
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kazunori Sango | + 1410 word(s) | 1410 | 2021-02-04 09:47:57 | | | |
| 2 | Vicky Zhou | -5 word(s) | 1405 | 2021-02-09 06:35:19 | | |
Video Upload Options
Aldose reductase (AR) is a member of the reduced nicotinamide adenosine dinucleotide phosphate (NADPH)-dependent aldo-keto reductase superfamily. It is also the rate-limiting enzyme of the polyol pathway, catalyzing the conversion of glucose to sorbitol, which is subsequently converted to fructose by sorbitol dehydrogenase. AR is highly expressed by Schwann cells in the peripheral nervous system (PNS). The excess glucose flux through AR of the polyol pathway under hyperglycemic conditions has been suggested to play a critical role in the development and progression of diabetic peripheral neuropathy (DPN).
1. Introduction
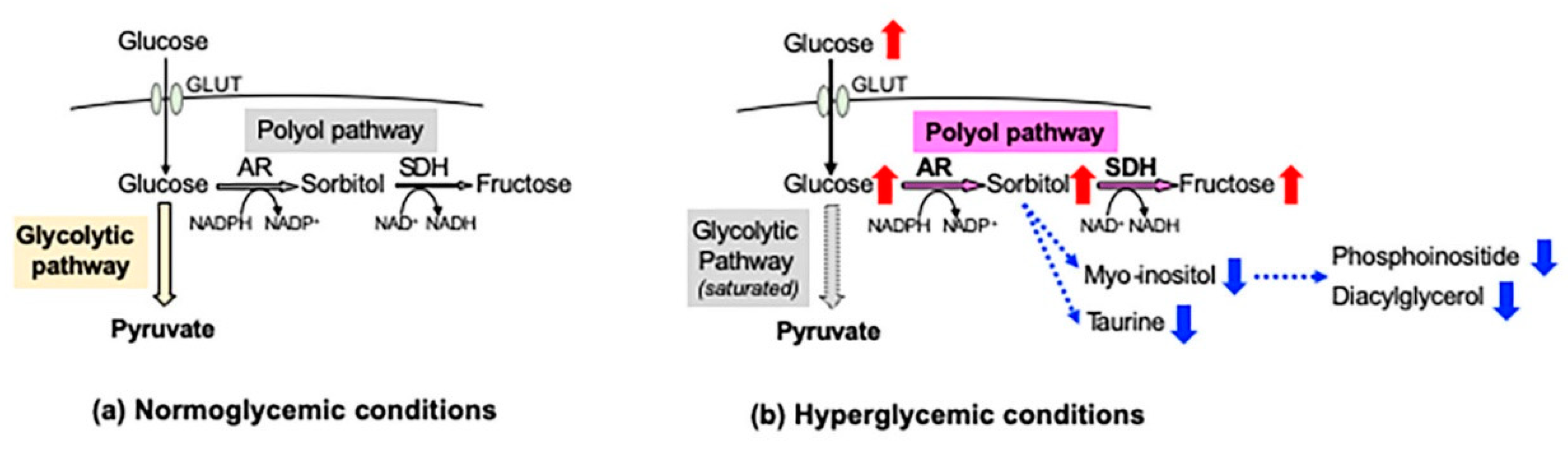
In the peripheral nervous system (PNS), blood glucose is incorporated into cells via glucose transporters (GLUT 1 and GLUT 3) in an insulin-independent manner. Under normoglycemic conditions, most of the cellular glucose is converted to glucose 6-phosphate by hexokinase and further metabolized into pyruvate through the glycolytic pathway. However, under hyperglycemic conditions, the saturation of the glycolytic pathway escalates the flux of glucose through the polyol pathway, the first and major collateral route of glucose metabolism. The polyol pathway, which initially identifies in the seminal vesicle [1], consists of the reduction step of glucose to sorbitol and the oxidation step of sorbitol to fructose: the first reduction step is catalyzed by aldose reductase (AR) in a reduced nicotinamide adenosine dinucleotide phosphate (NADPH)-dependent manner, and the second oxidation step is catalyzed by sorbitol dehydrogenase (SDH) in an NAD-dependent manner, respectively (Figure 1). Polyol pathway hyperactivity induces multiple metabolic imbalances, described in the following sections, and is thereby considered as a major cause of diabetic peripheral neuropathy (DPN) [2], as well as other complications [3][4]. AR, being the rate-limiting enzyme of the polyol pathway, has been studied intensively as a target protein for the prevention and treatment of DPN. Although numerous AR inhibitors (ARI) have been developed with promising results in the cell and animal models of DPN, most of the clinical trials using them ended in failure due to the adverse effects and/or limited drug potency [5]. Currently, epalrestat is the only ARI available for clinical use in Japan, but its use is limited to the patients with DPN at early stages and stable glycemic control [6]. Despite the intensive studies over the past four decades, the significance of AR and the polyol pathway in the pathogenesis of DPN and effective therapies against it remains unknown.
