
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angel Raya | + 4491 word(s) | 4491 | 2021-02-04 04:14:45 | | | |
| 2 | Conner Chen | -1 word(s) | 4490 | 2021-02-22 08:13:12 | | | | |
| 3 | Conner Chen | Meta information modification | 4490 | 2021-02-23 09:51:08 | | | | |
| 4 | Conner Chen | Meta information modification | 4490 | 2021-03-30 07:02:05 | | |
Video Upload Options
Cardiac tissue engineering is very much in a current focus of regenerative medicine research as it represents a promising strategy for cardiac disease modelling, cardiotoxicity testing and cardiovascular repair. Advances in this field over the last two decades have enabled the generation of human engineered cardiac tissue constructs with progressively increased functional capabilities. Numerous studies have demonstrated that the therapeutic benefits exerted by cells are mainly attributable to the release of complementary paracrine factors and the efficacy is limited as only a small percentage of transplanted cells engrafted in the infarcted tissue. Studies on animal models showed that combining cell therapy with tissue engineering techniques for the creation of cell sheets and patches, can increase stem cell survival and boost therapeutic action. Therefore, tissue engineering has been considered as a potential approach for cardiac regeneration after MI.
1. Cell Sheets
The cell sheet technique was first reported by Shimizu et al. in 2002 for creating a transplantable 3D cell patch [1]. Cell sheet technology, also referred to as scaffold-free system or “Cell Sheet Engineering” [1][2][3], is based on stacking monolayers (or sheets) of CMs cultured to confluency to form 3D tissue-like structures. By using cell culture surfaces coated with the temperature-responsive polymer poly(N-isopropylacrylamide) (PIPAAm), it is possible to readily detach intact cellular monolayers of CMs as cell sheets by lowering the temperature, without any enzymatic treatments. Overlaying these thin 2D monolayers then results in 3D cardiac constructs [4]. The benefits of this system have been analyzed in vivo in murine animal models of MI showing improvements in cell survival, cardiac function and tissue remodeling [5]. The use of cell sheets created new opportunities for in vitro tissue engineering and helped exploring new therapies and drugs for heart diseases [6][7]. Interestingly, Sekine et al. produced in vitro vascularized cardiac tissues with perfusable blood vessels by overlaying additional triple-layer cell sheets made by NRCMs cocultured with endothelial cells. Such sheets were then transplanted under the neck skin of nude rats and connected to the local vasculature. Constructs with vessel anastomoses survived and maintained their vascular structure up to two weeks after transplantation. However, the thickness of the constructs decreased over time indicating that uniform perfusion was insufficient for whole tissue survival. Moreover, no functional analyses were performed in the study to evaluate maturation at tissue level [8]. More recently, Kawatou et al. developed an in vitro drug-induced Torsade de Pointes arrhythmia model using 3D cardiac tissue sheets. Importantly, the authors showed the importance of using multi-layered 3D structures containing a hiPSC-derived heterogeneous cell mixture (CMs and non-myocytes) in order to recapitulate disease-related phenotypes in vitro [9]. In addition, phase II clinical trials have been performed by Japanese scientists to evaluate the efficacy and safety of autologous skeletal myoblast sheet transplantation in patients with advanced heart failure. They demonstrated that the transplantation of engineered tissue promoted left ventricular remodeling, improved the heart failure symptoms and prevented cardiac death with a 2 year follow-up period [10]. Also, the potential use of cell-sheets that contain allogeneic hiPSC-CMs for clinical transplantation is under investigation [11].
A clear advantage of cell sheet technology for therapeutic applications is the absence of biomaterials, which reduces the risk of immune rejection that could arise from xenobiotic or non-autologous materials, and that no suture is needed to ligate the construct to the injured heart. Moreover, sheet technology enables direct cell-to-cell communications between cells in the transplanted sheets and the host tissue, facilitating electrical communication and vascular network formation within the cell sheet structure. On the downside, it has been argued that the fragility of these sheets makes them difficult to manipulate during implantation onto the heart [12]. Although cardiac tissue sheets have many advantages over other tissue engineering methods, these structures are not thick enough to reproduce the high complexity of the native myocardial tissue.
