| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Barry V L Potter | + 1746 word(s) | 1746 | 2021-02-07 11:49:26 | | | |
| 2 | Barry V L Potter | Meta information modification | 1746 | 2021-02-09 14:59:10 | | |
Video Upload Options
Irosustat: (6-oxo-8,9,10,11-tetrahydro-7H-cyclohepta[c]chromen-3-yl) sulfamate; 6-oxo-8,9,10,11-tetrahydro-7H-cyclohepta-(c)(1)benzopyran-3-O-sulfamate; also known as STX64, 667 Coumate, BN83495, Oristusane is a tricyclic synthetic clinical drug of the aryl sulfamate ester class, designed mainly for applications in oncology as a steroid sulfatase inhibitor and has shown clinical benefit in patients.
1. The new concept
Irosustat [1] is an orally active, irreversible, nonsteroidal inhibitor of steroid sulfatase (STS) and member of the aryl sulfamate ester class of drugs [2] that is under development for the treatment of hormone-sensitive cancers such those of the breast, endometrium and prostate [1-3]. STS transforms hormonally inactive steroid sulfates into their active forms, Thus, hydrolysis of estrone sulfate into estrone is effected that can then be transformed into the more potent estradiol [4]. STS Inhibition is also the basis for the clinical development of an aryl sulfamate drug in endometriosis.
2. The synthesis process
The parent tricyclic coumarin 3-hydroxy-6-oxo-8,9,10,11-tetrahydro-7H-cyclohepta-[c]][1]benzopyran is prepared by a Pechmann reaction from the ketoester methyl 2-oxocycloheptane carboxylate with resorcinol in the presence of trifluoroacetic acid and conc. sulphuric acid as the condensing agent, added at a rate to keep the temperature below 10°C. After quenching with ice-water the precipitate is collected, washed with water, dissolved in acetone, the solution treated with activated charcoal, filtered and evaporated to give a residue that is recrystallised from acetone/hexane. To the coumarin in anhydrous DMF at 0°C under nitrogen is added sodium hydride followed by sulfamoyl chloride. The reaction mixture is stirred under nitrogen, poured into water and after extraction with ethyl acetate the organic portion washed with brine, dried, filtered and evaporated in vacuo. The crude product is recrystallized from ethyl acetate/hexane to give crystalline Irosustat. [4,5]
3. The structure
Irosustat was spectroscopically characterized using standard methods and was studied by single molecule X-ray crystallography [5].
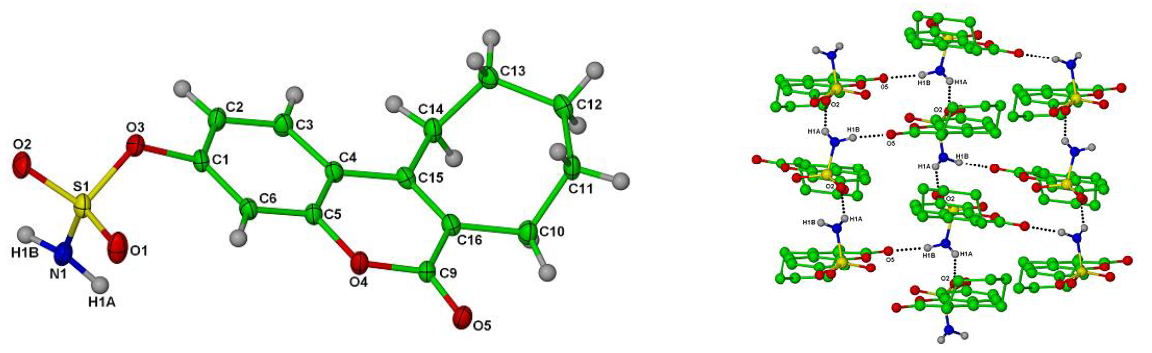
Single crystal X-ray structures of Irosustat showing the tricyclic nature of the drug, the sulfamate ester moiety crucial for inhibitory activity and overall crystal packing (CCDC codes: 826525 and 826525 respectively); reproduced with permission from [5].
4. The activities and bioactivities
Irosustat and aryl sulfamate esters are thought to modify irreversibly the active site formylglycine residue of STS [3]. By inhibiting STS, Irosustat prevents the conversion of hormonally inactive steroid sulfates such as DHEA sulfate (DHEAS) and estrone sulfate (E1S) into their respective active forms, DHEA and estrone which, in turn, can be transformed into more potent androgens and estrogens, respectively) [6]. Administration of 5 mg/day Irosustat to women with breast cancer for 5 days inhibited STS activity by up to 99% in breast tumour tissue and significantly decreased serum levels of estrone, estradiol, DHEA, androstenediol, androstenedione and testosterone, whereas levels of DHEA-S and E1S increased slightly [7]. Stable disease was also observed in patients. Despite Irosustat being quickly degraded in plasma ex vivo, this is prevented in vivo by its sequestration almost completely inside red blood cells after oral administration, being bound to carbonic anhydrase II (CA II), thus avoiding first pass metabolism [8]. The X-ray crystal structure of the drug bound to CAII has been determined [9].