2. Establishment of an AR-Deficient Schwann Cell Line IKARS1
2.1. Establishment and Characterization of IKARS1 Cells
Spontaneously immortalized Schwann cells were established not only from normal mice, but from murine disease models (e.g., Charcot–Marie-Tooth disease, neurofibromatosis and lysosomal storage diseases) [7]. These cell lines sufficiently represent the pathological features of the mutant mice, and they will be beneficial tools for studying the PNS lesions in the relevant diseases. In a similar manner, we obtained immortalized Schwann cell lines from AR-knockout C57BL/6 mice. Because our first trial to establish Schwann cell lines from wild-type littermates ended in failure, we employed one of the cell lines established from normal C57BL/6 mice in our institute as control of the AR-knockout cell line. These cell lines, termed IKARS1 (immortalized knockout AR Schwann cells 1) and 1970C3, respectively, showed distinct Schwann cell phenotypes similar to IMS32 cells, such as spindle-shaped morphology, expression of glial cell markers (S100β, p75 low-affinity neurotrophin receptor, etc.) and synthesis/secretion of neurotrophic factors [8]. Conditioned media collected from IKARS1 and 1970C3 cells enhanced neurite outgrowth from cultured adult mouse DRG neurons, and both cell lines secreted NGF and glial-cell-line-derived neurotrophic factor (GDNF) into the culture medium. The deficient AR activity in IKARS1 cells was confirmed by the galactose (25 mM)-induced increase in galactitol contents in 1970C3 cells but not in IKARS1 cells. A marked down-regulation of mRNA/protein expression of the enzymes downstream of AR in the polyol pathway (i.e., SDH and ketohexokinase (KHK)) in IKARS1 cells compared with 1970C3 cells (Figure 2) is consistent with the high-glucose-(30 mM)-induced increases in the sorbitol and fructose contents in 1970C3 cells but not IKARS1 cells. These findings indicate the absence of the polyol pathway in IKARS1 cells.
