| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tokuhiro Chano | + 2079 word(s) | 2079 | 2021-02-04 07:42:10 | | | |
| 2 | Rita Xu | -1296 word(s) | 783 | 2021-02-09 10:00:12 | | |
Video Upload Options
This entry summarizes the recent advances in the process and prevention of carcinogenesis, the characteristic nature of tumors, and the treatment of post-refractory ovarian clear cell carcinomas (OCCCs), which are highly linked to oxidative stress.
1. Introduction
Ovarian clear cell carcinomas (OCCCs) are resistant to conventional anti-cancer drugs; moreover, the prognoses of advanced or recurrent patients are extremely poor. OCCCs often arise from endometriosis associated with strong oxidative stress. Of note, the stress involved in OCCCs can be divided into the following two categories: (a) carcinogenesis from endometriosis to OCCC and (b) factors related to treatment after carcinogenesis. Antioxidants can reduce the risk of OCCC formation by quenching reactive oxygen species (ROS); however, the oxidant stress-tolerant properties assist in the survival of OCCC cells when the malignant transformation has already occurred. Moreover, the acquisition of oxidative stress resistance is also involved in the cancer stemness of OCCC.
The removal of oxidative stress suppresses the development of OCCCs in endometriosis. Strong antioxidants, such as vitamin A, carotenoids, or flavonoids, may help prevent carcinogenesis of OCCCs. However, the stress tolerance properties of OCCCs induce therapeutic resistance, making their treatment difficult. Antioxidants display bidirectional effects toward endometriosis and OCCCs. Elimination of oxidative stress, including by uptake of antioxidants, is highly effective in preventing progression from endometriosis to OCCCs, but, antioxidants are not suitable for treatment of established OCCCs, in which oxidative stress tolerance has accrued, providing therapeutic resistance.
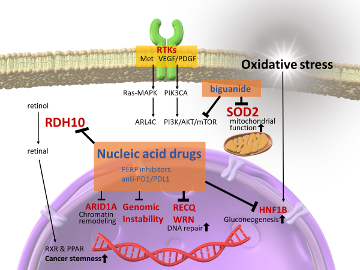
In OCCCs, downstream of receptor tyrosine kinases (RTKs), AT-rich interactive domain 1A (ARID1A)-related chromatin remodeling factors, and genomic instability, including MSI-H, are activated. These are currently being targeted.
In OCCC therapeutics, inhibition of oxidative stress tolerance molecules is essential. Therefore, other therapeutic strategies, such as nucleic acid-based drugs, RDH10, RECQL1, WRN, and HNF1B, should be targeted in the future to reduce cancer stemness, induce cancer-specific synthetic lethality, and reduce gluconeogenesis, together with a drug repositioning strategy against SOD2 anti-oxidative stress molecules.
