
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giacinto Triolo | + 1598 word(s) | 1598 | 2021-01-26 08:38:32 | | | |
| 2 | Vicky Zhou | Meta information modification | 1598 | 2021-02-05 11:02:01 | | |
Video Upload Options
Anterior-segment optical-coherence tomography (AS-OCT) is used to assess the iridocorneal angle and its alterations. The introduction of AS-OCT has led to improved assessments of the anatomy of the iridocorneal-angle and diagnoses of several mechanisms of angle closure which often result in raised intraocular pressure (IOP). Continuous advancements in AS-OCT technology and software, along with an extensive research in the field, have resulted in a wide range of possible parameters that may be used to diagnose and follow up on patients with this spectrum of diseases. However, the clinical relevance of such variables needs to be explored thoroughly.
1. Introduction
Although gonioscopy is currently the gold standard for clinical assessments and diagnoses of angle closure, being also characterized by the unique advantage of allowing a dynamic evaluation of the iridocorneal angle, it is an observer-dependent technique and, thus, is subject to intrinsic intra- and inter-individual variability. Furthermore, it may be challenging and uncomfortable for the patient, as it includes physical contact between the gonioscopic lens and the corneal surface. To overcome these limitations, anterior-segment optical-coherence tomography (AS-OCT) has recently emerged as an objective and noninvasive method to assess the anterior chamber and iridocorneal-angle anatomy.
However, there is no agreement with regard to which parameters extrapolated from the AS-OCT scan are of clinical relevance, nor has it been established whether AS-OCT is comparable to gonioscopy in detecting and monitoring iris and iridocorneal-angle anomalies [1][2][3].
2. Clinical Applications of AS-OCT
2.1. AS-OCT-Based Diagnosis of Primary-Angle Closure
A smaller ACW, ACA, and ACV, greater iris thickness (IT) and iris area (IArea), and increased LV have been associated with angle closure [4][5][6][7]. Nongipur et al. [2] performed gonioscopy and AS-OCT in 1368 subjects, including 295 (21.6%) who showed gonioscopic angle closure. They found that PAC patients were older, had a smaller ACW, ACA, ACV, a greater LV, and thicker irises compared to patients with open angles (p < 0.01 for all). According to these findings, they proposed a classification algorithm based on stepwise logistic regression that used a combination of these six AS-OCT parameters, obtained from a single horizontal scan, able to identify subjects with a gonioscopic PAC >95% of the time. Similarly, Winegarner et al. found that the ACD, ACV, and ACA showed a high diagnostic performance in detecting PAC-disease patients with areas under the receiver-operating-characteristic curve of 0.98, 0.97, and 0.93, respectively [8].
The results from the Chinese-American Eye Study showed that the mean intraocular pressure (IOP) increased as AS-OCT parameters, such as AOD, decreased, and that IOP and AS-OCT findings were inversely correlated in angle-closure patients but not in open-angle ones [9].
Kwon et al. [10] assessed long-term changes occurring to ACD, LV, and AOD in patients with angle closure due to different mechanisms, such as pupillary block (PB), plateau-iris configuration (PIC), thick peripheral iris roll (TPIR), and exaggerated lens vault (ELV). Their study population was followed up for 41 to 54 months, with an average follow-up length of four years. They found that the baseline ACD was shallowest in ELV, followed by PB, TPIR, and PIC, in that order. The PIC group showed significantly wider AOD than the other groups at the baseline. The ACD decreased and the LV increased over time in all groups, especially in the PIC group.
2.2. AS-OCT in Plateau-Iris Configuration
In the plateau-iris configuration (PIC), the iris root points anteriorly on the ciliary body or on the ciliary body itself are displaced more anteriorly than normal, critically narrowing the anterior-chamber recess by displacing the peripheral iris forward (Figure 1).