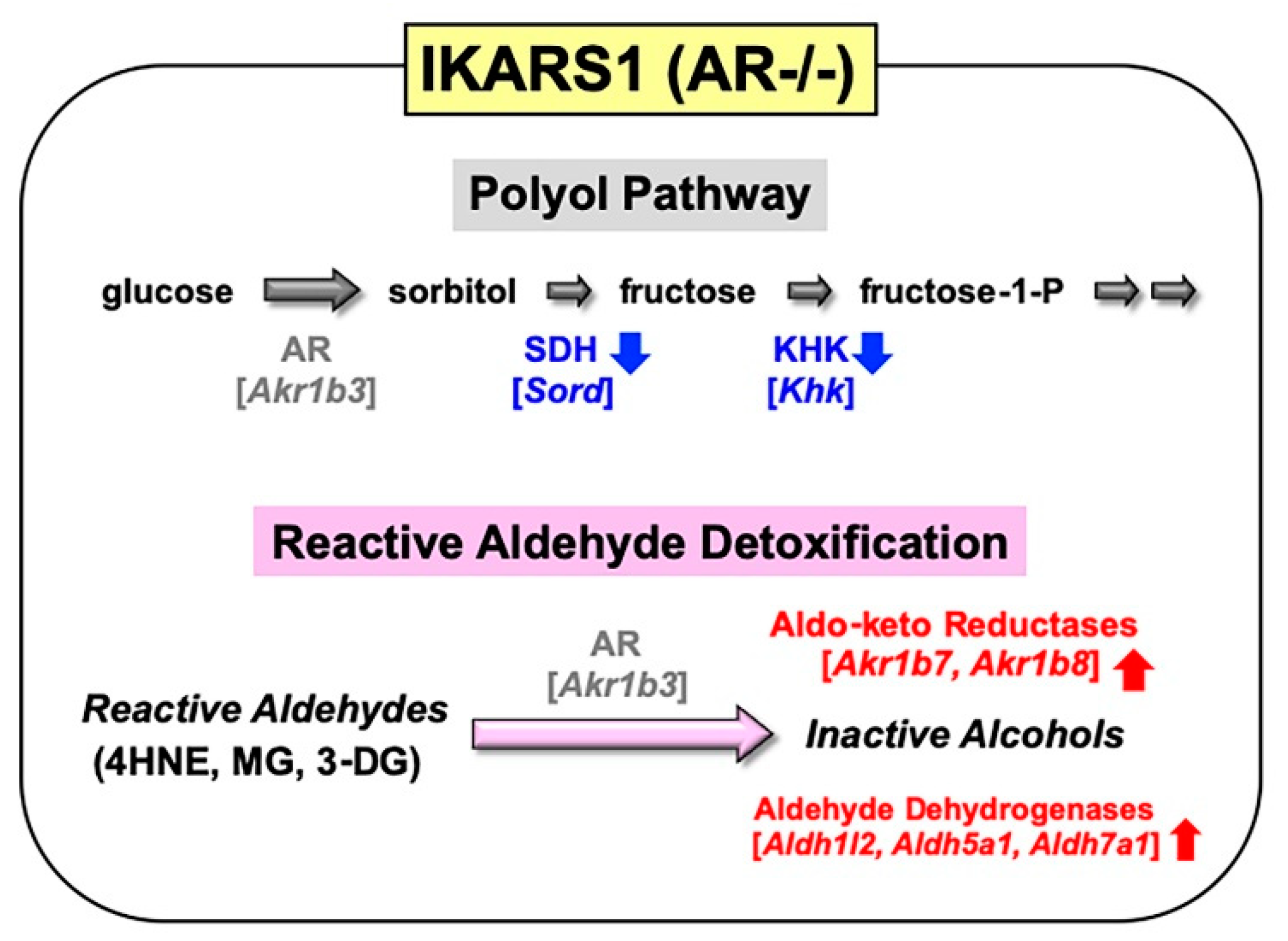
It is also noteworthy that mRNA expression of aldo-keto reductases (AKR1B7 and AKR1B8) and aldehyde dehydrogenases (ALDH1L2, ALDH5A1, and ALDH7A1) was significantly up-regulated in IKARS1 cells compared with 1970C3 cells (Figure 2). AKR1B7 and AKR1B8 are considered AR-related enzymes because of the high degree of sequence similarities to AR [9], and the AKRs and ALDHs that are up-regulated in IKARS1 cells appear to be involved in the reduction and oxidation of various aldehydes [10]. Although AR is suggested to be an efficient catalyst for the reduction in 4HNE, MG, and 3-DG, the cell viability assays revealed no significant differences in the relative survival ratios between IKARS1 and 1970C3 cells after exposure to these substances. The findings that AR gene deletion does not augment the aldehyde toxicity imply functional redundancies among AKRs and ALDHs regarding the metabolic disposal system of these aldehydes in Schwann cells. Because exposure to these aldehydes further up-regulated mRNA expression of AKR1B7 and AKR1B8, but not ALDHs, in IKARS1 cells, the reactive aldehyde detoxification can be taken over by AKR1B7 and AKR1B8 in the absence of AR.
2.2. Establishment of IWARS1 Cells and Future Studies with IKARS1 and IWARS1
The second trial to establish Schwann cell lines from wild-type littermates of AR-deficient C57BL/6 mice was successful. One of the cell lines termed IWARS1 (immortalized wild-type AR Schwann cells 1) possess distinct Schwann cell phenotypes similar to 1970C3 cells (Niimi et al., in preparation). Unlike IMS32 cells, neuregulin-β is required for the passage of IKARS1, 1970C3 and IWARS1 cells. The lower growth potency of these cells compared with IMS32 cells might be advantageous for myelination under co-culture with neurons, although we have not yet confirmed that they possess this capability.
