| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Frederic Michel Lamoth | + 2227 word(s) | 2227 | 2021-01-08 10:48:10 | | | |
| 2 | Rita Xu | -708 word(s) | 1519 | 2021-02-10 04:17:11 | | |
Video Upload Options
Invasive fungal infections (IFIs) are associated with high mortality rates and timely appropriate antifungal therapy is essential for good outcomes. Emerging antifungal resistance among Candida and Aspergillus spp., the major causes of IFI, is concerning and has led to the increasing incorporation of in vitro antifungal susceptibility testing (AST) to guide clinical decisions.
1. Introduction
Early appropriate antifungal therapy is a key determinant for the outcome of invasive fungal infections (IFIs). While first and alternative therapeutic choices have been well defined for the most frequent IFIs, such as invasive aspergillosis (IA) and invasive candidiasis (IC) [1][2][3][4][5], or other less frequent IFIs (e.g., mucormycosis, fusariosis, scedosporiosis) [6][7], much uncertainty remains about the role and interpretation of antifungal susceptibility testing (AST). For some fungal pathogens, antifungal susceptibility patterns are well known with limited intra-species variability (e.g., Scedosporium apiospermum). For others, the significance of minimal inhibitory concentration (MIC) in predicting outcome is notoriously weak and AST is not routinely recommended (e.g., Fusarium spp., Mucorales). However, for Candida spp. and Aspergillus spp., the two most frequent fungal pathogens, emergence of acquired antifungal resistance is a concern and definitions of clinical breakpoints (CBPs) are needed for the distinction between susceptible and resistant isolates in order to inform appropriate antifungal selection. Both the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) are working on establishing and updating CBPs for fungi. However, this task is complex, as illustrated by the reassessment and changes of CBPs over time, some discrepancy in CBP definitions between CLSI and EUCAST, and the absence of CBP definitions for some fungus/antifungal drug combinations (Table 1).
Table 1. Comparison of CLSI and EUCAST clinical breakpoints of antifungal drugs for most relevant Candida and Aspergillus spp. according to CLSI and EUCAST.
|
Species |
AMB |
FLC |
VRC |
POS |
CAS |
AND |
MCF |
|||||||
|
C. albicans |
ND |
1 |
2 (8) |
2 (8) |
0.12 (1) |
0.06 (0.5) |
ND |
0.06 |
0.25 (1) |
ND |
0.25 (1) |
0.03 |
0.25 (1) |
0.016 |
|
C. tropicalis |
ND |
1 |
2 (8) |
2 (8) |
0.12 (1) |
0.12 (0.5) |
ND |
0.06 |
0.25 (1) |
ND |
0.25 (1) |
0.06 |
0.25 (1) |
ND |
|
C. parapsilosis |
ND |
1 |
2 (8) |
2 (8) |
0.12 (1) |
0.12 (0.5) |
ND |
0.06 |
2 (8) |
ND |
2 (8) |
0.002 (8) |
2 (8) |
0.002 (4) |
|
C. glabrata |
ND |
1 |
32 (SDD) (64) |
0.002 (64) |
ND |
ND |
ND |
ND |
0.12 (0.5) |
ND |
0.12 (0.5) |
0.06 |
0.06 (0.25) |
0.032 |
|
C. krusei |
ND |
1 |
(R) |
(R) |
0.5 (2) |
ND |
ND |
ND |
0.25 (1) |
ND |
0.25 (1) |
0.06 |
0.25 (1) |
ND |
|
A. fumigatus |
ND |
1 (4) |
(R) |
(R) |
ND |
1 (4) |
ND |
0.125 (0.5) |
ND |
ND |
ND |
ND |
ND |
ND |
|
A. flavus |
ND |
ND |
(R) |
(R) |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
A. niger |
ND |
1 (4) |
(R) |
(R) |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
A. terreus |
(R) |
(R) |
(R) |
(R) |
ND |
ND |
ND |
0.125 (0.5) |
ND |
ND |
ND |
ND |
ND |
ND |
Clinical breakpoints (CBPs) of the Clinical and Laboratory Standards Institute (CLSI, left column) and European Committee on Antimicrobial Susceptibility Testing (EUCAST, right column). The numbers indicate the CBP [mg/L] for the distinction between susceptible “S” (≤the indicated value) vs. non-susceptible. If an intermediate “I” or “susceptible dose-dependent” (SDD) category has been defined, the resistance “R” cut-off (≥the indicated value) is mentioned in brackets. ND: no defined CBP (insufficient evidence), (R): the species is considered as intrinsically resistant (susceptibility testing not recommended).