5. The applications
Results of the "first-in-class" clinical trial in breast cancer of an STS inhibitor in humans were published in 2006 [7] and dose optimisation studies and further clinical data for this indication were reported [10]. The drug has reached phase II clinical trials in women with hormone-dependent breast cancer and with endometrial cancer and has also reached trials in men with prostate cancer. Development as a monotherapy for endometrial cancer in women with advanced/metastatic or recurrent estrogen-receptor positive endometrial cancer was halted after a analysis of trial data [11]. There were no statistically significant differences between Irosustat and the current standard of care the progestin megestrol acetate in response and survival rates. Results nevertheless showed clinical activity and a good safety profile for the drug, with 36% of patients on Irosustat alive without progression at 6 months; 11% showed responses and there was more stable disease noted (47%) compared to the current therapy. Irosustat also reached a phase I trial in the US for prostate cancer, being safe and well tolerated in male patients with castration-resistant prostate cancer and ongoing androgen deprivation therapy [12]. Irosustat effected nearly complete STS inhibition at three doses, showing pharmacodynamic proof of concept and with all patients there was notable suppression of endocrine parameters.[11] Clinical trials overseen by Cancer Research UK were designed to explore activity in early breast cancer (IPET trial) [13] and also in combination with an aromatase inhibitor (AI) (IRIS trial) [14]. The open-label phase II IRIS clinical trial explored the value of adding an STS inhibitor in addition to a first-line AI in patients with advanced breast cancer in postmenopausal women with ER+ locally advanced or metastatic breast cancer who had benefited from a first-line AI but were subsequently progressing. The trial reached its formal endpoints and the drug showed clinical benefit in patients. Irosustat was also assessed for the first time in ER+ early breast cancer through the pre-surgical window-of-opportunity IPET trial, a study in postmenopausal women with untreated early disease. Data from the latter trial showed clinical benefit, albeit in a small patient population, and were the first to demonstrate activity of Irosustat in early breast cancer. The results of both trials were published in 2017 and underpin further the scientific concept in favour of clinical STS inhibition. Larger studies are now required. Further clinical development continues and the current status was recently reviewed in 2018 [3].
6. The innovation
Dual novelty is demonstrated. The STS enzyme as a drug target is both an innovative approach to treating hormone-dependent diseases, driven from academia, and also aryl sulfamate esters such as Irosustat represent “first-in-class” drugs of a novel structure. The initial trial in breast cancer published in 2006 [7] was the first evaluation of an unsubstituted aryl sulfamate, Irosustat (then STX64), in human cancer patients. The overall approach has generated almost 20 clinical trials in multiple pathologies in both men and women, and STS inhibition has shown itself to be widely applicable in oncology and elsewhere and development of this drug class continues.
7. Stories behind this compound
The drug was first designed and synthesized in the group of Professor Barry V L Potter at the Department of Pharmacy & Pharmacology, University of Bath (now at Oxford University), working together with Professor Michael J Reed at Imperial College, London [2-4]. Initial development of aryl sulfamate-based drugs was undertaken through the university spin-out company Sterix Ltd and was overseen by Cancer Research UK. In 2004 Sterix Ltd was acquired by Ipsen and Irosustat continued in development through formal academic-industry partnerships by Ipsen with the University of Bath and Imperial College. The group first designed the potent steroidal STS inhibitors estrone and estradiol 3-O-sulfamate (E2MATE) [2] that was first studied in six phase I/II clinical trials as a pro-drug for hormone replacement therapy and oral contraception. STS inhibition was also found to be a potential new therapy for other estrogen-dependent conditions like endometriosis [15] and, following this, E2MATE was repurposed for this indication [16]. E2MATE was subsequently found to inhibit endometrial STS activity by 91% in a phase I trial [16]. In premenopausal women circulating levels of estradiol are not affected, indicating that E2MATE may have tissue-selective antiestrogenic effects in the endometrium. E2MATE has been in phase II clinical trials for endometriosis in Eastern Europe and results are awaited.
References
- Palmieri, C.; Januszewski, A.; Stanway. ; Coombes, R.C. Irosustat: a first-generation steroid sulfatase inhibitor in breast cancer. Expert Rev. Anticancer Ther. 2011, 11, 179-183.
- Thomas, M.P.; Potter, B.V.L. Discovery and Development of the Aryl O-Sulfamate Pharmacophore for Oncology and Women's Health. J. Med. Chem. 2015, 58(19), 7634–7658.
