
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammed Ghiboub | + 3447 word(s) | 3447 | 2021-02-07 03:17:40 | | | |
| 2 | Peter Tang | Meta information modification | 3447 | 2021-02-21 13:47:10 | | |
Video Upload Options
The increase in incidences of pediatric Crohn’s Disease (CD) worldwide has been strongly linked with dietary shifts towards a Westernized diet, ultimately leading to altered gut microbiota and disturbance in intestinal immunity and the metabolome. Multiple clinical studies in children with CD have demonstrated the high efficacy of nutritional therapy with exclusive enteral nutrition (EEN) to induce remission with an excellent safety profile. However, EEN is poorly tolerated, limiting its compliance and clinical application. This has spiked an interest in the development of alternative and better-tolerated nutritional therapy strategies. Several nutritional therapies have now been designed not only to treat the nutritional deficiencies seen in children with active CD but also to correct dysbiosis and reduce intestinal inflammation.
1. Introduction
Crohn’s disease (CD) is a chronic disorder that belongs to the group of inflammatory bowel diseases (IBD); it is characterized by transmural inflammation that can affect any area along the proximal-distal axis of the gastrointestinal tract (GI) [1][2][3]. Symptoms often involve abdominal pain, diarrhea, rectal blood loss, and fatigue, and the disease often leads to weight loss and malnutrition [4]. The incidence of CD is increasing worldwide, and disease-onset can occur at any age [5]. Up to 15% of CD patients are diagnosed before the age of 20 [6][7][8]. The incidence of pediatric CD is still increasing and varies from 2.5 to 11.4 per 100,000, although a recent meta-analysis concluded the incidence in Europe to be between 9–10 per 100,000. Few studies have reported on the prevalence of pediatric IBD, but overall, there is an estimated prevalence of 58/100,000, although the contribution of pediatric cases to the overall IBD burden for society has remained low due to the increasing prevalence of adult-onset disease [5][9][10]. While the etiology of CD may be similar between children and adults, children with CD typically have a more extensive/panenteric phenotype; however, the time of progression to stricturing and penetrating complications is similar [11][12]. As their disease course occurs during periods of growth and development, children are particularly vulnerable, and management strategies need to take growth characteristics into account [13][14]. CD is considered a multifactorial disorder, where genetics, environment, gut microbiota, and the immune system interplay to contribute to disease development [3][15][16]. However, despite the extensive research performed on CD, treatment remains focused on immune suppressive measures and its etiology not fully understood.
CD is characterized by the excessive infiltration of leukocytes into the inflamed mucosa and a high level of secreted proinflammatory cytokines [17][18]. Thus, medication regimens are focused on the use of immunomodulators or -suppressants (such as corticosteroids, methotrexates, thiopurines, and biologicals such antitumor necrosis factor alpha (TNFα)) to dampen immune system activity [9][19]. However, multiple side effects, such as the increased risk of infections and malignancy, are associated with the use of immuno-modulators and -suppressants [9]. In turn, the use of corticosteroids in pediatric CD is associated with growth retardation and reduced bone accrual [9][20][21][22].
Extensive studies have been performed to relate microbiome changes with active disease and/or response to treatment [23][24][25][26][27][28][29]. Indeed, microbiome manipulation by means of antibiotics has shown promise as a therapeutic strategy for treating pediatric CD in a randomized controlled trial (RCT) of azithromycin + metronidazole for luminal CD [9][19][30][31]. Antibiotics are also indicated to help maintain remission to anti-TNF in perianal CD [9][32][33]. Although various immunosuppressants and antibiotics can provide therapeutic benefit in CD, a minority of patients maintain remission after induction of remission without maintenance therapy. In addition, there is a substantial rate of primary nonresponse and loss-of-response to immunosuppressants, which leads to a high, unmet need for novel effective therapies [34][35][36][37].
A major factor in intestinal microbiota composition and ecology is diet. Diet has been found to strongly impact gut microbiota, which has been identified as a crucial player in regulating metabolism and the immune response [38][39][40]. Multiple studies have highlighted the impact of changes in dietary intake and consequences of food industrialization (such as the Western diet, which is rich in fats and carbohydrates) on the gut microbiome (dysbiosis) and on increasing pediatric CD incidence [3][5][26][41][42][43]. This has provided a strong rationale for further investigating nutrition as a potential therapy to induce or maintain remission in pediatric CD. While current medical therapy is mainly directed against inflammation, nutritional therapies can be directed toward the correction of dysbiosis and metabolome as well as to the reduction of inflammation [9]. Recent European guidelines have confirmed the central role of dietary therapy (notably exclusive enteral nutrition (EEN)) in the management of mild-to-moderate CD while emphasizing the need for rigorous clinical studies of novel dietary strategies (including the better-tolerated novel Crohn’s disease exclusion diet (CDED)) [9]. Over the past few years, a number of nutritional therapy strategies have been designed to reduce dietary exposure to foods that might adversely impact the microbiome, the intestinal barrier, and innate immunity [3][9][44].
2. Dietary Inflammatory Potential and Risk of Colitis
The persisting rise in incidences of IBD has gone hand in hand with the Westernization of different continents [24][26]. In particular, the Western diet has been largely studied and linked to an increased inflammatory state in a number of diseases, including IBD [40][45][46]. It includes high amounts of processed foods, red meat, high fat, sugar and additive exposure, and a lack of dietary fiber, fruit, and vegetables [39].
The loss of dietary fiber leads to less small chain fatty acid (SCFA) production and a reduction of the available energy source of gut epithelial cells [47]. This influences not only gut microbial composition but also function and, in turn, can impact host immunity [47]. High animal protein intake has been associated with an increased risk of IBD [48][49]. A high sugar diet has been shown to enhance susceptibility to colitis in mice by reducing SCFA and increasing gut permeability [50]. Food additives have also been found to play a negative role in intestinal inflammation, impairing antibacterial responses and suppressing antimicrobial defense mechanisms. They can promote colitis susceptibility in mice by increasing intestinal permeability and significant thinning of the mucous layer [51]. In addition, a positive correlation between emulsifier consumption and increased IBD incidence was found when studying data from different countries [52][53]. Dietary emulsifiers can increase the ability of CD-associated adherent-invasive E. coli to adhere to epithelial cells and promote intestinal inflammation in mice [54]. The intake of a diet high in red meat aggravates the severity of dextran sodium sulfate (DSS)-colitis, translated by higher disease activity and histopathological scores [55].