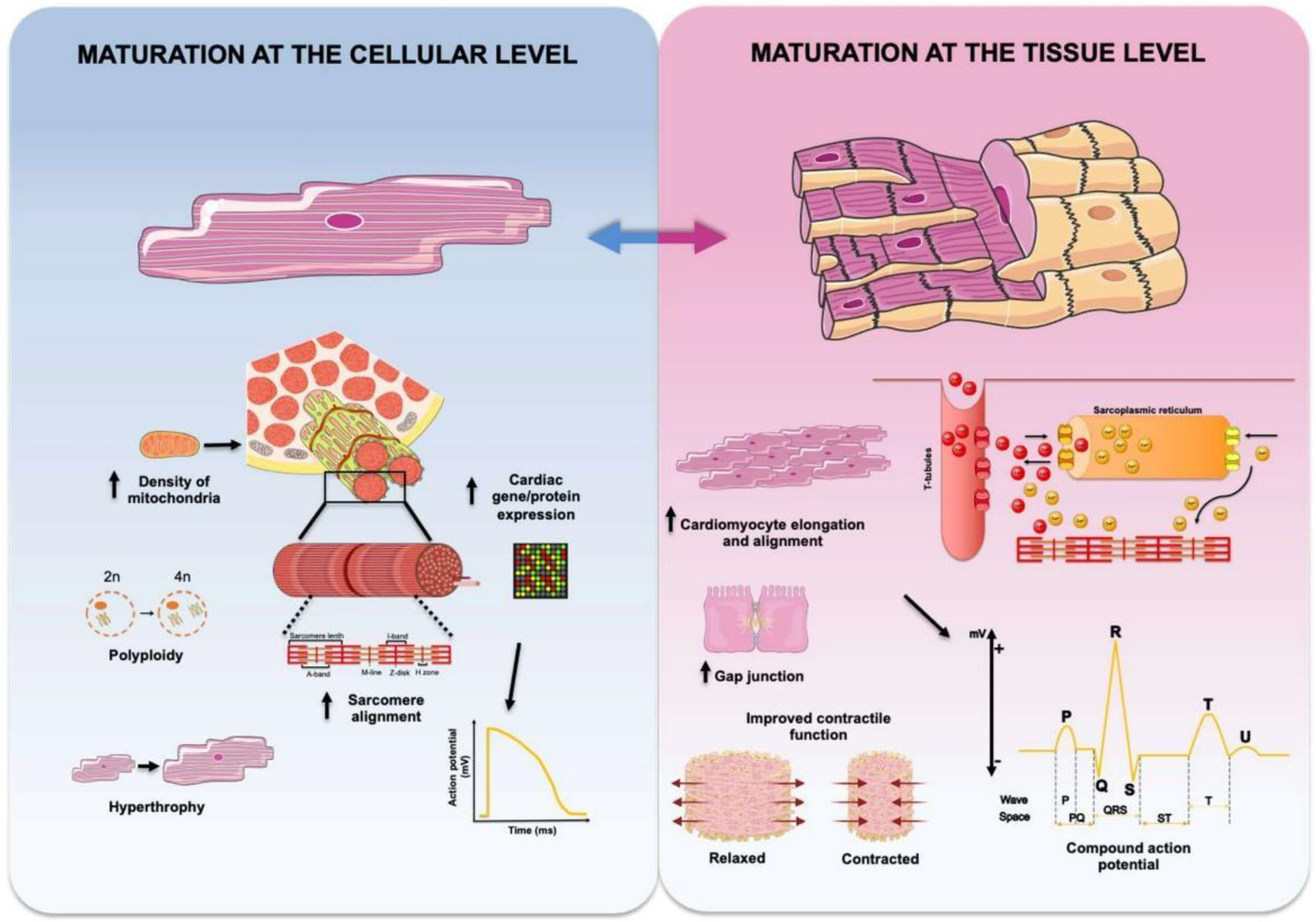
2. Scaffolds
ECT constructs made by repopulating cell-free scaffolds with suitable cells are usually referred to as cardiac patches. Scaffolds for CTE usually consist on a 3D polymeric porous structure that contributes to cell attachment and leads to the desirable cell interaction for further tissue formation [13][14][15][16][17][18][19][20][21]. Many different materials have been tested for the fabrication of scaffolds suitable for CTE, including natural and synthetic biomaterials. A commonly used synthetic material is polylactic acid (PLA), which is easily degradable forming lactic acid. PLA scaffolds were tested in some cardiovascular studies [22][23]. Another example is polyglycolic acid (PGA) and its copolymer with PLA poly(D,L-lactic-co-glycolic acid) (PLGA), an FDA approved biomaterial among the first tested for CTE due to its high porosity, biodegradability and processability [18][24][25]. However, it has been noted that the high stiffness of PLGA may limit the capacity of CMs to remodel the scaffold and ultimately impinge on their maturation [12]. Collagen, being the most abundant protein in the cardiac extracellular matrix (ECM), has a fibrillar structure that facilitates CM scaffolding. In addition to good biocompatibility, biodegradability and permeability, collagen also elicits low immunogenicity and can be engineered in various formats including high porosity scaffolds, all of which make of collagen the most commonly used biomaterial in scaffold-based CTE [26][27][14][28][15][29][30]. Other natural biomaterials used in this context include alginate, a polysaccharide derived from algae used in ECT constructs [16][31] due its high biocompatibility and appropriate chemical and mechanical properties [32], and albumin fibers, which have been used to create biocompatible scaffolds of high porosity and elasticity [33][34].