2. The Challenges of Fungal Clinical Breakpoints (Cbps) Definitions
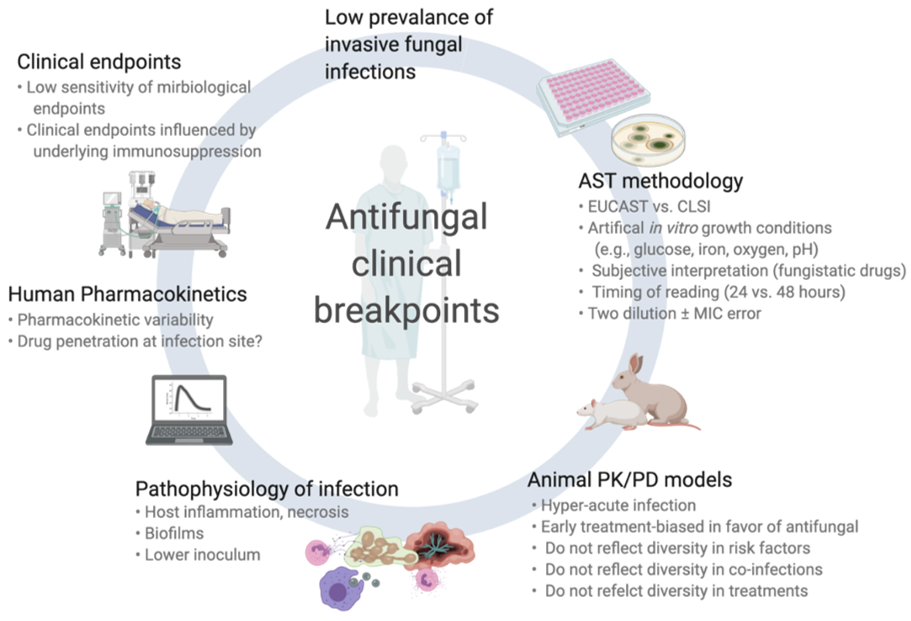
While often well established for antibacterials, CBPs for antifungals are associated with greater uncertainty. This is in part due to the relatively low prevalence of IFI (in particular mold infections) and some specific biological characteristics of fungal pathogens. In this section, we will review these specific aspects (summarized in Figure 1).
Figure 1. Challenges and pitfalls in the assessment of clinical breakpoints for fungi and antifungal drugs.
2.1. Antifungal Susceptibility Testing (AST) of Fungi
Some nuances of AST exist. First, the artificial in vitro conditions of testing by microbroth dilution method may differ considerably from the actual pathophysiological environment of IFIs. For example, invasive infections by molds affect mainly solid tissues (rather than biological fluids), have relatively low fungal inoculum (compared to the very high spore concentrations used in AST), and are often accompanied by tissue infarction and necrosis that might preclude appropriate drug penetration at the site of infection. In addition, the chemical composition of AST growth media differs from real pathogenic conditions regarding important elements for fungal growth (e.g., glucose, iron, oxygen, pH). Moreover, routine in vitro testing conditions do not take into account the possibility of biofilm formation (especially for Candida spp.).
