
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jia Xian Law | + 2120 word(s) | 2120 | 2021-02-04 05:18:41 | | | |
| 2 | Jia Xian Law | + 180 word(s) | 2300 | 2021-02-05 02:49:35 | | | | |
| 3 | Jia Xian Law | + 318 word(s) | 2438 | 2021-02-05 02:59:03 | | | | |
| 4 | Rita Xu | -587 word(s) | 1851 | 2021-02-05 05:14:34 | | |
Video Upload Options
Chondrocytes are the cells found in cartilage that responsible for the production and maintenance of the cartilaginous matrix, which consists of collagen, proteoglycans, glycoproteins and hyaluronan. Autologous chondrocyte implantation (ACI) is a new treatment modality with the potential to promote regeneration of worn cartilage. ACI has been used to treat osteoarthritis (OA), a degenerative joint disease that affects many people worldwide. Human platelet lysate (HPL) has been identified as a potential replacement for foetal bovine serum (FBS) for the expansion of chondrocytes.
1. Introduction
Having to go through daily chores and activities can be very stressful when the joints involved in daily locomotion cannot move as smoothly as it should and causing pain. This situation occurs more often with age due to imbalance between cartilage resorption and production as well as accumulation of injury and damage to the cartilage that serves as a cushion between two bones. The prevalence of symptomatic knee osteoarthritis (OA) among people aged 60 years and above is approximately 10–15% in the United States with women more prone to it compared to men [1]. This percentage is expected to further escalate in future due to the ageing population, the increasing number of people active in extreme sports and the obesity epidemic.
Several treatment modalities are available with the current knowledge and technology for the management of OA. Treatment for OA can be divided into non-pharmacological, pharmacological and invasive interventions [2]. Non-pharmacological treatments include education, controlled exercise, and lifestyle changes, while the pharmacological treatments include paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), topical analgesics, and hyaluronic injection. On the other hand, invasive interventions include osteochondral transplantation, microfracture, microdrilling, total knee replacement, and autologous chondrocyte implantation (ACI) [3]. In practice, treatment of OA starts with the less invasive options before proceeding to more invasive therapies.
ACI is a relatively new therapy introduced to repair the damaged knee cartilage. ACI is a two-stage operative procedure which involves the biopsy of cartilage tissue for chondrocyte expansion in vitro, followed by transplantation of the expanded cells to the affected joints under the periosteum or a synthetic graft [4][5]. ACI can reduce joint pain and promote cartilage regeneration. The implanted chondrocytes are expected to secrete the cartilage extracellular matrix (ECM) to repair the defect [6].
Human platelet lysate (HPL) has been identified as a potential replacement for foetal bovine serum (FBS) for the expansion of chondrocytes. HPL is more suitable for the clinical expansion of chondrocytes in the current good manufacturing practice (cGMP) facility as it is safer with no risk of animal pathogen transmission and carryover of animal protein that may elicit immune response [7]. Importantly, HPL is economical and will not increase the production cost. Similar to FBS, HPL is rich in biological factors that are essential in maintaining chondrocyte survival and proliferation in vitro [8].
2. Cartilage Anatomy and Physiology
Cartilage is a specialized elastic connective tissue wrapping the bony articular surface. Cartilage supplies a smooth, lubricated surface for articulation and to enable the conveyance of weights with a minimum frictional coefficient [9]. Together with synovial fluid, cartilage supports the frictionless motion of the joint. Cartilage tissue is hypocellular, aneural, alymphatic, and avascular [10]. Cartilage is composed of chondrocytes which are responsible for the production, organization, and maintenance of the cartilage ECM principally consists of water and macromolecules, including collagens, proteoglycans, and noncollagenous proteins [11]. Chondrocytes are called chondroblasts before they surround themselves with the matrix. Chondrocytes arise from mesenchymal stem cells and make about 2% of the total volume of articular cartilage [9]. As nutrition supply and waste removal of cartilage tissue are dependent on diffusion, which is a slow process, chondrocytes have a low metabolic rate and heal slowly after injury [12].