The combination of different approaches has enabled the development of scaffold systems of increasing complexity, thus bringing them morphologically closer to heart tissue. For example, researchers have generated fibrous scaffold with spatially distributed cues that enabled CM alignment within the patch [33][35][36]. However, major limitations of CTE still remain the generation of thick constructs (over ~100 µm in thickness) and the lack of electromechanical coupling between the cardiac patch and the host myocardium [37][12]. The generation of ECT constructs with a clinically relevant size requires ensuring that appropriate levels of oxygen and nutrients are maintained within the construct to satisfy the metabolic demand of CMs. Perfusion bioreactor systems pioneered by the Vunjak-Novakovic laboratory have proven to be of great value for the generation of thick ECT constructs full of viable cells with aerobic metabolism. In this case, cells were seeded and cultured in porous collagen scaffolds (11 mm in diameter, 1.5 mm in thickness) under continuous perfusion for 7 to 14 days, which led to the formation of contractile thick cardiac tissues [19][38]. More complex bioreactor systems designed to perfuse ECT constructs while also delivering electrical signals mimicking those in the native heart have also been developed [28][29][34]. Maidhof et al. used NRCMs seeded under perfusion into porous poly(glycerol sebacate) (PGS) scaffolds (8 mm in diameter, 1 mm in thickness), which were maintained under continuous perfusion at a flow rate of 18 µL/min and electrically stimulated at a frequency of 3 Hz. After 8 days, the combination of perfusion and electrical stimulation resulted in cell elongation, structural organization and improved contractility of ECT constructs [17]. Recently, our laboratories have reported the generation of 3D engineered thick human cardiac macrotissues (CardioSlices). Human iPSC-CMs were seeded together with human FBs into large 3D porous collagen/elastin scaffolds and cultured under perfusion and electrical stimulation in a custom-made bioreactor. Two weeks after culture, stimulated ECT constructs exhibited contractile and electrophysiological properties close to those of working human myocardium [39].