Altogether, these data suggest that an altered dietary pattern can lead to changes in the microbiota (dysbiosis) and altered gut homeostasis and host immunity by promoting inflammation and increasing susceptibility to colitis [38][40]. This indicates the potential of modulating dietary intake as therapy for IBD. To date, mostly preclinical studies in animal models and cell lines have studied the effect of different dietary components on the host; the data remain to be verified in humans [44][56][57][58][59].
3. Different Types of Nutritional Therapy and Their Efficacy
There are different types of nutritional therapy being used and explored in pediatric CD [3][9][44]. They differ in treatment duration, induced outcomes, and nutritional composition [60]. Here, we discuss the four main nutritional therapies, varying from the widely implemented EEN to more novel dietary/nutritional strategies.
3.1. Exclusive Enteral Nutrition (EEN)
EEN consists of a complete liquid formula diet that contains all nutritional requirements and excludes all regular table foods for a determined period of time [61][62]. The main types of EEN formula available are elemental, semielemental, and polymeric, which can be adapted for specific conditions like IBD [63][64][65]. The formulas differ in composition, size, and structure of proteins and fats [65][66]. The daily amount of EEN is based on the estimated energy requirement of an individual [67]. EEN can be administered orally (in case of a polymeric formula) or by a nasogastric tube in case of an inability to meet required daily intake (or to comply with payer-requirements in selected health care systems, where it is stipulated that medical nutrition is only reimbursed when it is delivered through a tube). The exact duration of EEN therapy for induction of remission varies from 6–8 weeks mostly, but there have been reports on the use of EEN for 4–12 weeks [9][61]. After the strict exclusion of solid food during EEN therapy, solid food is gradually reintroduced until a normal intake is reached [9][61].
EEN has been widely accepted as first-line therapy for induction of remission in mild-to-moderate pediatric CD [9]. Multiple studies and meta-analyses of data in children with CD have clearly shown that EEN leads to similar or even superior efficacy in induction of remission compared to corticosteroids [68][69][70]. More importantly, studies have shown that EEN may also be associated with superior mucosal healing and normal CRP remission [57][71][72], which, in turn, are associated with fewer subsequent complications [73]. In general, EEN induces remission in approximately 75–85% of children with mild-to-moderate CD [3][74]. In addition, EEN therapy has demonstrated an improved nutrition status, growth, mucosal healing, reduced fecal calprotectin, normal CRP remission, and a high safety profile [3][67][75]. The use of EEN for induction of remission does not come with increased use of biologicals or need for surgery but ultimately leads to a long-term avoidance of steroids in up to half of CD patients during their years of growth [21]. A nutrition-based induction strategy avoids steroid-related side-effects, such as growth retardation and risk of infections [76][77]. The efficacy of EEN-induced remission is independent of the formula types or the administration route [78][79]. However, a polymeric formula is preferred in daily practice due to lower costs, superior taste, and better tolerance [80].
EEN is associated with minimal and temporary side-effects. The most commonly reported side-effects are nausea, diarrhea, constipation, abdominal pain, bloating, and taste fatigue [81]. The only severe adverse event that has (rarely) been reported is refeeding syndrome, a potentially fatal metabolic complication that can occur when starting EEN in children with severe malnutrition but can be avoided with careful monitoring of electrolytes [82][83][84]. Although the vast majority of side-effects are minimal, the acceptability of the patient and their family is a major challenge in the use of EEN. The tolerance of EEN is estimated to be around 74%, where most patients will refuse to continue the treatment [3].
EEN has not only been investigated for induction of remission but also for maintaining remission. Belli et al. investigated the effect of intermittent nocturnal EEN during a one-year follow-up in children with CD with growth failure: 8 subjects received and completed EEN for one month, three times in total, with a three-month break in between each EEN regimen. They showed significant height and weight gains, along with a decreased PCDAI score and a decreased need for steroids [85]. Nevertheless, although EEN is the only well-established, evidence-based dietary therapy, with high rates of remission in pediatric CD [9], the use of EEN is not practical or appealing to continue long term. The rigorous requirements to avoid solid food and the resulting disturbance of normal dietary habits make EEN unsuitable as an effective approach to sustain remission [3]. In addition, there is a rapid loss of response and return of inflammation upon food reintroduction in patients after EEN induction therapy [76].
3.2. Partial Enteral Nutrition (PEN)
With the well-described beneficial effects of EEN, PEN has also been studied in the hope of creating a more patient-friendly, tolerable therapy to achieve induction or maintain remission [86]. PEN uses the same liquid formulas as EEN but for less than 100% of caloric needs, in addition to some regular daily food intake. The use of PEN, as investigated in clinical studies, varies from 10–90% of calculated caloric daily intake [86][87]. Different studies have shown an improvement of CD symptoms with the use of PEN for inducing remission. However, the effect is usually explained by symptomatic improvements like reduction in abdominal pain and weight gain [86]. Although these are important aspects that contribute to the overall health of the patient, they are not necessarily due to reduced inflammation and improved mucosal healing [86]. Indeed, PEN with a free diet was not able to induce remission and suppress inflammation in active CD [76][86]. It was also significantly less potent compared to EEN and anti-TNF for inducing mucosal healing and decreasing inflammation [88]. However, adherence rates to PEN are usually low, which could partially explain the lack of effect in different studies.
