. Nano carbon black-based screen printed sensor for carbofuran, isoprocarb, carbaryl and fenobucarb detection: Application to grain samples. Talanta 2018, 186, 389–396.
- Deroco, P.B.; Fatibello-Filho, O.; Arduini, F.; Moscone, D. Electrochemical determination of capsaicin in pepper samples using sustainable paper-based screen-printed bulk modified with carbon black. Electrochim. Acta 2020, 354, 136628.
- Mazzaracchio, V.; Tomei, M.R.; Cacciotti, I.; Chiodoni, A.; Novara, C.; Castellino, M.; Scordo, G.; Amine, A.; Moscone, D.; Arduini, F. Inside the different types of carbon black as nanomodifiers for screen-printed electrodes. Electrochim. Acta 2019, 317, 673–683.
- Talarico, D.; Arduini, F.; Constantino, A.; Del Carlo, M.; Compagnone, D.; Moscone, D.; Palleschi, G. Carbon black as successful screen-printed electrode modifier for phenolic compound detection. Electrochem. Commun. 2015, 60, 78–82.
- Talarico, D.; Arduini, F.; Amine, A.; Moscone, D.; Palleschi, G. Screen-printed electrode modified with carbon black nanopar-ticles for phosphate detection by measuring the electroactive phosphomolybdate complex. Talanta 2015, 141, 267–272.
- Cinti, S.; Neagu, D.; Carbone, M.; Cacciotti, I.; Moscone, D.; Arduini, F. Novel carbon black-cobalt phthalocyanine nano-composite as sensing platform to detect organophosphorus pollutants at screen-printed electrode. Electrochim. Acta 2016, 188, 574–581.
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello Filho, O. Electrochemical Biosensors Based on Nanostructured Carbon Black: A Review. J. Nanomater. 2017, 2017, 1–14.
- Portaccio, M.; Di Tuoro, D.; Arduini, F.; Moscone, D.; Cammarota, M.; Mita, D.; Lepore, M. Laccase biosensor based on screen-printed electrode modified with thionine—Carbon black nanocomposite, for Bisphenol A detection. Electrochim. Acta 2013, 109, 340–347.
- Arduini, F.; Palleschi, G. Disposable electrochemical biosensor based on cholinesterase inhibition with improved shelf-life and working stability for nerve agent detection. In Portable Chemical Sensors; Springer: Dordrecht, The Netherlands, 2012; pp. 261–278.
- Arduini, F.; Majorani, C.; Amine, A.; Moscone, D.; Palleschi, G. Hg2+ detection by measuring thiol groups with a highly sen-sitive screen-printed electrode modified with a nanostructured carbon black film. Electrochim. Acta 2011, 56, 4209–4215.
- Barron, E.S.G.; Bartlett, G.R.; Baker Miller, Z. The effect of nitrogen mustards on enzymes and tissue metabolism. J. Exp. Med. 1948, 87, 489–501.
- Karyakin, A.A. Prussian blue and its analogues: Electrochemistry and analytical applications. Electroanal. Int. J. Devoted Fun-dam. Pract. Asp. Electroanal. 2001, 13, 813–819.
- Cinti, S.; Arduini, F.; Vellucci, G.; Cacciotti, I.; Nanni, F.; Moscone, D. Carbon black assisted tailoring of Prussian Blue nano-particles to tune sensitivity and detection limit towards H2O2 by using screen-printed electrode. Electrochem. Commun. 2014, 47, 63–66.
- Arduini, F.; Scognamiglio, V.; Covaia, C.; Amine, A.; Moscone, D.; Palleschi, G. A choline oxidase amperometric bioassay for the detection of mustard agents based on screen-printed electrodes modified with Prussian blue nanoparticles. Sensors 2015, 15, 4353–4367.
- Newman, J.D.; Turner, A.P.F. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453.
- Arduini, F.; Amine, A.; Moscone, D.; Ricci, F.; Palleschi, G. Fast, sensitive and cost-effective detection of nerve agents in the gas phase using a portable instrument and an electrochemical biosensor. Anal. Bioanal. Chem. 2007, 388, 1049–1057.
- Arduini, F.; Neagu, D.; Dall’Oglio, S.; Moscone, D.; Palleschi, G. Towards a Portable Prototype Based on Electrochemical Cholinesterase Biosensor to be Assembled to Soldier Overall for Nerve Agent Detection. Electroanal. 2012, 24, 581–590.
- Pohanka, M.; Binder, J.; Kuca, K. Sarin Assay using Acetylcholinesterases and Electrochemical Sensor Strip. Def. Sci. J. 2009, 59, 300–304.
- Pohanka, M.; Adam, V.; Kizek, R. An acetylcholinesterase-based chronoamperometric biosensor for fast and relia-ble assay of nerve agents. Sensors 2013, 13, 11498–11506.
- Colozza, N.; Kehe, K.; Popp, T.; Steinritz, D.; Moscone, D.; Arduini, F. Paper-based electrochemical sensor for on-site detec-tion of the sulphur mustard. Environ. Sci. Pollut. Res. 2018, 1–12. doi:10.1007/s11356-018-2545-6.
- Cinti, S.; Minotti, C.; Moscone, D.; Palleschi, G.; Arduini, F. Fully integrated ready-to-use paper-based electrochemical bio-sensor to detect nerve agents. Biosens. Bioelectron. 2017, 93, 46–51.
- Yoon, Y.J.; Li, K.H.H.; Low, Y.Z.; Yoon, J.; Ng, S.H. Microfluidics biosensor chip with integrated screen-printed electrodes for amperometric detection of nerve agent. Sens. Actuators B Chem. 2014, 198, 233-238.
- Goud, K.Y.; Teymourian, H.; Sandhu, S.S.; Tostado, N.; Mishra, R.K.; Moore, L.C.; Harvey, S.P.; Wang, J. OPAA/fluoride bi-osensor chip towards field detection of G-type nerve agents. Sensors Actuators B: Chem. 2020, 320, 128344.