In addition to scaffolds made from synthetic or natural biomaterials, the use of matrices obtained by decellularizing native tissues has gained popularity for CTE. The process of decellularization allows obtaining natural ECM that can be used to mimic the native tissue structure. In essence, decellularized ECM could be recellularized with CMs or mixtures of CMs and other cell types, or with PSCs that would be differentiated in situ toward the desired cell types [40]. Tissues from a wide variety of sources including human, animals and plants have been used for this purpose [12]. The porcine heart is a prime example of tissue source for animal-derived decellularized scaffolds, due to its large size and to it being a preferred experimental model for cardiovascular research. In this case, it has been reported that the decellularization procedure allows obtaining a cardiac scaffold with preserved vasculature, mechanical integrity and biocompatibility [41]. Nevertheless, limitations noted with this approach include the extent of preservation of the ECM composition (which can be altered by the decellularization process), the difficulty in recellularizing the scaffold with clinically relevant numbers of CMs (in the order of billions), and the risk of eliciting immune rejection [12]. The issue of immune intolerance of animal-derived decellularized scaffolds has prompted research on plant-derived biomaterials as a source for ECT constructs [42]. Even though promising results have been reported using biomaterials derived from decellularized apple [43][44], spinach and parsley leaves [45], along with other cellulose-based scaffolds [46][47], further evaluation will be necessary to assess the usefulness of this type of materials for in vitro bioengineering and in vivo therapeutic applications.
Alternatively, human tissue might be a more appropriate source for decellularized ECM for therapeutic purposes, as it would address some of the limitations of animal- and/or plant-sourced materials described above [48][49][50][51]. In this respect, studies by Sanchez et al. demonstrated that the human acellular heart matrix can serve as a biocompatible scaffold for recellularization with parenchymal and vascular cells [52]. Moreover, Guyete et al. also used human decellularized heart tissue, which was in this case recellularized with iPSC-CMs and maintained in a custom-made bioreactor that provided coronary perfusion and mechanical stimulation. After 14 days in culture, the recellularized cardiac segment presented high metabolic activity and contractile function but exhibited low maturation state [53].
3. Hydrogels
Embedding suitable cells in hydrogels provide important 3D information cues and, in the context of CTE, the constructs generated in manner are typically known as cardiac grafts. Hydrogels are among the most widely studied types of biomaterials in CTE. In particular, hydrogel-based materials have been shown to provide structural/mechanical support to cells [54], promote vascularization [55] and cell migration, differentiation and proliferation [56], and to improve cardiac function after implantation in murine and porcine models of MI [57][58][59]. Hydrogels can be made from different biomaterials that are usually classified into three types: natural (type I collagen, fibrin, gelatin, alginate, keratin, among other), synthetic [polycaprolactone (PCL), polyethylene-glycol (PEG), PLA, PGA and their co-polymer PLGA], and hybrid hydrogels, which are made by combining natural and synthetic polymers [6][7]. Natural-based hydrogels are often preferred for generating ECT constructs because of their high bioactivity, biocompatibility and biodegradability [56].
Cardiac “bundles” are the most common structures generated when using hydrogel-based systems and are cylindrical ECT constructs in the form of cables, ribbons or rings [12]. These structures are usually formed by embedding CMs from various sources within hydrogels made up of fibrin, type I collagen or other biomaterials, and maintaining them in culture until constructs become spontaneously contractile. The formation of these bundles results in self-alignment and anisotropic organization of CMs, which is a hallmark of cell maturation. Moreover, these constructs provide an easy way to analyze the electrical and mechanical properties of CMs, thus enabling the readily evaluation of their maturation state and facilitating their use in drug screening and toxicity assessment platforms [60][61][62][63][64][65][66][67][68][69].