The effect of PEN alone or PEN combined with medical therapy for maintenance of remission has also been shown to be of benefit. These studies have been done mainly in adults [89][90]. Although some studies have shown benefits from the use of PEN for maintenance of remission in pediatric CD, the data remains inconclusive [91][92][93][94]. Short-term PEN as supportive treatment (in addition to regular therapy) for 4 weeks after induction of remission with medical therapy showed an improved nutritional status after 1 year in children with severe CD [94]. Similarly, nocturnal PEN for 4–5 nights/week, in addition to an ad libitum diet, showed prolongation of EEN- and corticosteroid-induced remission and improved linear growth in children and adolescents [91]. In the CERISIER trial conducted in Japan by Hisamatsu et al., PEN combined with an escalation of anti-TNF dosing in secondary loss-of-response was superior to dose-escalation alone [95]. The study was halted early after an intermediate analysis was performed on 15 patients that showed the benefits of combination therapy and the disadvantage of anti-TNF escalation alone. Compared with anti-TNF escalation alone, the combination group showed a tendency toward a superior response rate to infliximab (IFX; 10 mg/kg every 8 weeks), with week 56 as the primary endpoint, but, likely due to the early discontinuation of the study and the consequently reduced number of patients, significant differences at week 56 were not seen. This appears to be the first clinical trial to show the usefulness of combination therapy of enteral nutrition (EN) therapy with biologics for anti-TNF refractory CD [95].
In a recent meta-analysis based on studies in children and adults with CD, Gkikas et al. concluded that the consumption of more than 35% of caloric needs that come from EN is necessary to achieve clinical benefits in maintaining remission [96]. The ESPHGAN guideline recommends a daily caloric intake of at least 50% to reach therapeutic efficacy for effective prolongation of remission in low-risk CD [9]. Recently, different studies have investigated the role of PEN in combination with specific diets such as CDED and anti-inflammatory diet (AID)-CD, reaching promising results and showing the effectiveness of combination therapy with PEN and other specific diets [3][97][98][99].
3.3. Crohn’s Disease Exclusion Diet (CDED)
The recently described CDED is a whole-food diet coupled with PEN (MODULEN™ IBD, Nestlé) [3][99]. CDED is a structured diet designed to reduce exposure to dietary components that may negatively affect the microbiome, intestinal barrier, and intestinal immunity [3][99]. CDED limits exposure to animal fat, certain types of meat, gluten, maltodextrin, emulsifiers, sulfites, and certain monosaccharides [3][99]. CDED was described for the first time in a case series of adults and children with CD by Sigall-Boneh et al. [99]. Participants followed CDED, with additional PEN for 50% of calculated energy requirements for 6 weeks, followed by a step-down diet with an additional 25% of energy requirements by PEN [99]. Patients following CDED + PEN therapy reached high rates of clinical response and remission (around 80%) [99][100]. Interestingly, the majority of patients who refused additional PEN and followed only CDED also reached clinical remission [99]. PEN was initially added to the diet to guarantee the full caloric and nutritionally balanced intake of the subjects. Data from this study, however, indicated that PEN is not necessary to achieve remission [99]. This was confirmed in a recent RCT in adults, where the use of PEN was numerically but not statistically superior in remission induction [101]. However, the use of PEN is still preferred due to its nutritional status benefit and PEN being a major source of calcium during treatment with CDED [94][102].
The efficacy of CDED + PEN compared to EEN in inducing clinical remission in children with mild-to-moderate CD was recently shown in a multinational RCT [3]. CDED + PEN and EEN showed equal effectiveness in inducing remission at week 6, with remission rates around 85%. In addition, 80% of children with a response to EEN or CDED + PEN at week 3 reached clinical remission at week 6 [103]. However, CDED + PEN demonstrated better tolerance by children and their parents at week 6, addressing the adherence challenges of EEN. One out of 40 patients in the CDED + PEN group withdrew due to low compliance or intolerance to the diet. In the EEN group, 7 out 34 children discontinued, of which 6 refused to continue EEN. In addition, CDED + PEN led to better-sustained remission through to week 12, which was achieved in 75% of the children [3][100]. Importantly, CDED + PEN sustained remission was associated with sustained microbiome changes associated with remission [3][104]. In previous studies performed on both children and adults with CD, including patients with secondary loss of response to biologic therapy [98][99], combining CDED with immunomodulators often led to successful recapturing of remission in these patients. These results are promising as this specific group is in urgent need of alternative therapeutic options.
CDED shows promising therapeutic potential for pediatric CD, not only for induction of remission but also as maintenance therapy, drug de-escalation, or rescue therapy in children with loss of response to other treatments [100]. However, following the CDED also requires parental commitment to the planning and preparation of meals according to the dietary instructions. In addition, studies and data of its effects on mucosal healing and inflammation, as stated in the recently published ESPHGAN guideline, are eagerly awaited [9].
3.4. CD Treatment-with-Eating Diet (CD-TREAT)
The individualized food-based diet CD-TREAT is an ordinary (solid) food diet that recreates the composition of EEN (MODULEN™ IBD, Nestlé), excluding dietary components like gluten and lactose and matching others like carbohydrates and proteins [44]. The therapy aims to mimic EEN’s effects on the gut microbiome, metabolome, inflammation, and clinical outcomes [44]. In an RCT performed on 25 healthy volunteers (adults) that received EEN and CD-TREAT for 7 days, each with a 14-day washout period in between, the effects of CD-TREAT were comparable to EEN in terms of microbiome changes and the composition of a range of metabolites [44]. Many of these effects observed in human volunteers were confirmed in an animal model [44]. Additionally, in a pilot of 5 children with active CD, CD-TREAT administration for 8 weeks showed the benefit of inducing clinical remission and a reduction of fecal calprotectin [44]. There was high adherence to the diet during the study, which did arrange for meals to be prepared and distributed for free by a local provider to participants [44]. Although these results are promising, they remain to be confirmed in an ongoing and sufficiently powered RCT [105].
3.5. Recommendations for Clinical Practice
New ESPHGAN-ECCO guidelines recommend EEN as the first choice of therapy for the induction of remission for mild-to-moderate luminal CD [9]. CDED + PEN can be considered a better-tolerated alternative with a similar rate of induction of remission by 6 weeks and improved maintenance of remission at 12 weeks [3]. PEN alone is not recommended for induction of remission but can be used to prolong remission or as a short-term bridge between therapies [9]. Other dietary strategies will need further research before incorporation into daily clinical practice can be recommended.