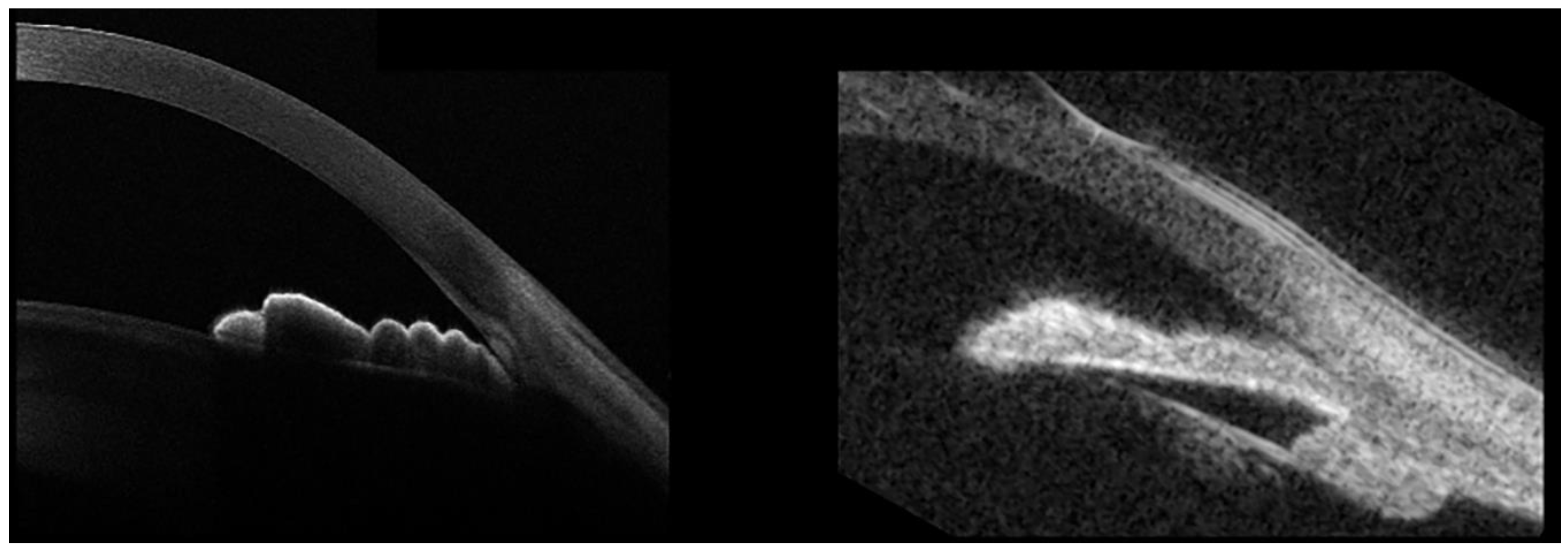
PIC is often associated with temporary, appositional angle closure and subsequent raised IOP, eventually leading to glaucoma. It is important to recognize PIC, because it may require additional treatment to the laser-peripheral iridotomy (LPI) usually performed in primary-angle-closure eyes, meaning argon-laser peripheral iridoplasty (ALPI), although the efficacy of this procedure remains debated. Although the diagnosis of PIC is mainly gonioscopic, this technique can be particularly tricky in evaluating the iris root position. In these cases, AS-OCT may offer an objective, noninvasive, and easy tool with which to achieve an accurate diagnosis. While AS-OCT is certainly helpful to evaluate AC and angle details with high-image resolution, it does not allow us to identify an anteriorly-displaced ciliary-body position due to its limited capacity of deep penetration (Figure 1, left panel). Conversely, ultrasound biomicroscopy (UBM) is characterized by poor definition in evaluating the AC and angle-anatomical findings, but it allows good visualization of the ciliary-body position (Figure 1, right). Thus, AS-OCT and UBM provide complimentary information in the evaluation of patients with PIC.
Based on AS-OCT findings, Verma et al. reported that roughly one-third showed incidents of plateau-iris configuration among a group of patients with primary-angle-closure glaucoma [11], suggesting the importance of this imaging technique to evaluate alternative mechanisms to pupillary block in patients with narrow angles. They also identified the iris thickness at 750 microns from the scleral spur (IT750) as the only variable significantly associated with plateau iris.
Interestingly, Crowell et al. reported that lens and pupil parameters evaluated on AS-OCT scans (such as pupil arc, lens vault, and pupil diameter) showed the greatest diagnostic power to discriminate between pupillary block and PIC mechanisms [12].