Chondrocytes at different zones of the cartilage vary in number, shape and size. In the superficial zone, the chondrocytes appear to be flatter and smaller and generally have a greater density compared to the cells deeper in the matrix. Each chondrocyte provides a unique microenvironment and is accounted for the turnover of the ECM in its immediate vicinity. This microenvironment confines the chondrocyte within its own matrix and prevents migration of the cells to adjacent sites of cartilage. Chondrocytes can sense the changes in ECM structure and react by modulating the matrix anabolism and catabolism and remodelling as the cells substitute the matrix macromolecules lost through degradation [9].
Cartilage is categorized into hyaline cartilage, elastic cartilage and fibrocartilage based on its ECM composition [13]. Generally, hyaline cartilage is the one that is found in joints and helps to ease joint movement by minimizing the friction.
3. Osteoarthritis
OA is a common yet treatable joint disorder. Development of OA is multifactorial as it can be due to age, obesity, gender, knee injury, over and repetitive use of the knee, muscle weakness and joint laxity [1]. The risk of OA has been reported to increase in elderly, women and people who are obese, physically inactive and with history of knee trauma [14][15]. In addition, OA is also related to genetic and occupational risk factors [16]. Individual with abnormal joint anatomy, joint instability, inadequate muscle strength or disturbance of joint or muscle innervation also have an increased risk of OA [17].
OA can manifest with joint pain when used while stiffness is felt at rest. Restricted range of motion due to pain will greatly reduce the patient’s quality of life [18]. In a normal adult, cartilage degeneration in OA happened in two phases, i.e., biosynthetic phase and degradative phase [19][20]. In biosynthetic phase, a variety of anabolic cytokines and growth factors such as bone morphogenic proteins (BMPs), insulin-like growth factor-1 (IGF-1) and transforming growth factor-β (TGF-β) stimulate the chondrocytes to synthesis ECM. In the degradative phase, enzymes like matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motif’ (ADAMTS) in the presence of inflammatory cytokines digest the ECM and inhibit its synthesis. Physiologically, there exists a strict regulation of matrix synthesis that leads to a balance between these two phases. However, in OA, the presence of inflammatory cytokines increases the synthesis of proteolytic enzymes, decreases the matrix metalloproteinases inhibitors and reduces the matrix synthesis, causing an imbalance in the two phases. In other words, OA is defined by the loss of cartilage tissue through degradation of collagen type II (Col II) and proteoglycan components in the ECM [21].
4. Autologous Chondrocyte Implantation
Throughout the years, many techniques, including microfracture, microdrilling, abrasion chondroplasty, and debridement, have been developed to promote cartilage regeneration [3]. Disappointingly, treatment with these techniques resulted in the formation of fibrocartilage with poorer mechanical properties compared to original hyaline cartilage. Thus, ACI was introduced to promote hyaline cartilage regeneration.
ACI is a technique used to repair the damaged cartilage through the implantation of chondrocytes. The procedure involves the collection of cartilage tissue for chondrocyte isolation and expansion in vitro before implanting the cells back to the chondral defect [22]. In the first generation, the implanted chondrocytes are covered with a periosteal flap which is sutured to the surrounding cartilage tissue. The use of periosteal flap has several issues, including the fragile nature of the tissue that renders it difficult to handle during the surgery and periosteal hypertrophy that leads to postoperative failure. Very soon thereafter, the second-generation ACI which uses the collagenous scaffold to replace periosteal flap was introduced. The third generation, also known as matrix-associated ACI (MACI), involved the suspension of chondrocytes within a hydrogel scaffold or chondrocytes seeded on a scaffold. In addition, the chondrocytes also can be cultured as a spheroid to stimulate self-secretion of ECM prior transplantation. The application of MACI eliminates the need for a native or synthetic periosteal patch and allows easier control of cell distribution on the defect. More importantly, MACI can be used to treat more severe osteochondral defect [6][23].
Key limitations of ACI that have yet to be resolved are the dedifferentiation of expanded chondrocytes and the long culturing period of 2–3 months to fetch the number of cells needed. It is well known that chondrocytes expanded in vitro will undergo dedifferentiation which reduces the cells’ capacity to regenerate hyaline cartilage. Figure 1 shows the factors that affect chondrocyte dedifferentiation and redifferentiation in vitro. The characteristic of chondrocytes changed in the presence of different serum supplements.
