| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lyes BENNAMOUN | + 1527 word(s) | 1527 | 2021-02-02 04:30:03 | | | |
| 2 | Bruce Ren | -21 word(s) | 1506 | 2021-02-09 02:38:06 | | |
Video Upload Options
The application of thermal storage materials in solar systems involves materials that utilize sensible heat energy, thermochemical reactions, or phase change materials, such as hydrated salts, fat-ty acids paraffin, and non-paraffin like glycerol.
1. Introduction
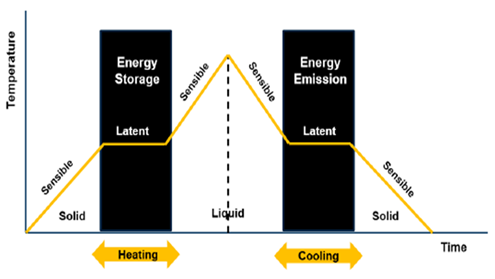
A major challenge encountered by the application of solar heating systems is the continuity of their operation when there is no sunshine, i.e., during off-sunshine periods [1][2][3]. In response, researchers have either adopted supplementary heating systems using electric heaters or biomass furnaces [4] or have integrated thermal storage materials [4]. The application of thermal storage materials involves materials (rocks, bricks, water, concretes, etc.) that utilize their sensible heat energy, thermochemical reactions, and those that apply both sensible and latent heat energy (phase change materials) such as hydrated salts, fatty acid paraffin and non-paraffins like glycerol, etc. [5][6]. The advantage of phase change materials (PCM) is that they evolve large energy density over a narrow temperature range as the material changes from liquid to solid or vice versa [4] as shown in Figure 1. Kenesarin and Mahkamov [5] and Sharma and Sagara [7] have comprehensively reviewed available PCMs and their applications as thermal storage in the solar energy system and their application as thermal storage materials in energy storage systems in housing, spacecraft, vehicles, space heating, desalination, solar dryers, water heating, etc. In their discussion, they classified the PCMs as organic materials, mainly present as fatty acids with melting temperatures of −5 to 71 °C and inorganic materials present mostly as hydrated salts. They also analyzed the structural compatibility of the PCM chemical composition and concluded that research on inorganic PCMs needed time for commercial application. This is because PCMs, especially hydrated salts, suffer from the problem of supercooling (“phase irreversibility”) and phase segregation in the thermal process [8]. This has been observed for sodium sulfate decahydrate (Na2SO4·10H2O) when used in solar drying [9], though researchers have suggested adding nucleating agents, thickening agents, direct contact heat transfer, encapsulation, rotating storage, and rotary storage devices [8] to address this issue.
Figure 1. Phase change transition of PCM [10].
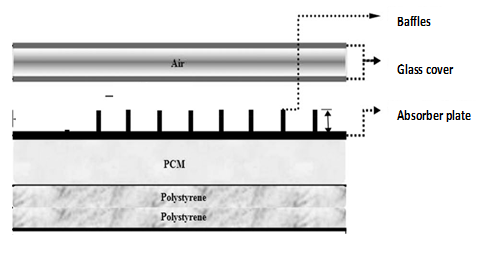
Despite this shortcoming, the large energy density carrier of PCMs has made them attractive in solar heating systems while researchers are working to overcome the problems associated with hydrated salts [11]. However, the performance of solar systems adopting PCMs as thermal storage can only be assessed if the thermal behavior of the PCM while in the system is known. Several studies dealing with this aspect have considered different configurations and positions of the PCM in the system. This thermal behavior of the PCM has been established in relationship with its position within the system. For example, Ndukwu et al. placed the PCM on the solar collector chamber during charging and moved it into the drying chamber during discharging in a solar dryer, while Ortega and Carmona [12] and Ghiami et al. [13] placed their PCMs under the collector as shown in Figure 2. Nkwetta, and Haghighat [14] encapsulated the PCM in the solar system while Li et al. [15] placed the PCM in a storage tank. Abdulmunen et al. [16] embedded the solar fin into a PCM in a solar collector.