- Potter, B.V.L. SULFATION PATHWAYS: Steroid Sulfatase Inhibition by Aryl Sulfamates: Clinical Progress, Mechanism, and Future Prospects. J Mol Endocrinol. 2018, 61, T233–T252.
- Woo, L.W.L.; Purohit, A.; Malini, B.; Reed, M.J.; Potter, B.V.L. Potent active site-directed inhibition of steroid sulphatase by tricyclic coumarin-based sulphamates. Chemistry & Biology 2000, 7 (10), 773–791.
- Woo, L. W. L.; Ganeshapillai. D.; Thomas, M.P.; Sutcliffe, O. B.; Malini, B.; Mahon, M. F.; Purohit, A.; Potter, B. V. L. Structure-Activity Relationship of the Clinical Steroid Sulfatase Inhibitor Irosustat (STX64, BN83495). ChemMedChem. 2011, 6, 2019–2034.
- Reed, M.J.; Purohit, A.; Woo, L.W.L.; Newman, S.P.; Potter, B.V.L. Steroid sulfatase: molecular biology, regulation, and inhibition. Rev. 2005, 26, 171-202.
- Stanway, S.; Purohit, A.; Woo, L.W.L.; Sufi S; Vigushin, D.; Ward, R.; Wilson, R.; Stanczyk, F.Z.; Dobbs, N.; Kulinskaya, E.; Elliott, M.; Potter, B.V.L. Reed, M.J.; Coombes. R. C. Phase I study of STX64 (667 Coumate) in breast cancer patients: the first study of a steroid sulphatase inhibitor. Clin. Cancer Res. 2006, 12, 1585–1592.
- Ireson, C.R.; Chander, S.K.; Purohit, A.; Parish, D.C.; Woo, L.W.L.; Potter, B.V.L.; Reed M.J. Pharmacokinetics of the nonsteroidal steroid sulphatase inhibitor 667 COUMATE and its sequestration into red blood cells in rats. Brit. J. Cancer 2004, 91, 1399–1404.
- Lloyd, M.D.; Pederick R.L.; Natesh, R.; Woo, L.W.L.; Purohit A.; Reed M.J.; Acharya K.R.; Potter, B.V.L. Crystal structure of human carbonic anhydrase II at 1.95 Å resolution in complex with 667-Coumate, a novel anti-cancer agent. Biochem. J. 2005 385, 715–720.
- Coombes, R.C.; Cardoso, F.; Isambert, N. ; Lesimple, T.; Soulié, P.; Peraire, C. ; Fohanno, V.; Kornowski, A. ; Ali, T. ; Schmid, P. phase I dose escalation study to determine the optimal biological dose of irosustat, an oral steroid sulfatase inhibitor, in postmenopausal women with estrogen receptor-positive breast cancer. Breast Cancer Res. Treat. 2013,140, 73–82.
- Pautier, P.; Vergote, I.; Joly, F.; Melichar, B.; Kutarska, E.; Hall, G.; Lisyanskaya, A.; Reed, N.; Oaknin, A.; Ostapenko, V. et al. A phase 2, randomized, open-label study of Irosustat versus megestrol acetate in advanced endometrial cancer. Int. J. Cancer. 2017, 27, 258–266.
- Denmeade, S.; George D.; Liu G.; Peraire C.; Geniaux A.; Baton F.; Ali T.; Chetaille A. phase I pharmacodynamics dose escalation study of steroid sulphatase inhibitor Irosustat in patients with prostate cancer. Eur. J. Cancer 2011, 47, 2011 S499.
- Palmieri, C.; Szydlo, R.; Miller, M.; Barker, L.; Patel, N.H.; Sasano, H.; Barwick, T.; Tam, H.; Hadjiminas, D.; Lee J. et al. IPET study: an FLT-PET window study to assess the activity of the steroid sulfatase inhibitor irosustat in early breast cancer. Breast Cancer Res. Treat. 2017 166, 527–539.
- Palmieri, C.; Stein, R.C.; Liu, X.; Hudson, E.; Nicholas, H.; Sasano, H.; Guestini, F.; Holcombe, C.; Barrett, S.; Kenny, L, et al. IRIS study: a phase II study of the steroid sulfatase inhibitor Irosustat when added to an aromatase inhibitor in ER-positive breast cancer patients. Breast Cancer Res. Treat. 2017, 165, 343–353.
- Purohit, A.; Fusi, L.; Brosens, J.; Woo, L.W.L.; Potter, B.V.L.; Reed, M.J. Inhibition of steroid sulphatase activity in endometriotic implants by 667 COUMATE: a potential new therapy. Reprod. 2008, 23, 290-297.
- Pohl, O.; Bestel, E.; Gotteland, J.-P. Synergistic effects of E2MATE and norethindrone acetate on sulfatase inhibition: a randomized phase I proof-of-principle clinical study in women of reproductive age. Reprod. Sci. 2014, 21, 1256-1265.