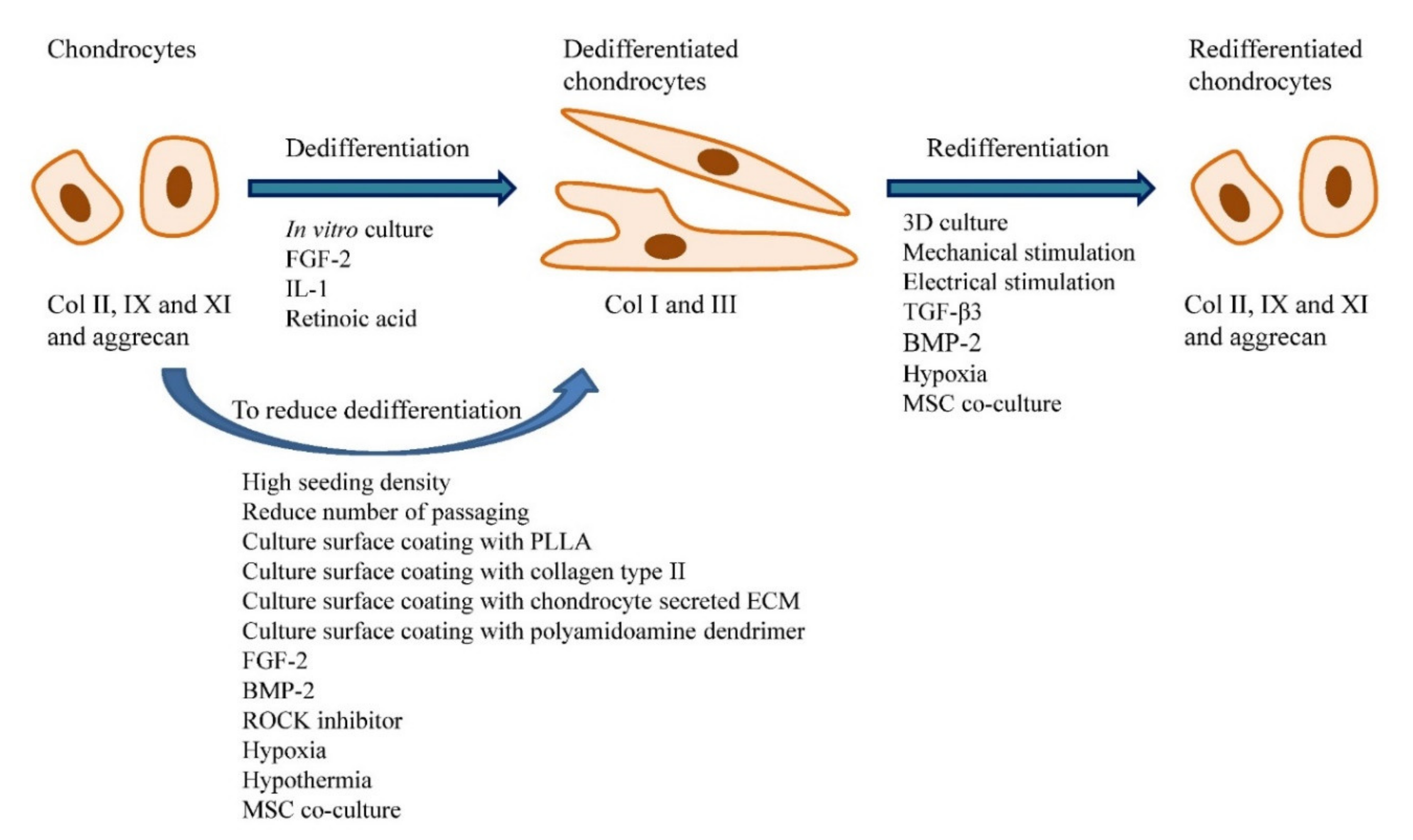
Figure 1. Factors that affect chondrocyte dedifferentiation and redifferentiation in vitro. Chondrocytes cultured in vitro will become dedifferentiated and the process is accelerated in the presence of FGF-2, IL-1 and retinoic acid. Strategies such as seeding the cells at high density, reduce the frequency of passaging, culture surface coating with specific substrates (i.e., PLLA, collagen type II, chondrocyte secreted ECM and polyamidoamine dendrimer), the addition of specific chemicals (i.e., FGF-2, BMP-2 and ROCK inhibitor), cell culture at low oxygen tension and low temperature, and co-culture with MSCs can reduce chondrocytes dedifferentiation. The dedifferentiated chondrocytes can be redifferentiated through 3D cell culture, application of the mechanical and electrical stimulus, the addition of certain chemicals (i.e., TGF-β1 and BMP-2), hypoxic culture and MSC co-culture.