Recent reviews in the literature have also presented the thermal behavior of PCM in solar heaters in terms of their thermo-physical properties, sensible and latent heat energy [17], thermal storage abilities [18][19], application in buildings [20], application in solar absorption refrigeration systems [21], integration of nano-fluids [22][23][24], progress in thermal storage and recovery of industrial waste heat [25] and application in cold storage [26][27]. However, to properly understand the thermodynamic performance of the system units, energy and exergy analysis approach has always been adopted. Many researchers are of the opinion that exergy analysis provides a better understanding of the thermodynamic performance of energy systems and it is the future of research if the efficiency of energy systems is to be improved. Jegadheeswaran et al. [28] have emphasized the need of exergy studies based on the analysis and continuous update on exergy evaluation techniques for different system applications. The analysis was based on the configuration of the heat flow, the position or method of integration of the latent heat storage material in the system and the heat carrying fluids. Elucidating exergy analysis methods is vital in improving the operation of the thermodynamic systems and designing energy systems to obtain higher efficiency. Therefore, this study is intended to present a review of the exergy based thermodynamic analysis for some solar system configurations that have adopted PCMs as thermal storage. This is to present a guide for further studies on this area.
2. Thermodynamic Performance of Energy Systems
The evaluation of the thermodynamic performance of energy systems can be performed using the energy conservation principle which is based on the first law of thermodynamics or the concept of exergy based on the second law [29]. Exergy is the maximum extractable amount of work from a thermodynamic system as it equilibrates with the surroundings [30][31]. Obtaining higher energy output from a thermodynamic system makes the operation attractive. During energy flow, losses occurring within the system components which affects the total energy output from these systems. The magnitude of these losses varies from one component to another and at different points along the energy streams. Accounting for these losses based on individual components as embodied in the concept of exergy helps to know the components that requires improvement for better optimization and design of the energy systems, which the first law does not give room for. In a properly insulated solar system integrated with a PCM, the energy analysis during the PCM cycle will give 100% efficiency because the latent heat of solidification and melting which is equal to the output and input energy is the same. However, there is irreversibility which will lead to energy destruction. Exergy does not only identify irreversibility (therefore cannot be conserved) but also where it occurs and the degree of this irreversibility. This will lower the output exergy which is why the exergy efficiency is lower than energy efficiency since both is expressed as a ratio of output to input of each adopted principle. The more exergy is destroyed within the system, the more entropy is generated, which indicates the unavailability of thermal energy for conversion into mechanical work. Consequently, exergy is proportional to the produced entropy, as the system approaches equilibrium with the surroundings, which decreases the efficiency of the system to zero.
Figure 2. PCM embedded below the collector (Source: Ghiami et al. ).
Most importantly, exergy is related to the concept of environmental sustainability. Minimizing system losses (increased exergy efficiency) will limit energy waste into the surroundings. Ndukwu et al. have reported that exergy waste might upstage the thermodynamic equilibrium of the environment by interfering with the presence of atmospheric CO2, which can lead to solar re-radiation. Thus, exergy analysis evaluates energy both quantitatively and qualitatively [32][33]. In the analysis of the exergy of most solar heating systems with or without sensible heat, three exergy streams have always been identified, viz: exergy of the solar radiation, exergy of the heat transfer fluid and exergy exchange within the product of which the stream is applied to in the case of drying as shown in Equations (1)–(3) below [34][35]:
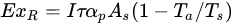 |
(1) |
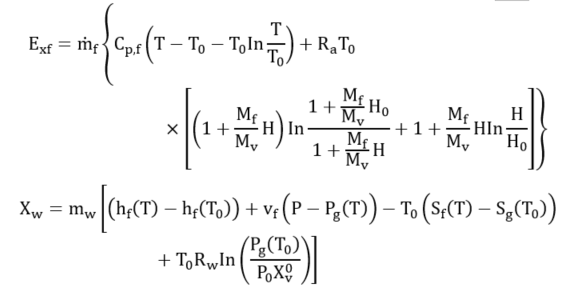 |
(2)
(3) |
|
|