2. Molecular Characteristics in OCCCs Related to Anti-Oxidative Pathway
As mentioned above, in OCCCs, which arise from endometriosis under massive oxidative stress, abnormalities are often detected in genes associated with the oxidative stress response and ROS metabolism. Several antioxidant molecules are involved in OCCC carcinogenesis. Among them, the overexpression of hepatocyte nuclear factor 1 homeobox B (HNF1B), a major homeobox-containing protein, also known as transcription factor-2, is highly important. Under hypoxia and acidosis, HNF1B can modify and adapt cancer cells to survive through a process between gluconeogenesis and glycolysis, commonly known as the Warburg effect. Tsuchiya et al. first reported HNF1B overexpression in OCCCs and showed that reduced HNF1B expression considerably increased the apoptosis rate in two OCCC cell lines. Overexpression of HNF1B was observed in endometrial tissues adjacent to OCCC tumors, suggesting that HNF1B overexpression is an early event in OCCC carcinogenesis. Kato et al. found that hypomethylation of the CpG island of HNF1B induced its overexpression in OCCCs, indicating that overexpression in OCCC was also caused by epigenetic changes rather than by mutations. Moreover, recent research has revealed that HNF1B promotes the dedifferentiation of cancer stem-like cells (CSCs) via activation of the Notch pathway and enhancing the invasive potential and epithelial–mesenchymal transition in cancer cells. Anti-oxidative pathways are deeply involved in carcinogenesis and therapeutic resistance in OCCCs. As oxidative stress tolerance represents therapeutic resistance, OCCCs usually exhibit poor and fatal prognoses, even during gradual progression. OCCC has low sensitivity to platinum- and taxane-based chemotherapy. Therefore, the prognosis of OCCCs is extremely poor, particularly in its advanced stages. Previous studies have revealed the role of HNF1B in driving the expression of several characteristic genes associated with OCCCs, stimulating metabolic changes to promote gluconeogenesis, glycogen accumulation, and aerobic glycolysis, inducing chemotherapeutic resistance by suppressing sulfatase-1 (Sulf-1), an extracellular sulfatase catalyzing the 6-O desulfation of heparan sulfate glycosaminoglycans, and reducing the activity of immunological checkpoints against tumors. Thus, HNF1B plays an important role in therapeutic resistance via oxidative stress tolerance in OCCCs (Figure 1).
Figure 1. Activating pathways and targeting proposals in ovarian clear cell carcinomas. In ovarian clear cell carcinomas, downstream of receptor tyrosine kinases (RTKs), AT-rich interactive domain 1A (ARID1A)-related chromatin remodeling factors, and genomic instability, including MSI-H, are activated. These are currently being targeted. However, other therapeutic strategies, such as nucleic acid-based drugs, RDH10, RECQL1, WRN, and HNF1B, should be targeted in the future to reduce cancer stemness, induce cancer-specific synthetic lethality, and reduce gluconeogenesis, together with a drug repositioning strategy against SOD2 anti-oxidative stress molecules.
Although therapeutic approaches should still be improved against OCCCs, multi-combinatorial treatments including nucleic acid-based drugs directed to the transcriptional profile of each OCCC are expected to improve the outcomes of patients.
References
- Available online: http://gco.iarc.fr/today/home (accessed on December 15th 2020).
- Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html (accessed on December 15th 2020).
- Available online: https://ganjoho.jp/reg_stat/statistics/stat/short_pred.html (accessed on December 15th 2020).
- Yamagami, W.; Nagase, S.; Takahashi, F.; Ino, K.; Hachisuga, T.; Aoki, D.; Katabuchi, H. Clinical statistics of gynecologic cancers in Japan. Gynecol. Oncol. 2017, 28, doi:10.3802/jgo.2017.28.e32.
- Itamochi, H.; Kigawa, J.; Terakawa, N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008, 99, 653–658, doi:10.1111/j.1349-7006.2008.00747.x.
- Shih, I.-M.; Kurman, R.J. Ovarian Tumorigenesis. J. Pathol. 2004, 164, 1511–1518, doi:10.1016/s0002-9440(10)63708-x.
- Köbel, M.; Reuss, A.; Du Bois, A.; Kommoss, S.; Kommoss, F.; Gao, D.; Kalloger, S.E.; Huntsman, D.G.; Gilks, C.B. The biological and clinical value of p53 expression in pelvic high-grade serous carcinomas. Pathol. 2010, 222, 191–198, doi:10.1002/path.2744.
- Kaldawy, A.; Segev, Y.; Lavie, O.; Auslender, R.; Sopik, V.; Narod, S.A. Low-grade serous ovarian cancer: A review. Oncol. 2016, 143, 433–438, doi:10.1016/j.ygyno.2016.08.320.
- Seidman, J.D.; Kurman, R.J.; Ronnett, B.M. Primary and Metastatic Mucinous Adenocarcinomas in the Ovaries. J. Surg. Pathol. 2003, 27, 985–993, doi:10.1097/00000478-200307000-00014.