Although a component of pure pupillary block is often present in patients with PIC, the effects of LPI alone are, at best, controversial. Leaung et al. were among the first in 2005 to report a case of a PAC patient not responding to LPI, although their AS-OCT scans showed PIC [13]. On this patient, ALPI was performed, which proved effective in widening the iridocorneal angles and reducing the IOP.
More recently, Kwon J et al. confirmed that LPI was ineffective in widening the iridocorneal angle in this group of patients [10]. Ramakrishnan et al. reported the effect of ALPI on eyes with plateau-iris configuration, on which LPI had already been revealed to be unsuccessful [14]. They found that on eyes with peripheral anterior synechiae, laser-peripheral iridoplasty can be very effective, not only in lowering IOP and number of IOP-lowering medications, but also in increasing AOD500, TISA500, and the scleral spur angle.
2.3. AS-OCT Changes after Laser-Peripheral Iridotomy and Cataract Extraction
In a prospective, observational, randomized, controlled trial of 176 PACS eyes undergoing LPI, this procedure resulted in an increased AOD, TISA, ARA, ACA, and ACV, but not in an increased ACW, ACD, or LV [15]. Similarly, in a prospective interventional case series of 66 PACS eyes, Esfandiari et al. reported that the AOD, ARA, and TISA at 500 mm all increased by 48% to 73% (all p < 0.001) after LPI. Conversely, the LV and iris volume did not change significantly [16].
The IMPACT Study, which is a longitudinal, prospective, double-randomized research study, showed similar findings on 39 PAC/PACS eyes [17]. Kwon et al. reported a significant AOD increase in PB and TIPR patients but not in PIC and ELV patients [10]. Moghimi et al. evaluated the effects of LPI on eyes with acute primary-angle closure (APAC) and their fellow eyes [18]. LPI resulted in angle widening with a significant increase in AOD in both eyes with APAC and fellow eyes. Central ACD and ACA significantly increased, and LV decreased, in eyes with APAC, but not in their fellow eyes.
Although relatively short-term changes to AS-OCT parameters are well established after LPI, the same is not the case with long-term changes. For instance, Lee et al. noticed that the AOD, TISA, and ARA significantly increased within two weeks following LPI, but had returned to their pretreatment values at the 18-month follow-up [19]. This finding basically confirmed the previous evidence from the Zhongshan Angle-Closure Prevention Trial, evaluating long-term changes to the iridocorneal angle after LPI in primary-angle-closure suspects [20]. In this randomized, controlled trial, the angle configuration was evaluated using gonioscopy, as well as angle-opening distance at 250, 500, and 750 microns from the scleral spur (AOD250, AOD500, AOD750, respectively), trabecular iris-space area (TISA500, TISA750), and angle-recess area (ARA) measured on AS-OCT images. A significant increase in all AS-OCT parameters was reported two weeks after LPI. Nevertheless, between 2 weeks and 18 months after LPI, a significant decrease in angle width was observed in treated eyes, although this finding was not statistically significant in the first six months. In untreated eyes, the angle width consistently decreased across all follow-up visits after LPI, with a more rapid longitudinal decrease compared to treated eyes.
Yan C et al. used AS-OCT to compare the effects of LPI vs. lens extraction on the anterior-chamber anatomy in PACS eyes [21]. They found that the ACD increased and the LV decreased significantly in the lens-extraction group, but not in the LPI group. The effects of lens extraction on the anatomy of the anterior chamber have already been reported. Huang G et al. showed that ACD and AOD500 increased after cataract surgery, both in patients with preoperatively narrow and open angles, and found a high correlation with IOP-lowering in the former group [22]. Similar findings were confirmed later [23][24], while Nolan et al. reported an increase in the mean AOD500 (88.2%) and TISA750 (94.4%) after cataract extraction in primary-angle-closure glaucoma eyes [25]. More recently, Lee et al. demonstrated that ARA500 and TISA500 increased significantly in healthy eyes and normal-tension glaucoma eyes, with the changes in these parameters being linearly and inversely correlated with postoperative IOP changes [26].