However, when a PCM is applied in a solar heating system, the exergy analysis includes the phase change exergy. In most exergy analysis of solar systems involving the solar collector (receiver) and PCM storage, researchers have separated the two components and analyzed their respective exergy streams. Pertaining to the exergy analysis for PCMs factors such as thermal storage, mass ratio, melting (charging) temperature, discharging (solidification) temperature, mass flow rate and the number of transfer units are taken into consideration, assuming that the PCM is a reservoir of thermal energy with constant melting temperature while the temperature of flowing air varies along with its flow path [32]. This is because the process of charging and discharging of a PCM involves the receiver transferring the high temperature into the heat transfer fluid which was at low temperature. This temperature mix raises the temperature of the heat transfer fluid (HTF), partly absorbed by the PCM. The PCM melts at its melting temperature. As the HTF exhausts and returns-back to the receiver or the ambient depending on the system design to complete the charging cycle, the PCM cools by releasing the latent heat of fusion. Hence, the exergy analysis is evaluated during both the charging and discharging periods. The assessment of thermodynamic performance of any system integrated with PCM requires the operational configuration of the system, including the trajectory of the HTF, point of heat contact of the PCM, geometric and other design parameters of the system. Exergy efficiency is carried out to determine the maximum recoverable energy from the system since entropy generation destroys efficiency of the system. Nevertheless, adoption of the PCM though helps the functionality of the system, however, it generates entropy (Equations (4) and (5)) in the heat storage process during the charging and discharging of the PCM and also due to HTF flow across the heat exchanger. Li et al. expressed the entropy generated for PCM as follows:
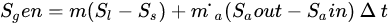 |
(4) |
El-Dessouky and Faisal [36] gave the entropy of PCM during charging as:
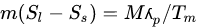 |
(5) |
References
- Simo-Tagne, M.; Ndukwu, M.C.; Zoulalian, A.; Bennamoun, L.; Kifani-Sahban, F.; Rogaume, Y. Numerical analysis and val-idation of a natural convection mix-mode solar dryer for drying red chili under variable conditions. Renew. Energy 2020, 151, 659–673.
- Ndukwu, M.C.; Bennamoun, L.; Abam, F.I. Experience of solar drying in Africa: Presentation of designs, operations and models. Food Eng. Rev. 2018, 10, 211–244.
- Ndukwu, M.C.; Onyenwigwe, D.; Abam, F.I.; Eke, A.B.; Dirioha, C. Development of a low-cost wind-powered active solar dryer integrated with glycerol as thermal storage. Renew. Energy 2020, 154, 553–568.
- Ndukwu, M.C.; Bennamoun, L.; Abam, F.I.; Eke, A.B.; Ukoha, D. Energy and exergy analysis of a solar dryer integrated with sodium sulfate decahydrate and sodium chloride as thermal storage medium. Renew. Energy 2017, 113, 1182–1192.
- Kenisarin, M.; Mahkamov, K. Solar energy storage using phase change materials. Renew. Sustain. Energy Rev. 2007, 11, 1913–1965.
- Oliver, A.; Neila, F.J.; García-Santos, A. PCM choosing and classification according to their characteristics for their applica-tion for thermal energy storage systems. Mater. Constr. 2010, 62, 131–140.
- Sharma, S.D.; Sagara, K. Latent heat storage materials and systems: A review. Int. J. Green Energy 2005, 2, 1–56.
- Hasnain, S.M. Review on sustainable thermal energy technologies, Part II: Cool 6 thermal storage. Energy Conv. Manag. 1998, 39, 1139–1153.
- Ndukwu, M.C.; Bennamoun, L. Potential of integrating Na2SO4 • 10H2O pellets in solar drying system. Dry Technol. 2018, 36, 1017–1030.
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273.
- Marliacy, P.; Solimando, R.; Bouroukba, M.; Schuffenecker, L. Thermodynamics of crystallization of sodium sulphate deca-hydrate in H2O±NaCl±Na2SO4: Application to Na2SO4.10H2O-based latent heat storage materials, Thermochim. Acta. 2000, 344, 85–94.
- Ortega, A.R.; Carmona, M. Exergy analysis of a flat plate solar collector with latent heat storage by phase change material for water heating applications at low temperature. Contemporary Urban. Aff. 2017, 1, 43–48.
- Ghiami, A.; Kianifar, A.; Aryana, K.; Edalatpour, M. Energy and Exergy Analysis of a Single-Pass Sequenced Array Baffled Solar Air Heater with Packed Bed Latent Storage Unit for Nocturnal Use; Heat Transfer—Asian Research, 2017, 46, 546-568..
- Nkwetta, D.N.; Haghighat, F. Thermal energy storage with phase change material- A state of the art review. Sustain. Cities Soc. 2014, 10, 87–100.