4. Potential Mechanisms of Action of Nutritional-Therapy-Induced Remission
Nutritional therapies have shown a great ability to attenuate intestinal inflammation and induce mucosal healing in pediatric CD patients [3][44][71][99]. However, the mechanisms by which they induce and sustain remission remain unclear [106]. The enhancement in nutritional status, promoting an anti-inflammatory response and increasing the production of innate defense proteins, restraining luminal antigen exposure, improving gut permeability, and changes in gut microbiota have been suggested as potential mechanisms [107]. Some of the potential mechanisms of action of nutritional-therapy-induced remission are illustrated in Figure 1.
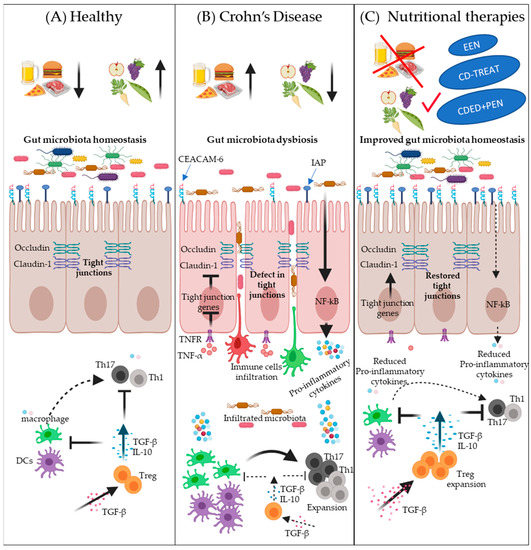
Figure 1. Schematic overview of some potential mechanisms of nutritional-therapy-induced remission in Crohn’s disease (CD). Panel (A) depicts the intestinal homeostasis in health associated with a healthy diet, i.e., less intake of a Westernized diet (normal microbiota diversity, normal expression of IAP and CEACAM-6, less interactions between bacteria and epithelial cells, normal expression of tight junction proteins such as occludin and claudin-1, less penetration of epithelium by potentially pathogenic bacteria and infiltration of immune cells, better immune surveillance by Tregs, and normal expression of TGF-β). Panel (B) illustrates the potential effects of a Westernized diet on the development of CD: low expression of antigen-related cell adhesion molecule (CEACAM-6) and intestinal alkaline phosphatase (IAP), decreased tight junction proteins expression, defects in intestinal barrier function, increased bacterial adherence to epithelial cells, stimulation of epithelial cells to express proinflammatory cytokines, increased bacterial and immune cells infiltration, reduced TGF-β, decreased Treg cells expansion, increased expansion of proinflammatory T-cells, and elevated proinflammatory cytokines expression by immune cells. Panel (C) describes the potential of nutritional therapies to correct dysbiosis and to reduce intestinal inflammation. Nutritional therapies can enhance microbial diversity and increase the expression of CEACAM-6 and IAP to prevent the adherence of bacteria with epithelial cells. Nutritional therapies can also reduce proinflammatory-cytokine-induced barrier dysfunction, such as TNF-α, thus normalizing the expression of tight junction proteins and improving intestinal barrier function, which, in turn, leads to less infiltration of microbes and immune cells. TGF-β supplementation has a beneficial effect on T-cell polarization into a Treg phenotype, which inhibits T-cell polarization into Th1 and Th17 subsets as well as dampening inflammatory macrophage function.
References
- Li Yim, A.; Duijvis, Y.; Ghiboub, M.; Sharp, C.; Ferrero, E.; Mannens, M.; D’Haens, G.R.; de Jonge, W.J.; Te Velde, A.A.; Henneman, P. Whole-Genome DNA Methylation Profiling of CD14+ Monocytes Reveals Disease Status and Activity Differences in Crohn’s Disease Patients. J. Clin. Med. 2020, 9, 1055.
- Lemberg, D.A.; Day, A.S. Crohn disease and ulcerative colitis in children: An update for 2014. J. Paediatr. Child Health 2014, 51, 266–270.
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8.
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pr. 2017, 2017, 1–11.
- oberts, S.E.; Thorne, K.; Thapar, N.; Broekaert, I.; Benninga, M.A.; Dolinsek, J.; Mas, E.; Miele, E.; Orel, R.; Pienar, C.; et al. A Systematic Review and Meta-analysis of Paediatric Inflammatory Bowel Disease Incidence and Prevalence Across Europe. J. Crohns Coliti 2020, 14, 1119–1148.
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060.
- Van Limbergen, J.; Haskett, J.; Griffiths, A.M.; Critch, J.; Huynh, H.; Ahmed, N.; de Bruyn, J.C.; Issenman, R.; El-Matary, W.; Walters, T.D.; et al. Toward enteral nutrition for the treatment of pediatric Crohn disease in Canada: A workshop to identify barriers and enablers. Can. J. Gastroenterol. Hepatol. 2015, 29, 351–356.
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.-P. Long-Term Evolution of Disease Behavior of Crohn’s Disease. Inflamm. Bowel Dis. 2002, 8, 244–250.
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohns Coliti 2020.
- Burgess, C.J.; Henderson, P.; Jones, G.-R.; Lees, C.W.; Wilson, D.C. Paediatric Patients (Less Than Age of 17 Years) Account for Less Than 1.5% of All Prevalent Inflammatory Bowel Disease Cases. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 521–523.
- Van Limbergent, J.; Russell, R.; Drummond, H.E.; Aldhous, M.C.; Round, N.K.; Nimmo, E.R.; Smith, L.; Gillett, P.M.; McGrogan, P.; Weaver, L.T.; et al. Definition of Phenotypic Characteristics of Childhood-Onset Inflammatory Bowel Disease. Gastroenterol. 2008, 135, 1114–1122.