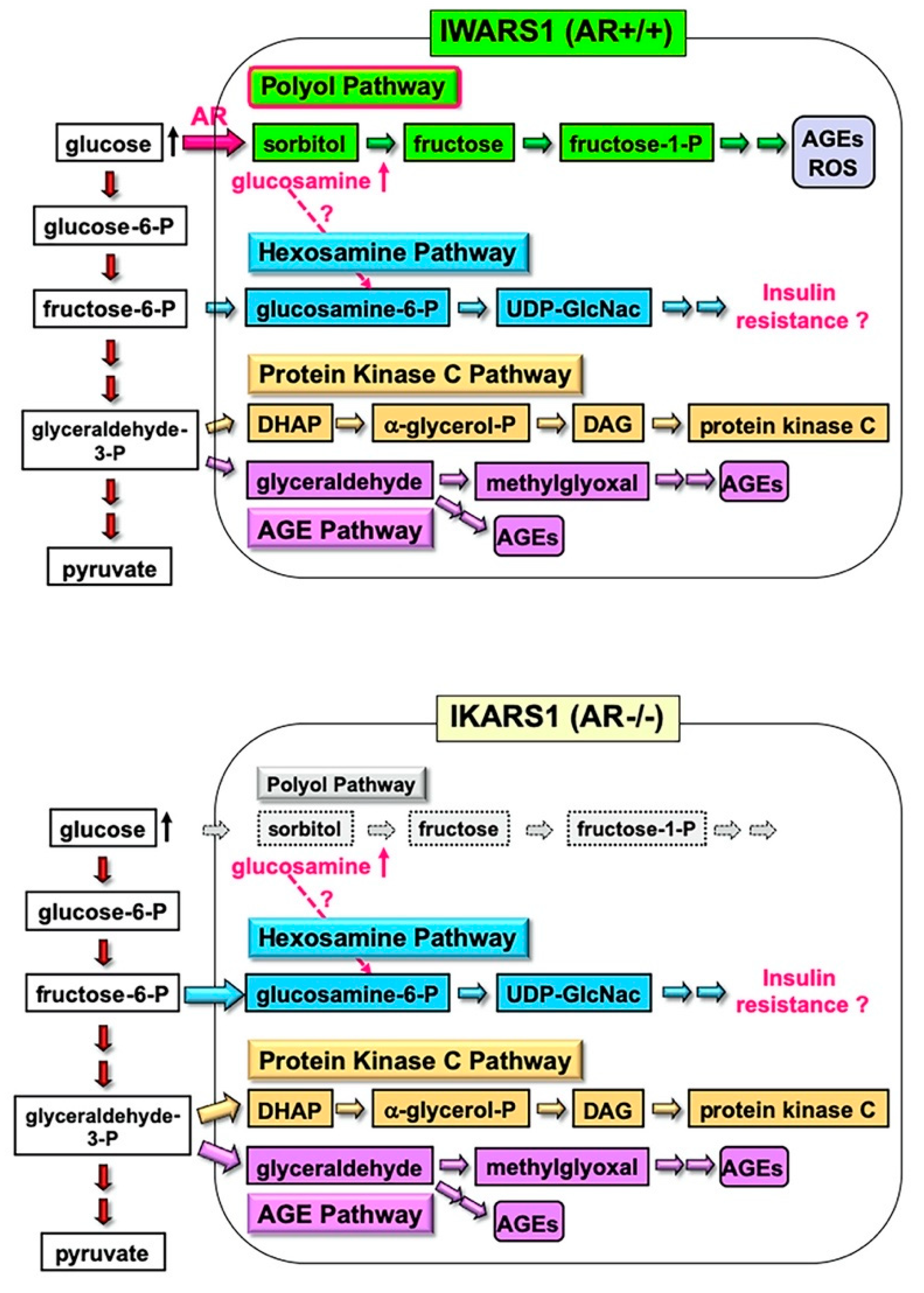
The IWARS1 cells are “true controls” of IKARS1 cells, and these cell lines can be utilized to explore various collateral glucose-utilizing pathways (polyol pathway, hexosamine pathway, PKC pathway and AGE pathway) in relation to AR (Figure 3). The AR knockout mice, when rendered diabetes by streptozotocin, showed defense against neurological manifestations by 12 weeks after onset of diabetes [11]. However, Mizukami et al. (2020) [12] recently reported the reduced NCV in AR knockout mice with 16 weeks of diabetes, suggesting that other pathways, downstream or independent of the polyol pathway, are involved in the pathogenesis of DPN with long duration of hyperglycemia. They also observed an accumulation of glucosamine in the sciatic nerves of diabetic AR knockout and wild-type mice and direct toxicity of glucosamine toward cultured DRG neurons and IMS32 Schwann cells. While how glucosamine is up-regulated under diabetic conditions remains unclear, glucosamine can be metabolized to glucosamine-6-phosphate, constituents of the hexosamine pathway, the second collateral glycolysis pathway [2][4]. IKARS1 and IWARS1 cells will be essential reagents to determine the involvement of the AR and hexosamine pathway in the pathogenesis of DPN, as well as other pathogenic factors of DPN related to the polyol pathway, such as glycated, oxidative and nitrosative stress.