- Li, Y.Q.; He, Y.; Wang, Z.; Xu, C.; Wanga, W. Exergy analysis of two phases change materials storage system for solar ther-mal power with finite-time thermodynamics. Renew. Energy 2012, 39, 447–454.
- Abdulmunem, R.A.; Jaba, M.H.; Samin, P.M.; Rahman, H.A.; Hussien, H.A. Analysis of Energy and Exergy for the Flat Plate Solar Air Collector with Longitudinal Fins Embedded in Paraffin Wax Located in Baghdad Center. Int. J. Heat. Technol. 2019, 37, 1180–1186.
- Hasnain, S.M. Review on sustainable thermal energy storage technologies. Part I: Heat storage materials and techniques. En-ergy Conver Manag. 1988, 39, 1127–1138.
- Zalba, B.; Marin, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283.
- Kalogirou, S.A.; Karellas, S.; Badescu, V.; Braimakis, K. Exergy analysis on solar thermal systems: A better understanding of their sustainability. Renew. Energy 2016, 85, 1328–1333.
- Mehling, H.; Cabeza, L.F. Heat and cold storage with PCM, 1st ed.; Berlin Springer Publication Corporation: Berlin, Germany, 2008..
- Mahinian, O.; Kianifar, A.; Kalogirou, S.A.; Pop, I.; Wongwises, S. A review of the applications of nanofluids in solar energy. Int. J. Heat. Mass. Trans. 2013, 57, 582–594.
- Bozorgan, N.; Shafahi, M. Performance evaluation of nanofluids in solar energy: A review of the recent literature. Micro. Nano Syst. Lett. 2015, 3, 1–15.
- Liu, L.; Su, D.; Tang, Y.; Fang, G. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2016, 62, 305–317.
- Jarimi, H.; Aydin, D.; Yanan, Z.; Ozankaya, G.; Chen, X.; Riffat, S. Review on the recent progress of thermochemical materi-als and processes for solar thermal energy storage and industrial waste heat recovery. Int. J. Low-Carbon Technol. 2019, 14, 44–69.
- Oró, E. Review on phase change materials (PCMs) for cold thermal energy storageApplications. Appl. Energy 2012, 99, 513–533.
- Saito, A. Recent advances in research on cold thermal energy storage. Int. J. Refrig. 2002, 25, 177–189.
- Zhai, X.Q.; Wang, X.L.; Wang, T.; Wang, R.Z. A review on phase change cold storage in air-conditioning system: Materials and applications. Renew. Sustain. Energy Rev. 2013, 22, 108–120.
- Jegadheeswaran, S.; Pohekar, S.D.; Kousksou, T. Exergy based performance evaluation of latent heat thermal storage system: A review. Renew. Sustain. Energy Rev. 2010, 14, 2580–2595.
- Ranjan, K.R.; Kaushik, S.C.; Panwar, N.L. Energy and exergy analysis of passive solar distillation systems. Int. J. Low-Carbon Technol. Adv. Access 2013, 11, 211–221.
- Kianifar, A.; Heris, S.Z.; Mahian, O. Exergy and economic analysis of a pyramid-shaped solar water purification system: Ac-tive and passive cases. Energy 2012, 38, 31–36.
- Kurtbas, I.; Durmus, A. Efficiency and exergy analysis of a new solar air heater. Renew. Energ. 2004, 29, 1489–1501.
- Domański, R.; Fellah, G. Exergy as A Tool for Designing and Operating Thermal Storage Units; Biuletyn Instytutu Techniki Cieplnej Politechniki Warszawskiej: Warsaw, Poland, 1995.
- Aghbaslou, F.; Badia, F.; Illa, J. Exergetic optimization of solar collector and thermal energy storage system. Int. J. Heat. Mass. Trans. 2006, 49, 1255–1263.
- Hatami, S.; Payehaneh, G.; Mehrpanahi, A. Energy and exergy analysis of an indirect solar dryer based on a dynamic model. J. Clean. Prod. 2020, 244, 118809.
- Ndukwu, M.C.M.; Simo-Tagne, F.I.; Abam, O.S.; Onwuka, S.; Prince, L. Bennamoun. Exergy, environmental and economic analysis of hybrid solar-biomass dryer integrated with copper tubing as heat exchanger. Heliyon 2020, 6, e03401.