Traditionally, foetal bovine serum (FBS) is used to expand the chondrocytes. However, the use of FBS is not ideal for the expansion of cells mean for clinical applications as it possesses the risk of animal pathogen transmission and animal protein transfer to host. Human platelet lysate (HPL) appears to be a suitable alternative to FBS as it is rich in biological factors that enhance cell proliferation. Table 1 lists the pros and cons of using FBS, HPL and defined medium for in vitro cell expansion. Thus far, HPL has been found to be superior in promoting chondrocyte proliferation compared to FBS. However, both HPL and FBS cannot prevent chondrocyte dedifferentiation. Discrepant results have been reported for the maintenance of chondrocyte redifferentiation potential by HPL. These differences are likely due to the diversity in the HPL preparation methods. In the future, more studies on HPL need to be performed to develop a standardized technique which is capable of producing HPL that can maintain the chondrocyte redifferentiation potential reproducibly.
Table 1. Pros and cons of FBS, HPL and defined medium in cell culture.
| FBS | HPL | Defined Medium | |
|---|---|---|---|
| Pros | Abundant and easily available | Easy to produce | Components are well-defined |
| Cheaper compared to HPL and chemically defined medium | A universal growth supplement that is suitable for most human and animal cells | No batch-to-batch variation | |
| A universal growth supplement that is suitable for most human and animal cells | Contains most of the factors required for cell survival and proliferation | No risk of disease transmission | |
| Contains most of the factors required for cell survival and proliferation | No risk of xenogeneic immune reaction | No risk of xenogeneic/allogeneic protein contamination | |
| Used by most of the studies and publications | Can be prepared using autologous blood | No unintended interaction with test substances | |
| Cons | Components are ill-defined | Components are ill-defined | Not available for certain cells |
| Batch-to-batch variation | Batch-to-batch variation (reduced by pooling) | Some defined medium required extra coating to promote cell attachment | |
| A potential source of animal microbial contaminants | Unintended interaction with test substances | More expansive compared to FBS and HPL | |
| Risk of animal protein contamination on cells prepared for clinical usage | Fewer vendors distribute the product | Time consuming and difficult to develop | |
| Unintended interaction with test substances | Potential eliciting allogeneic immune response | ||
| Ethical concerns with animal welfare | Risk of transmitting human viruses |
References
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Geriatr. Med. 2010, 26, 355–369.
- Yusuf, E. Pharmacologic and Non-Pharmacologic Treatment of Osteoarthritis. Treat. Opt. Rheumatol. 2016, 2, 111–125.
- Ogura, T.; Merkely, G.; Bryant, T.; Winalski, C.S.; Minas, T. Autologous Chondrocyte Implantation “Segmental-Sandwich” Technique for Deep Osteochondral Defects in the Knee: Clinical Outcomes and Correlation with Magnetic Resonance Imaging Findings. J. Sports Med. 2019, 7, 2325967119847173.
- Haleem, A.M.; Chu, C.R. Advances in Tissue Engineering Techniques for Articular Cartilage Repair. Tech. Orthop. 2010, 20, 76–89.
- Batty, L.; Dance, S.; Bajaj, S.; Cole, B.J. Autologous Chondrocyte Implantation: An Overview of Technique and Outcomes. ANZ J. Surg. 2011, 81, 18–25.
- Davies, R.L.; Kuiper, N.J. Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering 2019, 6, 22.