- Köbel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Ionescu, D.N.; Rajput, A.; et al. Ovarian Carcinoma Subtypes Are Different Diseases: Implications for Biomarker Studies. PLoS Med. 2008, 5, e232, doi:10.1371/journal.pmed.0050232.
- Bouchard-Fortier, G.; Panzarella, T.; Rosen, B.; Chapman, W.; Gien, L.T. Endometrioid Carcinoma of the Ovary: Outcomes Compared to Serous Carcinoma After 10 Years of Follow-Up. Obstet. Gynaecol. Can. 2017, 39, 34–41, doi:10.1016/j.jogc.2016.10.006.
- Kuo, K.-T.; Mao, T.-L.; Jones, S.; Veras, E.; Ayhan, A.; Wang, T.-L.; Glas, R.; Slamon, D.; Velculescu, V.E.; Kuman, R.J.; et al. Frequent Activating Mutations of PIK3CA in Ovarian Clear Cell Carcinoma. J. Pathol. 2009, 174, 1597–1601, doi:10.2353/ajpath.2009.081000.
- Jones, S.; Wang, T.-L.; Shih, I.-M.; Mao, T.-L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A.; Vogelstein, B.; et al. Frequent Mutations of Chromatin Remodeling Gene ARID1A in Ovarian Clear Cell Carcinoma. Science 2010, 330, 228–231, doi:10.1126/science.1196333.
- Tsuchiya, A.; Sakamoto, M.; Yasuda, J.; Chuma, M.; Ohta, T.; Ohki, M.; Yasugi, T.; Taketani, Y.; Hirohashi, S. Expression Profiling in Ovarian Clear Cell Carcinoma. J. Pathol. 2003, 163, 2503–2512, doi:10.1016/s0002-9440(10)63605-x.
- Zondervan, K.; Becker, C.M.; Missmer, S.A. Endometriosis. Engl. J. Med. 2020, 382, 1244–1256, doi:10.1056/nejmra1810764.
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: Apooled analysis of case–control studies. Lancet Oncol. 2012, 13, 385–394, doi:10.1016/s1470-2045(11)70404-1.
- Nezhat, F.; Datta, M.S.; Hanson, V.; Pejovic, T.; Nezhat, C.; Nezhat, C. The relationship of endometriosis and ovarian malignancy: A review. Steril. 2008, 90, 1559–1570, doi:10.1016/j.fertnstert.2008.08.007.
- Landskron, G.; De La Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. Immunol. Res. 2014, 2014, 1–19, doi:10.1155/2014/149185.
- Balkwill, F.R.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545, doi:10.1016/s0140-6736(00)04046-0.
- Worley, J.M.J.; Welch, W.R.; Berkowitz, R.S.; Ng, S.-W. Endometriosis-Associated Ovarian Cancer: A Review of Pathogenesis. J. Mol. Sci. 2013, 14, 5367–5379, doi:10.3390/ijms14035367.
- Bulun, S.E. Endometriosis. Engl. J. Med. 2009, 360, 268–279, doi:10.1056/nejmra0804690.
- Yamaguchi, K.; Mandai, M.; Oura, T.; Matsumura, N.; Hamanishi, J.; Baba, T.; Matsui, S.; Murphy, S.K.; Konishi, I. Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene 2010, 29, 1741–1752, doi:10.1038/onc.2009.470.
- Toyokuni, S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009, 100, 9–16, doi:10.1111/j.1349-7006.2008.01001.x.
- Yamaguchi, K.; Mandai, M.; Toyokuni, S.; Hamanishi, J.; Higuchi, T.; Takakura, K.; Fujii, S. Contents of Endometriotic Cysts, Especially the High Concentration of Free Iron, Are a Possible Cause of Carcinogenesis in the Cysts through the Iron-Induced Persistent Oxidative Stress. Cancer Res. 2008, 14, 32–40, doi:10.1158/1078-0432.ccr-07-1614.