References
- Hers, H.G. The mechanism of the transformation of glucose in fructose in the seminal vesicles. Biochem. Biophys. Acta 1956, 22, 202–203.
- Yagihashi, S. Glucotoxic Mechanisms and Related Therapeutic Approaches. Int. Rev. Neurobiol. 2016, 127, 121–149.
- González, R.G.; Barnett, P.; Aguayo, J.; Cheng, H.M.; Chylack, L.T. Direct measurement of polyol pathway activity in the ocular lens. Diabetes 1984, 33, 196–199.
- Yabe-Nishimura, C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol. Rev. 1998, 50, 21–33.
- Grewal, A.S.; Bhardwaj, S.; Pandita, D.; Lather, V.; Sekhon, B.S. Updates on Aldose Reductase Inhibitors for Management of Diabetic Complications and Non-diabetic Diseases. Mini-Rev. Med. Chem. 2015, 16, 120–162.
- Hotta, N.; Kawamori, R.; Fukuda, M.; Shigeta, Y. The Aldose Reductase Inhibitor–Diabetes Complications Trial Study Group Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: Multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet. Med. 2012, 29, 1529–1533.
- Sango, K.; Tsukamoto, M.; Utsunomiya, K.; Watabe, K. Chapter 10. Spontaneously Immortalized Adult Rodent Schwann Cells as Valuable Tools for the Study of Peripheral Nerve Degeneration and Regeneration. In Schwann Cell Development and Pathology; Sango, K., Yamauchi, J., Eds.; Springer: Tokyo, Japan, 2014; pp. 147–170.
- Niimi, N.; Yako, H.; Takaku, S.; Kato, H.; Matsumoto, T.; Nishito, Y.; Watabe, K.; Ogasawara, S.; Mizukami, H.; Yagihashi, S.; et al. A spontaneously immortalized Schwann cell line from aldose reductase-deficient mice as a useful tool for studying polyol pathway and aldehyde metabolism. J. Neurochem. 2018, 144, 710–722.
- Yu, T.; Li, L.; Chen, T.; Liu, Z.; Liu, H.; Li, Z. Erythropoietin Attenuates Advanced Glycation Endproducts-Induced Toxicity of Schwann Cells In Vitro. Neurochem. Res. 2015, 40, 698–712.
- Singh, M.; Kapoor, A.; Bhatnagar, A. Oxidative and reductive metabolism of lipid-peroxidation derived carbonyls. Chem. Interact. 2015, 234, 261–273.
- Ho, E.C.; Lam, K.S.L.; Chen, Y.S.; Yip, J.C.; Arvindakshan, M.; Yamagishi, S.-I.; Yagihashi, S.; Oates, P.J.; Ellery, C.A.; Chung, S.S.; et al. Aldose Reductase-Deficient Mice Are Protected From Delayed Motor Nerve Conduction Velocity, Increased c-Jun NH2-Terminal Kinase Activation, Depletion of Reduced Glutathione, Increased Superoxide Accumulation, and DNA Damage. Diabetes 2006, 55, 1946–1953.
- Mizukami, H.; Osonoi, S.; Takaku, S.; Yamagishi, S.-I.; Ogasawara, S.; Sango, K.; Chung, S.; Yagihashi, S. Role of glucosamine in development of diabetic neuropathy independent of the aldose reductase pathway. Brain Commun. 2020, 2, fcaa168.