- Kugathasan, S.; Denson, L.A.; Walters, T.D.; Kim, M.-O.; Marigorta, U.M.; Schirmer, M.; Mondal, K.; Liu, C.; Griffiths, A.; Noe, J.D.; et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: A multicentre inception cohort study. Lancet 2017, 389, 1710–1718.
- Jakobsen, C.; Bartek, J.; Wewer, V.; Vind, I.; Munkholm, P.; Groen, R.; Paerregaard, A. Differences in phenotype and disease course in adult and paediatric inflammatory bowel disease—A population-based study. Aliment. Pharmacol. Ther. 2011, 34, 1217–1224.
- Kelsen, J.; Baldassano, R.N. Inflammatory bowel disease: The difference between children and adults. Inflamm. Bowel Dis. 2008, 14, S9–S11.
- Verstockt, B.; Smith, K.G.; Lee, J.C. Genome-wide association studies in Crohn’s disease: Past, present and future. Clin. Transl. Immunol. 2018, 7, e1001.
- Boyapati, R.; Satsangi, J.; Ho, G.-T. Pathogenesis of Crohn’s Disease. F1000 Prime Rep. 2015, 7, 44.
- Ghiboub, M.J.; Koster, P.D.; Craggs, A.Y.F.; Li Yim, A.; Shillings, S.; Hutchinson, R.P.; Bingham, K.; Gatfield, I.L.; Hageman, G.; Yao, G.; et al. Modulation of macrophage inflammatory function through selective inhibition of the epigenetic reader protein SP140. bioRxiv 2020.
- Clough, J.; Omer, O.S.; Tasker, S.; Lord, G.M.; Irving, P.M. Regulatory T-cell therapy in Crohn’s disease: Challenges and advances. Gut 2020, 69, 942–952.
- Sulz, M.C.; Burri, E.; Michetti, P.; Rogler, G.; Peyrin-Biroulet, L.; Seibold, F. Treatment Algorithms for Crohn’s Disease. Digestion 2020, 101, 43–57.
- Grover, Z.; Lewindon, P. Two-Year Outcomes After Exclusive Enteral Nutrition Induction Are Superior to Corticosteroids in Pediatric Crohn’s Disease Treated Early with Thiopurines. Dig. Dis. Sci. 2015, 60, 3069–3074.
- Connors, J.; Basseri, S.; Grant, A.; Giffin, N.; Mahdi, G.; Noble, A.; Rashid, M.; Otley, A.R.; Van Limbergen, J. Exclusive Enteral Nutrition Therapy in Paediatric Crohn’s Disease Results in Long-term Avoidance of Corticosteroids: Results of a Propensity-score Matched Cohort Analysis. J. Crohns Coliti 2017, 11, 1063–1070.
- Cohen-Dolev, N.; Sladek, M.; Hussey, S.; Turner, D.; Veres, G.; Koletzko, S.; de Carpi, J.M.; Staiano, A.; Shaoul, R.; Lionetti, P.; et al. Differences in Outcomes Over Time With Exclusive Enteral Nutrition Compared With Steroids in Children With Mild to Moderate Crohn’s Disease: Results From the GROWTH CD Study. J. Crohns Colitis 2018, 12, 306–312.
- Clooney, A.G.; Eckenberger, J.; Laserna-Mendieta, E.; Sexton, K.A.; Bernstein, M.T.; Vagianos, K.; Sargent, M.; Ryan, F.J.; Moran, C.; Sheehan, D.; et al. Ranking microbiome variance in inflammatory bowel disease: A large longitudinal intercontinental study. Gut 2020.
- Zuo, T.; Ng, S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 2247.
- Jones, C.M.A.; Connors, J.; Dunn, K.A.; Bielawski, J.P.; Comeau, A.M.; Langille, M.G.I.; Van Limbergen, J. Bacterial Taxa and Functions Are Predictive of Sustained Remission Following Exclusive Enteral Nutrition in Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2020, 26, 1026–1037.
- Lo, C.-H.; Lochhead, P.; Khalili, H.; Song, M.; Tabung, F.K.; Burke, K.E.; Richter, J.M.; Giovannucci, E.L.; Chan, A.T.; Ananthakrishnan, A.N. Dietary Inflammatory Potential and Risk of Crohn’s Disease and Ulcerative Colitis. Gastroenterology 2020, 159, 873–883.e1.
- Ananthakrishnan, A.N.; Luo, C.; Yajnik, V.; Khalili, H.; Garber, J.J.; Stevens, B.W.; Cleland, T.; Xavier, R.J. Gut Microbiome Function Predicts Response to Anti-integrin Biologic Therapy in Inflammatory Bowel Diseases. Cell Host Microbe 2017, 21, 603–610.e3.
- Effenberger, M.; Reider, S.; Waschina, S.; Bronowski, C.; Enrich, B.; Adolph, T.E.; Koch, R.; Moschen, A.R.; Rosenstiel, P.; Aden, K.; et al. Microbial Butyrate Synthesis Indicates Therapeutic Efficacy of Azathioprine in IBD Patients. J. Crohns Colitis 2020.
- Aden, K.; Rehman, A.; Waschina, S.; Pan, W.-H.; Walker, A.; Lucio, M.; Nunez, A.M.; Bharti, R.; Zimmerman, J.; Bethge, J.; et al. Metabolic Functions of Gut Microbes Associate With Efficacy of Tumor Necrosis Factor Antagonists in Patients With Inflammatory Bowel Diseases. Gastroenterology 2019, 157, 1279–1292.e11.
- Sprockett, D.; Fischer, N.; Boneh, R.S.; Turner, D.; Kierkus, J.; Sladek, M.; Escher, J.C.; Wine, E.; Yerushalmi, B.; Dias, J.A.; et al. Treatment-Specific Composition of the Gut Microbiota Is Associated With Disease Remission in a Pediatric Crohn’s Disease Cohort. Inflamm. Bowel Dis. 2019, 25, 1927–1938.