References
- Nongpiur, M.E.; Sakata, L.M.; Friedman, D.S.; He, M.; Chan, Y.-H.; Lavanya, R.; Wong, T.Y.; Aung, T. Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology 2010, 117, 1967–1973.
- Nongpiur, M.E.; Haaland, B.A.; Friedman, D.S.; Perera, S.A.; He, M.; Foo, L.-L.; Baskaran, M.; Sakata, L.M.; Wong, T.Y.; Aung, T. Classification Algorithms Based on Anterior Segment Optical Coherence Tomography Measurements for Detection of Angle Closure. Ophthalmology 2013, 120, 48–54.
- Lavanya, R.; Foster, P.J.; Sakata, L.M.; Friedman, D.S.; Kashiwagi, K.; Wong, T.-Y.; Aung, H.T.; Alfred, T.; Gao, H.; Ee, A.G.; et al. Screening for Narrow Angles in the Singapore Population: Evaluation of New Noncontact Screening Methods. Ophthalmology 2008, 115, 1720–1727.e2.
- Nemeth, G.; Vajas, A.; Kolozsvari, B.; Berta, A.; Modis, L., Jr. Anterior chamber depth measurements in phakic and pseudophakic eyes: Pentacam versus ul-trasound device. J. Cataract. Refract. Surg. 2006, 32, 1331–1335.
- Narayanaswamy, A.; Sakata, L.M.; He, M.; Friedman, D.S.; Chan, Y.-H.; Lavanya, R.; Baskaran, M.; Foster, P.J.; Aung, T. Diagnostic Performance of Anterior Chamber Angle Measurements for Detecting Eyes With Narrow Angles. Arch. Ophthalmol. 2010, 128, 1321.
- Wang, B.; Sakata, L.M.; Friedman, D.S.; Chan, Y.-H.; He, M.; Lavanya, R.; Wong, T.-Y.; Aung, T. Quantitative Iris Parameters and Association with Narrow Angles. Ophthalmology 2010, 117, 11–17.
- Wang, B.-S.; Narayanaswamy, A.; Amerasinghe, N.; Zheng, C.; He, M.; Chan, Y.-H.; Nongpiur, M.E.; Friedman, D.S.; Aung, T. Increased iris thickness and association with primary angle closure glaucoma. Br. J. Ophthalmol. 2010, 95, 46–50.
- Winegarner, A.; Miki, A.; Kumoi, M.; Ishida, Y.; Wakabayashi, T.; Sakimoto, S.; Usui, S.; Matsushita, K.; Nishida, K. Anterior segment Scheimpflug imaging for detecting primary angle closure disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 257, 161–167.
- Xu, B.Y.; Burkemper, B.; Lewinger, J.P.; Jiang, X.; Pardeshi, A.A.; Richter, G.; Torres, M.; McKean-Cowdin, R.; Varma, R. Correlation between Intraocular Pressure and Angle Configuration Measured by OCT. Ophthalmol. Glaucoma 2018, 1, 158–166.
- Kwon, J.; Sung, K.R.; Han, S. Long-term Changes in Anterior Segment Characteristics of Eyes with Different Primary Angle-Closure Mechanisms. Am. J. Ophthalmol. 2018, 191, 54–63.
- Verma, S.; Nongpiur, M.E.; Oo, H.H.; Atalay, E.; Goh, D.; Wong, T.T.; A Perera, S.; Aung, T. Plateau Iris Distribution Across Anterior Segment Optical Coherence Tomography Defined Subgroups of Subjects With Primary Angle Closure Glaucoma. Investig. Opthalmol. Vis. Sci. 2017, 58, 5093–5097.
- Crowell, E.L.; Chuang, A.Z.; Bell, N.P.; Blieden, L.S.; Feldman, R.M. Using Anterior Segment Optical Coherence Tomography (ASOCT) Parameters to Determine Pupillary Block versus Plateau Iris Configuration. J. Glaucoma 2020.
- Leung, C.K.-S.; Chan, W.; Ko, C.; Chui, S.; Woo, J.; Tsang, M.; Tse, R. Visualization of Anterior Chamber Angle Dynamics Using Optical Coherence Tomography. Ophthalmology 2005, 112, 980–984.