- Li, J.L.; Okada, S.; Hamazaki, S.; Ebina, Y.; Midorikawa, O. Subacute nephrotoxicity and in-duction of renal cell carcinoma in mice treated with ferric nitrilotriacetate. Cancer Res. 1987, 47, 1867–1869.
- Liu, M.; Okada, S. Induction of free radicals and tumors in the kidneys of Wistar rats by ferric ethylenediamine-N,N’-diacetate. 1994, 15, 2817–2821, doi:10.1093/carcin/15.12.2817.
- Munksgaard, P.S.; Blaakaer, J. The association between endometriosis and ovarian cancer: A review of histological, genetic and molecular alterations. Oncol. 2012, 124, 164–169, doi:10.1016/j.ygyno.2011.10.001.
- Melin, A.-S.; Lundholm, C.; Malki, N.; Swahn, M.-L.; Sparén, P.; Bergqvist, A. Hormonal and surgical treatments for endometriosis and risk of epithelial ovarian cancer. Acta Obstet. et Gynecol. Scand. 2013, 92, 546–554, doi:10.1111/aogs.12123.
- Yoshino, O.; Minamisaka, T.; Ono, Y.; Tsuda, S.; Samejima, A.; Shima, T.; Nakashima, A.; Koga, K.; Osuga, Y.; Saito, S. Three cases of clear-cell adenocarcinoma arising from endometrioma during hormonal treatments. Obstet. Gynaecol. Res. 2018, 44, 1850–1858, doi:10.1111/jog.13702.
- Modugno, F.; Ness, R.B.; Allen, G.O.; Schildkraut, J.M.; Davis, F.G.; Goodman, M.T. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. J. Obstet. Gynecol. 2004, 191, 733–740, doi:10.1016/j.ajog.2004.03.035.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23 257 women with ovarian cancer and 87 303 controls. Lancet 2008, 371, 303–314, doi:10.1016/s0140-6736(08)60167-1.
- Nishida, Y.; Yamashita, E.; Miki, W. Quenching activities of common hydrophilic and lip-ophilic antioxidants against singlet oxygen using chemiluminescence detection System. Carotenoid Sci. 2007, 11, 16–20.
- Martin, H.D.; Ruck, C.; Schmidt, M.; Sell, S.; Beutner, S.; Mayer, B.; Walsh, R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999, 71, 2253–2262, doi:10.1351/pac199971122253.
- Kuroki, T.; Ikeda, S.; Okada, T.; Maoka, T.; Kitamura, A.; Sugimoto, M.; Kume, S. Astaxanthin ameliorates heat stress-induced impairment of blastocyst development In Vitro: Astaxanthin colocalization with and action on mitochondria. Assist. Reprod. Genet. 2013, 30, 623–631, doi:10.1007/s10815-013-9987-z.
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Zhang, J.; Reinhart, G.A.; Chew, B.P. Astaxanthin modulates age-associated mitochondrial dysfunction in healthy dogs1. Anim. Sci. 2013, 91, 268–275, doi:10.2527/jas.2012-5341.
- Ames, B.N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science 1983, 221, 1256–1264, doi:10.1126/science.6351251.
- Dušinská, M.; Kažimírová, A.; Barančoková, M.; Beňo, M.; Smolkova, B.; Horská, A.; Rašlová, K.; Wsólová, L.; Collins, A. Nutritional supplementation with antioxidants decreases chromosomal damage in humans. 2003, 18, 371–376, doi:10.1093/mutage/geg002.
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. J. Cancer 2007, 121, 2381–2386, doi:10.1002/ijc.23192.
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive Effects of Astaxanthin on Lipid Profiles and Oxidative Stress in Overweight Subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369, doi:10.1007/s11130-011-0258-9.
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. Nutr. Biochem. 2010, 21, 381–389, doi:10.1016/j.jnutbio.2009.01.011.
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biophys. Res. Commun. 2008, 366, 892–897, doi:10.1016/j.bbrc.2007.12.019.
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Metab. 2010, 7, 18, doi:10.1186/1743-7075-7-18.