Different AST methods are used in routine across countries or local laboratories. CLSI and EUCAST methods are recognized as the standard procedures and MICs derived from these methods are used for definitions of epidemiological cut-off values (ECVs) and CBPs [8][9][10][11]. CLSI and EUCAST procedures exhibit some notable differences (e.g., related to glucose content, spore inoculum, and reading interpretation), which may explain some differences between their respective CBPs. Moreover, these methods are manual and fastidious with an accepted margin of errors of up to +/− two dilutions, which may considerably impact MIC classification and interpretation. As a consequence, many laboratories use commercially available microbroth dilution method (e.g., Sensititre YeastOneTM, Vitek-2TM) or alternative methods (E-tests, agar disk diffusion), which may result in significant differences in MIC results, despite relatively good essential agreements [12][13][14][15]. Important interlaboratory discrepancies have also been notified regarding AST of caspofungin for Candida spp, which resulted in the withdrawal of CBPs recommendations by EUCAST [16]. Another common issue with AST consists of the difficulties in MIC determination for some drug/fungus, which may lead to discrepant results as a consequence of different subjective interpretation from the reader. This is principally the case for antifungal drugs for which there is a fungistatic activity and a trailing effect (e.g., Candida spp. and azoles, Aspergillus spp. and echinocandins) or a paradoxical effect at increased concentrations (Candida spp. and Aspergillus spp. with echinocandins). Moreover, the timing of reading (24 vs. 48 h), which is not consistent across studies, may affect MIC determination.
Finally and most importantly, AST results for fungi are usually obtained after a significant delay due to their slower growth rate compared to bacteria (i.e., several days to one week). Their interpretation for patient management is not made in “real time” and their impact on outcome is therefore limited, since early and appropriate antifungal therapy is of paramount importance for success.
2.2. Animal Pharmacodynamic/Pharamacokinetic (Pk/Pd) Models
In view of the paucity of clinical data, murine Pk/Pd models are important for assessing the correlation between drug exposure/MIC and therapeutic response. These data are also taken into account for CBP definitions. However, an element of artificiality also exists in these in vivo models as it compares to the complex clinical scenarios of IFIs in humans. For example, much higher fungal inocula are administered to mice, an innately non-susceptible species to fungal diseases, and only following intensive immunosuppressive regimens (e.g., myelotoxic drugs and corticosteroids) in order to induce a quick and intense (rapidly fatal) infection. These conditions do not reflect the diversity of immunosuppressive and other co-morbid conditions in humans, the variety of IFI types and localizations (e.g., pulmonary vs. cerebral aspergillosis, candidemia vs. non-candidemic IC) and the actual timing and course of infection.
2.3. Pathophysiology of Invasive Fungal Infections (IFIs)
The distinct features of IFI (compared to bacterial infections) represent the most important aspect of interpreting MIC results for fungi. Mold pathogens are relatively infrequently isolated in culture and MICs are therefore lacking [17]. The diagnosis of IFI (in particular for molds) is complex and associated with some degree of uncertainty with a high rate of possible/probable infections and a lower rate of proven infections, which may bias outcome analyses towards later diagnosis when the fungal burden is high. The timing of diagnosis and initiation of antifungal therapy is also crucial and frequent delays in IFI diagnosis have a considerable impact on outcome. The localization and extension of infection may also affect the therapeutic response. For instance, drug penetration within the different organs commonly affected by IFI (e.g., lungs, brain, or skin for IA, blood, or peritoneal cavity for IC) may be quite different [18]. Most importantly, the outcome of IFI is highly influenced by non-pharmacologic parameters, such as host variables (type, severity and potential for recovery of underlying diseases and immunosuppression) or adjunct therapies such as surgical interventions.
The assessment of therapeutic response in IFI is also difficult. While objective criteria can be monitored in candidemia (e.g., clearance of blood cultures), the outcome evaluation of invasive mold infections essentially relies on radiological interpretation, which may be confounded by other infectious or non-infectious (e.g., sequela of surgery, inflammatory reaction) radiological patterns with frequent initial worsening of lesions at the time of neutrophil recovery. Moreover, the assessment of response for invasive mold infections requires prolonged follow-up (weeks to months) and overall survival may be affected by multiple intercurrent infections/events in these patients with severe underlying diseases, such as cancer.