- Ramakrishnan, R.; Mitra, A.; Kader, M.A.; Das, S. To Study the Efficacy of Laser Peripheral Iridoplasty in the Treatment of Eyes With Primary Angle Closure and Plateau Iris Syndrome, Unresponsive to Laser Peripheral Iridotomy, Using Anterior-Segment OCT as a Tool. J. Glaucoma 2016, 25, 440–446.
- How, A.C.; Baskaran, M.; Kumar, R.S.; He, M.; Foster, P.J.; Lavanya, R.; Wong, H.-T.; Chew, P.T.; Friedman, D.S.; Aung, T. Changes in Anterior Segment Morphology after Laser Peripheral Iridotomy: An Anterior Segment Optical Coherence Tomography Study. Ophthalmology 2012, 119, 1383–1387.
- Esfandiari, H.; Pakravan, M.; Amouhashemi, N.; Yaseri, M.; Torkian, P.; Jadidi, K.; Loewen, N.A. Low iris and anterior chamber volume is associated with deepening after laser peripheral iridotomy in primary angle closure suspects. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 2173–2179.
- Zhekov, I.; Pardhan, S.; Bourne, R.R. Ocular coherence tomography-measured changes over time in anterior chamber angle and diurnal intraocular pressure after laser iridotomy: IMPACT study. Clin. Exp. Ophthalmol. 2018, 46, 895–902.
- Moghimi, S.; Bijani, F.; Chen, R.I.; Yasseri, M.; He, M.; Lin, S.C.; Weinreb, R.N. Anterior Segment Dimensions Following Laser Iridotomy in Acute Primary Angle Closure and Fellow Eyes. Am. J. Ophthalmol. 2017, 186, 59–68.
- Lee, K.S.; Sung, K.R.; Shon, K.; Sun, J.H.; Lee, J.R. Longitudinal Changes in Anterior Segment Parameters After Laser Peripheral Iridotomy Assessed by Anterior Segment Optical Coherence Tomography. Investig. Opthalmol. Vis. Sci. 2013, 54, 3166–3170.
- Jiang, Y.; Chang, D.S.; Zhu, H.; Khawaja, A.P.; Aung, T.; Huang, S.; Chen, Q.; Muñoz, B.; Grossi, C.M.; He, M.; et al. Longitudinal Changes of Angle Configuration in Primary Angle-Closure Suspects. Ophthalmology 2014, 121, 1699–1705.
- Yan, C.; Han, Y.; Yu, Y.; Wang, W.; Lyu, D.; Tang, Y.; Yao, K. Effects of lens extraction versus laser peripheral iridotomy on anterior segment morphology in primary angle closure suspect. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1473–1480.
- Huang, G.; Gonzalez, E.; Peng, P.-H.; Lee, R.; Leeungurasatien, T.; He, M.; Porco, T.; Lin, S. Anterior Chamber Depth, Iridocorneal Angle Width, and Intraocular Pressure Changes After Phacoemulsification. Arch. Ophthalmol. 2011, 129, 1283–1290.
- Pereira, F.A.S.; Cronemberger, S. Ultrasound biomicroscopic study of anterior segment changes after phacoemulsification and foldable intraocular lens implantation. Ophthalmology 2003, 110, 1799–1806.
- Nonaka, A.; Kondo, T.; Kikuchi, M.; Yamashiro, K.; Fujihara, M.; Iwawaki, T.; Yamamoto, K.; Kurimoto, Y. Angle Widening and Alteration of Ciliary Process Configuration after Cataract Surgery for Primary Angle Closure. Ophthalmology 2006, 113, 437–441.
- Nolan, W.P.; See, J.L.; Aung, T.; Friedman, D.S.; Chan, Y.-H.; Smith, S.D.; Zheng, C.; Huang, D.; Foster, P.J.; Chew, P.T.K. Changes in Angle Configuration After Phacoemulsification Measured by Anterior Segment Optical Coherence Tomography. J. Glaucoma 2008, 17, 455–459.
- Lee, W.; Bae, H.W.; Kim, C.Y.; Seong, G.J. The change of anterior segment parameters after cataract surgery in normal-tension glaucoma. Int. J. Ophthalmol. 2017, 10, 1239–1245.