- Kavitha, K.; Kowshik, J.; Kishore, T.K.K.; Baba, A.B.; Nagini, S. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. et Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 4433–4444, doi:10.1016/j.bbagen.2013.05.032.
- Kowshik, J.; Baba, A.B.; Giri, H.; Reddy, G.D.; Dixit, M.; Nagini, S. Astaxanthin Inhibits JAK/STAT-3 Signaling to Abrogate Cell Proliferation, Invasion and Angiogenesis in a Hamster Model of Oral Cancer. PLoS ONE 2014, 9, e109114, doi:10.1371/journal.pone.0109114.
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009, 283, 108–117, doi:10.1016/j.canlet.2009.03.031.
- Tanaka, T.; Makita, H.; Ohnishi, M.; Mori, H.; Satoh, K.; Hara, A. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin. Cancer Res. 1995, 55, 4059–4064.
- Brito, A.; Ribeiro, M.; Abrantes, A.M.; Pires, A.; Teixo, R.; Tralhão, J.; Botelho, M.F. Quercetin in Cancer Treatment, Alone or in Combination with Conventional Therapeutics? Med. Chem. 2015, 22, 3025–3039, doi:10.2174/0929867322666150812145435.
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Rep. 2016, 6, 24049, doi:10.1038/srep24049.
- Satyan, K.; Swamy, N.; Dizon, D.S.; Singh, R.; Granai, C.O.; Brard, L. Phenethyl isothiocyanate (PEITC) inhibits growth of ovarian cancer cells by inducing apoptosis: Role of caspase and MAPK activation. Oncol. 2006, 103, 261–270, doi:10.1016/j.ygyno.2006.03.002.
- Wattenberg, L.W. Inhibitory effects of benzyl isothiocyanate administered shortly before diethylnitrosamine or benzo[a]pyrene on pulmonary and forestomach neoplasia in A/J mice. 1987, 8, 1971–1973, doi:10.1093/carcin/8.12.1971.
- Long, Y.; Fei, H.; Xu, S.; Wen, J.; Ye, L.; Su, Z. Association about dietary vitamin C intake on the risk of ovarian cancer: A meta-analysis. Rep. 2020, 40, doi:10.1042/bsr20192385.
- L’Espérance, K.; Datta, G.D.; Qureshi, S.; Koushik, A. Vitamin D Exposure and Ovarian Cancer Risk and Prognosis. J. Environ. Res. Public Health 2020, 17, 1168, doi:10.3390/ijerph17041168.
- Leng, Y.; Zhou, H.; Meng, F.; Tian, T.; Xu, J.; Yan, F. Association of vitamin E on the risk of ovarian cancer: A meta-analysis. Rep. 2019, 39, doi:10.1042/bsr20193311.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The Effect of Vitamin E and Beta Carotene on the Incidence of Lung Cancer and Other Cancers in Male Smokers. Engl. J. Med. 1994, 330, 1029–1035, doi:10.1056/nejm199404143301501.
- Wang, Q.; He, C. Dietary vitamin A intake and the risk of ovarian cancer: A meta-analysis. Rep. 2020, 40, 40, doi:10.1042/BSR20193979.
- Hua, X.; Yu, L.; You, R.; Yang, Y.; Liao, J.; Chen, D.; Yu, L. Association among Dietary Flavonoids, Flavonoid Subclasses and Ovarian Cancer Risk: A Meta-Analysis. PLoS ONE 2016, 11, e0151134, doi:10.1371/journal.pone.0151134.
- Chang, E.T.; Lee, V.S.; Canchola, A.J.; Clarke, C.A.; Purdie, D.M.; Reynolds, P.; Anton-Culver, H.; Bernstein, L.; Deapen, D.; Peel, D.; et al. Diet and Risk of Ovarian Cancer in the California Teachers Study Cohort. J. Epidemiol. 2007, 165, 802–813, doi:10.1093/aje/kwk065.