- Levine, A.; Kori, M.; Kierkus, J.; Boneh, R.S.; Sladek, M.; Escher, J.; Wine, E.; Yerushalmi, B.; Dias, J.A.; Shaoul, R.; et al. Azithromycin and metronidazole versus metronidazole-based therapy for the induction of remission in mild to moderate paediatric Crohn’s disease: A randomised controlled trial. Gut 2018, 68, 239–247.
- Ledder, O. Antibiotics in inflammatory bowel diseases: Do we know what we’re doing? Transl. Pediatr. 2019, 8, 42–55.
- Dewint, P.; Hansen, B.E.; Verhey, E.; Oldenburg, B.; Hommes, D.W.; Pierik, M.; Ponsioen, C.I.J.; Van Dullemen, H.M.; Russel, M.; Van Bodegraven, A.A.; et al. Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn’s disease: A randomised, double-blind, placebo controlled trial (ADAFI). Gut 2014, 63, 292–299.
- Rutgeerts, P. A critical assessment of new therapies in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2002, 17, S176–S185.
- Papadakis, K.A.; Shaye, O.A.; Vasiliauskas, E.A.; Ippoliti, A.; Dubinsky, M.C.; Loane, J.; Paavola, J.; Lee, S.K.; Price, J.; Targan, S.R.; et al. Safety and Efficacy of Adalimumab (D2E7) in Crohn’s Disease Patients with an Attenuated Response to Infliximab. Am. J. Gastroenterol. 2005, 100, 75–79.
- Feagan, B.G.; Greenberg, G.R.; Wild, G.; Fedorak, R.N.; Paré, P.; McDonald, J.W.D.; Cohen, A.; Bitton, A.; Baker, J.; Dubé, R.; et al. Treatment of Active Crohn’s Disease With MLN0002, a Humanized Antibody to the α4β7 Integrin. Clin. Gastroenterol. Hepatol. 2008, 6, 1370–1377.
- Sandborn, W.J.; Feagan, B.G.; Fedorak, R.N.; Scherl, E.; Fleisher, M.R.; Katz, S.; Johanns, J.; Blank, M.; Rutgeerts, P. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2008, 135, 1130–1141.
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124.
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838.
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738.
- Mokkala, K.; Houttu, N.; Cansev, T.; Laitinen, K. Interactions of dietary fat with the gut microbiota: Evaluation of mechanisms and metabolic consequences. Clin. Nutr. 2020, 39, 994–1018.
- Lo, C.-H.; Khalili, H.; Song, M.; Lochhead, P.; Burke, K.E.; Richter, J.M.; Giovannucci, E.L.; Chan, A.T.; Ananthakrishnan, A.N. Healthy Lifestyle Is Associated With Reduced Mortality in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 87–95.e4.
- Levine, A.; Rhodes, J.M.; Lindsay, J.O.; Abreu, M.T.; Kamm, M.A.; Gibson, P.R.; Gasche, C.; Silverberg, M.S.; Mahadevan, U.; Boneh, R.S.; et al. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1381–1392.
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e6.
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033.
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811.
- Daïen, C.I.; Pinget, G.V.; Tan, J.; Macia, L. Detrimental Impact of Microbiota-Accessible Carbohydrate-Deprived Diet on Gut and Immune Homeostasis: An Overview. Front. Immunol. 2017, 8, 548.
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Carbonnel, F. Animal Protein Intake and Risk of Inflammatory Bowel Disease: The E3N Prospective Study. Am. J. Gastroenterol. 2010, 105, 2195–2201.
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Zakostelska, Z.J.; Cermakova, M.; Sediva, B.; et al. Diet Rich in Animal Protein Promotes Pro-inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019, 10, 919.
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.H.; Hotte, N.; Madsen, K. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 2019, 9, 12294.
- Chassaing, B. Involvement of food additives in intestinal inflammation and metabolic syndrome in mice. Med. Sci. 2015, 31, 586–588.
- Roberts, C.L.; Rushworth, S.L.; Richman, E.; Rhodes, J.M. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J. Crohns Colitis 2013, 7, 338–341.
- Sandall, A.M.; Cox, S.R.; Lindsay, J.O.; Gewirtz, A.T.; Chassaing, B.; Rossi, M.; Whelan, K. Emulsifiers Impact Colonic Length in Mice and Emulsifier Restriction is Feasible in People with Crohn’s Disease. Nutrients 2020, 12, 2827.
- Viennois, E.; Bretin, A.; Dubé, P.E.; Maue, A.C.; Dauriat, C.J.; Barnich, N.; Gewirtz, A.T.; Chassaing, B. Dietary Emulsifiers Directly Impact Adherent-Invasive E. coli Gene Expression to Drive Chronic Intestinal Inflammation. Cell Rep. 2020, 33, 108229.
- Le Leu, R.K.; Young, G.P.; Hu, Y.; Winter, J.; Conlon, M.A. Dietary Red Meat Aggravates Dextran Sulfate Sodium-Induced Colitis in Mice Whereas Resistant Starch Attenuates Inflammation. Dig. Dis. Sci. 2013, 58, 3475–3482.
- Beattie, R.M.; Bentsen, B.S.; MacDonald, T.T. Childhood Crohn’s disease and the efficacy of enteral diets. Nutrition 1998, 14, 345–350.
- Fell, J.M.; Paintin, M.; Arnaud-Battandier, F.; Beattie, R.M.; Hollis, A.; Kitching, P.; Donnet-Hughes, A.; Macdonald, T.T.; Walker-Smith, J.A. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2000, 14, 281–289.
- Yamamoto, T.; Nakahigashi, M.; Umegae, S.; Kitagawa, T.; Matsumoto, K. Impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: Cytokine production and endoscopic and histological findings. Inflamm. Bowel. Dis. 2005, 11, 580–588.
- Schwerd, T.; Frivolt, K.; Clavel, T.; Lagkouvardos, I.; Katona, G.; Mayr, D.; Uhlig, H.H.; Haller, D.; Koletzko, S.; Bufler, P. Exclusive enteral nutrition in active pediatric Crohn disease: Effects on intestinal microbiota and immune regulation. J. Allergy Clin. Immunol. 2016, 138, 592–596.