The rarity of some IFIs makes that many clinical pharmacodynamic studies present very heterogeneous data pooling different type of IFI (IC, IA, and other IFIs) or different species within a same genus (e.g., Candida albicans and non-albicans Candida spp.) or different grading of IFI (proven/probable or possible, empiric treatment for suspected IFI without documentation). Moreover, uniform therapeutic approaches are needed for outcome analyses and IFIs often require multiple lines of different antifungal treatments or drug combinations. Because of the delay in culture results, initial antifungal therapy is usually empirical and then switched to targeted treatment.
References
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Microbiol. Infect. 2012, 18 (Suppl. S7), 19–37.
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Infect. Dis. 2016, 62, e1–e50.
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Infect. Dis. 2016, 63, e1–e60.
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Microbiol. Infect. 2018, 24 (Suppl. S1), e1–e38.
- Ullmann, A.J.; Akova, M.; Herbrecht, R.; Viscoli, C.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Microbiol. Infect. 2012, 18 (Suppl. S7), 53–67.
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the european confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect. Dis. 2019, 19, e405–e421.
- Tortorano, A.M.; Richardson, M.; Roilides, E.; van Diepeningen, A.; Caira, M.; Munoz, P.; Johnson, E.; Meletiadis, J.; Pana, Z.D.; Lackner, M.; et al. Escmid and ecmm joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., scedosporium spp. And others. Microbiol. Infect. 2014, 20 (Suppl. S3), 27–46.
- M59ed2e: Epidemiological Cut off Values for Antifungal Susceptibility Testing, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Mic and Zone Diameter Distributions and Ecoffs. Available online: Http://www.Eucast.Org/mic_distributions_and_ecoffs/ (accessed on 1 October 2020).
- M60ed1e: Performance Standards for Antifungal Susceptibility Testing of Yeasts, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; fourth international supplement; CLSI document m27-4. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; p. 32.
- Cuenca-Estrella, M.; Gomez-Lopez, A.; Mellado, E.; Rodriguez-Tudela, J.L. Correlation between the procedure for antifungal susceptibility testing for candida spp. Of the european committee on antibiotic susceptibility testing (eucast) and four commercial techniques. Microbiol. Infect. 2005, 11, 486–492.
- Espinel-Ingroff, A.; Turnidge, J.; Alastruey-Izquierdo, A.; Botterel, F.; Canton, E.; Castro, C.; Chen, Y.C.; Chen, Y.; Chryssanthou, E.; Dannaoui, E.; et al. Method-dependent epidemiological cutoff values for detection of triazole resistance in candida and aspergillus species for the sensititre yeastone colorimetric broth and etest agar diffusion methods. Agents Chemother. 2018, 63(1),e01651-18.
- Lamoth, F.; Alexander, B.D. Comparing etest and broth microdilution for antifungal susceptibility testing of the most-relevant pathogenic molds. Clin. Microbiol. 2015, 53, 3176–3181.
- Meletiadis, J.; Geertsen, E.; Curfs-Breuker, I.; Meis, J.F.; Mouton, J.W. Intra- and interlaboratory agreement in assessing the in vitro activity of micafungin against common and rare candida species with the eucast, clsi, and etest methods. Agents Chemother. 2016, 60, 6173–6178.
- Espinel-Ingroff, A.; Arendrup, M.C.; Pfaller, M.A.; Bonfietti, L.X.; Bustamante, B.; Canton, E.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Fothergill, A.; et al. Interlaboratory variability of caspofungin mics for candida spp. Using clsi and eucast methods: Should the clinical laboratory be testing this agent? Agents Chemother. 2013, 57, 5836–5842.
- Tarrand, J.J.; Lichterfeld, M.; Warraich, I.; Luna, M.; Han, X.Y.; May, G.S.; Kontoyiannis, D.P. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. J. Clin. Pathol. 2003, 119, 854–858.
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Microbiol. Rev. 2014, 27, 68–88.