- Bieback, K. Platelet Lysate as Replacement for Fetal Bovine Serum in Mesenchymal Stromal Cell Cultures. Med. Hemother. 2013, 40, 326–335.
- Sykes, J.G.; Kuiper, J.; Richardson, J.B.; Wright, K.T.; Kuiper, N.J. Impact of Human Platelet Lysate on the Expansion and Chondrogenic Capacity of Cultured Human Chondrocytes for Cartilage Cell Therapy. Cartil. 2018, 26, S103.
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468.
- Karuppal, R. Current Concepts in the Articular Cartilage Repair and Regeneration. Orthop. 2017, 14, A1–A3.
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The Minor Collagens in Articular Cartilage. Protein Cell 2017, 8, 560–572.
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular Cartilage: From Formation to Tissue Engineering. Sci. 2016, 4, 734–767.
- Shen, S.; Chen, M.; Guo, W.; Li, H.; Li, X.; Huang, S.; Luo, X.; Wang, Z.; Wen, Y.; Yuan, Z.; et al. Three Dimensional Printing-Based Strategies for Functional Cartilage Regeneration. Tissue Eng. Part B Rev. 2019, 25, 187–201.
- Van Tunen, J.A.C.; Peat, G.; Bricca, A.; Larsen, L.B.; Søndergaard, J.; Thilsing, T.; Roos, E.M.; Thorlund, J.B. Association of Osteoarthritis Risk Factors with Knee and Hip Pain in a Population-Based Sample of 29–59 Year Olds in Denmark: A Cross-Sectional Analysis. BMC Musculoskelet. Disord. 2018, 19, 300.
- Poulsen, E.; Goncalves, G.H.; Bricca, A.; Roos, E.M.; Thorlund, J.B.; Juhl, C.B. Knee Osteoarthritis Risk Is Increased 4-6 Fold after Knee Injury—A Systematic Review and Meta-Analysis. J. Sports Med. 2019, 53, 1454–1463.
- Yucesoy, B.; Charles, L.E.; Baker, B.; Burchfiel, C.M. Occupational and Genetic Risk Factors for Osteoarthritis: A Review. Work 2015, 50, 261–273.
- Buckwalter, J.A.; Mankin, H.J. Articular Cartilage: Degeneration and Osteoarthritis, Repair, Regeneration, and Transplantation. Course Lect. 1998, 47, 487–504.
- Lespasio, M.J.; Piuzzi, N.S.; Husni, M.E.; Muschler, G.F.; Guarino, A.; Mont, M.A. Knee Osteoarthritis: A Primer. J. 2017, 21, 16–183.
- Sandell, L.J.; Aigner, T. Articular Cartilage and Changes in Arthritis: Cell Biology of Osteoarthritis. Arthritis Res. Ther. 2001, 3, 107.
- Wu, C.; Tian, B.O.; Qu, X.; Liu, F.; Tang, T.; Qin, A.N.; Zhu, Z.; Dai, K. MicroRNAs Play a Role in Chondrogenesis and Osteoarthritis. J. Mol. Med. 2014, 34, 13–23.
- Shi, Y.; Hu, X.; Cheng, J.; Zhang, X.; Zhao, F.; Shi, W.; Ren, B.; Yu, H.; Yang, P.; Li, Z.; et al. A Small Molecule Promotes Cartilage Extracellular Matrix Generation and Inhibits Osteoarthritis Development. Commun. 2019, 10, 1914.
- Niemeyer, P.; Albrecht, D.; Andereya, S.; Angele, P.; Ateschrang, A.; Aurich, M.; Baumann, M.; Bosch, U.; Erggelet, C.; Fickert, S.; et al. Autologous Chondrocyte Implantation (ACI) for Cartilage Defects of the Knee: A Guideline by the Working Group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee 2016, 23, 426–435.
- Hoburg, A.; Löer, I.; Körsmeier, K.; Siebold, R.; Niemeyer, P.; Fickert, S.; Ruhnau, K. Matrix-Associated Autologous Chondrocyte Implantation Is an Effective Treatment at Midterm Follow-up in Adolescents and Young Adults. J. Sports Med. 2019, 7, 2325967119841077.