- Sabino, J.; Lewis, J.D.; Colombel, J.-F. Treating Inflammatory Bowel Disease With Diet: A Taste Test. Gastroenterology 2019, 157, 295–297.
- Ashton, J.J.; Gavin, J.; Beattie, R.M. Exclusive enteral nutrition in Crohn’s disease: Evidence and practicalities. Clin. Nutr. 2019, 38, 80–89.
- Levine, A. Exclusive Enteral Nutrition: Clues to the Pathogenesis of Crohn’s Disease. Issues Complementary Feed. 2014, 79, 131–140.
- Escuro, A.A.; Hummell, A.C. Enteral Formulas in Nutrition Support Practice: Is There a Better Choice for Your Patient? Nutr. Clin. Pract. 2016, 31, 709–722.
- Erskine, J.M.; Lingard, C.D.; Sontag, M.K.; Accurso, F.J. Enteral nutrition for patients with cystic fibrosis: Comparison of a semi-elemental and nonelemental formula. J. Pediatr. 1998, 132, 265–269.
- Limketkai, B.N.; Shah, N.D.; Sheikh, G.N.; Allen, K. Classifying Enteral Nutrition: Tailored for Clinical Practice. Curr. Gastroenterol. Rep. 2019, 21, 47.
- Lochs, H.; Allison, S.; Meier, R.; Pirlich, M.; Kondrup, J.; Schneider, S.; Berghe, G.V.D.; Pichard, C. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, Definitions and General Topics. Clin. Nutr. 2006, 25, 180–186.
- Critch, J.; Day, A.S.; Otley, A.R.; King-Moore, C.; Teitelbaum, J.E.; Shashidhar, H. Use of Enteral Nutrition for the Control of Intestinal Inflammation in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 298–305.
- Van Limbergen, J.; Koot, B.G.P.; De Winter, J.P. Fool me once… treatment exposure to achieve remission in pediatric IBD. Eur. J. Nucl. Med. Mol. Imaging 2020, 179, 1921–1924.
- Heuschkel, R.B.; Menache, C.C.; Megerian, J.T.; Baird, A.E. Enteral Nutrition and Corticosteroids in the Treatment of Acute Crohn’s Disease in Children. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 8–15.
- Dziechciarz, P.; Horvath, A.; Shamir, R.; Szajewska, H. Meta-analysis: Enteral nutrition in active Crohn’s disease in children. Aliment. Pharmacol. Ther. 2007, 26, 795–806.
- Moriczi, M.; Pujol-Muncunill, G.; Martín-Masot, R.; Treviño, S.J.; Segarra Cantón, O.; Sangrador, C.O.; Quintana, L.P.; Santana, D.G.; Martínez, A.R.; Camps, A.R.; et al. Predictors of Response to Exclusive Enteral Nutrition in Newly Diagnosed Crohn’s Disease in Children: PRESENCE Study from SEGHNP. Nutrients 2020, 12, 1012.
- Pigneur, B.; Ruemmele, F.M. Nutritional interventions for the treatment of IBD: Current evidence and controversies. Ther. Adv. Gastroenterol. 2019, 12, 1756284819890534.
- Reinink, A.R.; Lee, T.C.; Higgins, P.D. Endoscopic Mucosal Healing Predicts Favorable Clinical Outcomes in Inflammatory Bowel Disease: A Meta-analysis. Inflamm. Bowel. Dis. 2016, 22, 1859–1869.
- Day, A.S.; Lopez, R.N. Exclusive enteral nutrition in children with Crohn’s disease. World J. Gastroenterol. 2015, 21, 6809–6816.
- Adamji, M.; Day, A.S. An overview of the role of exclusive enteral nutrition for complicated Crohn’s disease. Intest. Res. 2019, 17, 171–176.
- Logan, M.; Clark, C.M.; Ijaz, U.Z.; Gervais, L.; Duncan, H.; Garrick, V.; Curtis, L.; Buchanan, E.; Cardigan, T.; Armstrong, L.; et al. The reduction of faecal calprotectin during exclusive enteral nutrition is lost rapidly after food re-introduction. Aliment. Pharmacol. Ther. 2019, 50, 664–674.
- Yu, Y.; Chen, K.-C.; Chen, J. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: A meta-analysis. World J. Pediatr. 2019, 15, 26–36.
- Ludvigsson, J.F.; Krantz, M.; Bodin, L.; Stenhammar, L.; Lindquist, B. Elemental versus polymeric enteral nutrition in paediatric Crohn’s disease: A multicentre randomized controlled trial. Acta Paediatr. 2004, 93, 327–335.
- Rubio, A.; Pigneur, B.; Garnier-Lengliné, H.; Talbotec, C.; Schmitz, J.; Canioni, D.; Goulet, O.; Ruemmele, F.M. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment. Pharmacol. Ther. 2011, 33, 1332–1339.
- Wall, C.L.; Gearry, R.B.; Day, A.S. Polymeric formula is more palatable than elemental formula to adults with Crohn’s disease. e-SPEN J. 2014, 9, e200–e203.
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolaček, S.; et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708.
- Mehanna, H.; Moledina, J.; Travis, J. Refeeding syndrome: What it is, and how to prevent and treat it. BMJ 2008, 336, 1495–1498.
- Afzal, N.; Addai, S.; Fagbemi, A.; Murch, S.; Thomson, M.; Heuschkel, R. Refeeding syndrome with enteral nutrition in children: A case report, literature review and clinical guidelines. Clin. Nutr. 2002, 21, 515–520.
- Akobeng, A.K.; Thomas, A.G. Refeeding Syndrome Following Exclusive Enteral Nutritional Treatment in Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 364–366.
- Belli, D.; Seidman, E.; Bouthillier, L.; Weber, A.; Roy, C.; Pletincx, M.; Beaulieu, M.; Morin, C. Chronic intermittent elemental diet improves growth failure in children with Crohn’s disease. Gastroenterology 1988, 94, 603–610.
- Johnson, T.; Macdonald, S.; Hill, S.M.; Thomas, A.; Murphy, M.S. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: A randomised controlled trial. Gut 2006, 55, 356–361.
- Gupta, K.; Noble, A.; Kachelries, K.E.; Albenberg, L.; Kelsen, J.R.; Grossman, A.B.; Baldassano, R.N. A Novel Enteral Nutrition Protocol for the Treatment of Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2013, 19, 1374–1378.
- Lee, D.; Baldassano, R.N.; Otley, N.; Albenberg, L.; Griffiths, A.M.; Compher, C.; Chen, E.Z.; Li, H.; Gilroy, E.; Nessel, L.; et al. Comparative Effectiveness of Nutritional and Biological Therapy in North American Children with Active Crohn’s Disease. Inflamm. Bowel. Dis. 2015, 21, 1786–1793.
- Takagi, S.; Utsunomiya, K.; Kuriyama, S.; Yokoyama, H.; Takahashi, S.; Iwabuchi, M.; Kinouchi, Y.; Hiwatashi, N.; Funayama, Y.; Sasaki, I.; et al. Effectiveness of an ’half elemental diet’ as maintenance therapy for Crohn’s disease: A randomized-controlled trial. Aliment. Pharmacol. Ther. 2006, 24, 1333–1340.
- Yamamoto, T.; Nakahigashi, M.; Saniabadi, A.R.; Iwata, T.; Maruyama, Y.; Umegae, S.; Matsumoto, K. Impacts of long-term enteral nutrition on clinical and endoscopic disease activities and mucosal cytokines during remission in patients with Crohn’s disease: A prospective study. Inflamm. Bowel. Dis. 2007, 13, 1493–1501.
- Wilschanski, M.; Sherman, P.; Pencharz, P.; Davis, L.; Corey, M.; Griffiths, A. Supplementary enteral nutrition maintains remission in paediatric Crohn’s disease. Gut 1996, 38, 543–548.
- Schulman, J.M.; Pritzker, L.; Shaoul, R. Maintenance of Remission with Partial Enteral Nutrition Therapy in Pediatric Crohn’s Disease: A Retrospective Study. Can. J. Gastroenterol. Hepatol. 2017, 2017, 5873158.
- Gavin, J.; Marino, L.; Ashton, J.J.; Beattie, R.M. Patient, parent and professional perception of the use of maintenance enteral nutrition in Paediatric Crohn’s Disease. Acta Paediatr. 2018, 107, 2199–2206.
- Kang, Y.; Kim, S.; Kim, S.Y.; Koh, H. Effect of short-term partial enteral nutrition on the treatment of younger patients with severe Crohn’s disease. Gut Liver 2015, 9, 87–93.
- Hisamatsu, T.; Kunisaki, R.; Nakamura, S.; Tsujikawa, T.; Hirai, F.; Nakase, H.; Watanabe, K.; Yokoyama, K.; Nagahori, M.; Kanai, T.; et al. Effect of elemental diet combined with infliximab dose escalation in patients with Crohn’s disease with loss of response to infliximab: CERISIER trial. Intest. Res. 2018, 16, 494–498.
- Gkikas, K.; Gerasimidis, K.; Milling, S.; Ijaz, U.Z.; Hansen, R.; Russell, R.K. Dietary Strategies for Maintenance of Clinical Remission in Inflammatory Bowel Diseases: Are We There Yet? Nutrients 2020, 12, 2018.
- Urlep, D.; Benedik, E.; Brecelj, J.; Orel, R. Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn’s disease: Results of a prospective cohort study. Eur. J. Nucl. Med. Mol. Imaging 2019, 179, 431–438.
- Boneh, R.S.; Shabat, C.S.; Yanai, H.; Chermesh, I.; Ben-Avraham, S.; Boaz, M.; Levine, A. Dietary Therapy With the Crohn’s Disease Exclusion Diet is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. J. Crohns Colitis 2017, 11, 1205–1212.
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel. Dis. 2014, 20, 1353–1360.
- Levine, A.; El-Matary, W.; Van Limbergen, J. A Case-Based Approach to New Directions in Dietary Therapy of Crohn’s Disease: Food for Thought. Nutrients 2020, 12, 880.
- Yanai, H.; Levine, A.; Boneh, R.S.; Maharshak, N.; Kopylov, U.; Wardi, J.; Abramas, L.; Fliss-Isakov, N.; Gik, T.; Dotan, I.; et al. Crohn’s disease exclusion diet induces remission in adults with mild to moderate Crohn’s disease: Preliminary report from a randomized controlled trail (CDED-AD trail), in United European Gastroenterology week. United Eur. Gastroenterol. J. 2020, 8, 466–467.
- Ruemmele, F.M.; Veres, G.; Kolho, K.; Griffiths, A.; Levine, A.; Escher, J.; Dias, J.A.; Barabino, A.; Braegger, C.; Bronsky, J.; et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohns Colitis 2014, 8, 1179–1207.
- Boneh, R.S.; Van Limbergen, J.; Wine, E.; Assa, A.; Shaoul, R.; Milman, P.; Cohen, S.; Kori, M.; Peleg, S.; On, A.; et al. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients With Active Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2020.
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392.
- Turner, D.; Griffiths, A.M.; Wilson, D.; Mould, D.R.; Baldassano, R.N.; Russell, R.K.; Dubinsky, M.; Heyman, M.B.; De Ridder, L.; Hyams, J.; et al. Designing clinical trials in paediatric inflammatory bowel diseases: A PIBDnet commentary. Gut 2020, 69, 32–41.
- Nahidi, L.; Day, A.S.; Lemberg, D.A.; Leach, S.T. Paediatric Inflammatory Bowel Disease: A Mechanistic Approach to Investigate Exclusive Enteral Nutrition Treatment. Scientifica 2014, 2014, 423817.
- El-Matary, W. Enteral Nutrition as a Primary Therapy of Crohn’s Disease: The Pediatric Perspective. Nutr. Clin. Pr. 2009, 24, 91–97.