In a pioneering approach, Eschenhagen and Zimmermann generated cardiac bundles (which they termed engineered heart tissues, or EHTs) by casting a mixture of NRCMs and a blend of type I collagen type I and Matrigel into cylindrical molds. Under conditions of mechanical stretching, the resulting ring-shaped constructs exhibited improved contractile function and a high degree of cardiac myocyte differentiation [70]. In subsequent work, five of such rings were stacked on a custom-made structure creating multiloop tissue constructs that survived after implantation and improved the cardiac function of infarcted rats [59]. Using the same principle, Kensah et al. produced cardiac bundles by seeding NRCMs with FBs in a collagen/Matrigel hydrogel into a Teflon casting mold between two titanium rods and subjected to mechanical and/or chemical stimulation [71]. Similarly, human ECT constructs have also been generated by casting a cell/hydrogel suspension in different types of molds between or around flexible posts. Schaaf et al. used hESC-CMs in a fibrin hydrogel seeded into an agarose casting mold between 2 elastic silicone posts for 5 weeks [72]. Controlling the 3D microenvironment has been further reported to induce spatial organization and promote CM maturation in hydrogel-based systems. In a study by Thavandiran et al., hESC-CMs and hESC-derived cardiac FBs were seeded in a collagen/Matrigel hydrogel into polydimethylsiloxane (PDMS) microwells with integrated posts. The authors compared two-well designs side by side: an elongated microwell containing posts in both extremes (capable of inducing uniaxial mechanical stress) and a square well containing posts around the edges (thus effecting biaxial mechanical conditioning). These studies demonstrated that constructs on elongated microwells showed comparatively better aligned sarcomeres and more elongated and longitudinally oriented CMs [73]. In turn, the Bursac laboratory created thin (~70 µm in thickness) 3D sheet-like constructs of large surface dimensions (7 × 7 mm) by casting hESC-derived CMs in fibrin hydrogels into PDMS molds with hexagonal posts, resulting in improved maturation at the functional (conduction velocities of up to 25 cm/s and contractile forces and stresses of 3.0 mN and 11.8 mN/mm2, respectively) and structural (increased sarcomeric organization and expression of cardiac genes) level [37]. Similarly, Turnbull et al. generated human ECT constructs with hESC-derived cells mixed in a collagen/Matrigel hydrogel in rectangular PDMS casting molds with integrated posts at each end and removable inserts. Forty-eight hours after casting, the inserts were removed from the mold, allowing the self-assembly of the tissues between the two flexible posts, which were used as force sensors. The resulting tissues exhibited typical features of human newborn myocardium tissue including contractile, structural and molecular characteristics [74].
Similar to hESC-CMs, iPSC-CMs also have been successfully cultured in hydrogel-based structures [13][62][75][76][77][64][65][78][66]. The Radisic laboratory pioneered the use of hiPSC-CMs to generate human ECT constructs by developing a platform in which cells in a collagen hydrogel organized around a surgical suture in a PDMS channel. The resulting 3D microstructures (3 mm2) were termed Biowires and contained aligned CMs that exhibited well-developed striations and showed improved cardiac tissue function after electrical stimulation [62]. A further improvement to this platform was the use of a polytetrafluoroethylene tube that allowed perfusion of the ECT microstructures and facilitated their use for drug toxicity testing [79]. More importantly, three independent studies reported in 2017 on the generation of clinical-size cardiac tissues by using hydrogel-based systems and hiPSC-CMs. Shadrin et al. generated human cardiac tissues of 36 × 36 mm that showed electromechanical properties close to those of working myocardium (conduction velocity of 30 cm/s and specific forces of 20 mN/mm2) by seeding hiPSC-CMs in a fibrin hydrogel into square PDMS molds [13]. Using a mixture of type I collagen and Matrigel with hiPSC-derived CMs and endothelial and vascular cells (in a 3:1:1 ratio), Nakane et al. generated rectangular ECT constructs with different shapes (bundles and mesh junctions, parallel bundles, plain sheets) and sizes (from 15 × 15 mm to 30 × 30 mm). They analyzed the association of CM orientation and survival with construct architecture and found that bundles and mesh junctions resulted in the highest myofiber alignment and lowest percentage of dead cells. Moreover, functional integration was observed after 4 weeks of transplantation onto rat uninjured hearts [64]. Large ECT constructs (35 × 34 mm) were also generated in the Zimmermann laboratory by seeding hiPSC-CMs and FBs in a collagen hydrogel on a 3D-printed construct holder with flexible poles in a hexagonal casting mold [65]. In a further refinement of this approach, Gao et al. generated human ECT constructs of 4 × 2 cm comprising hiPSC-derived CMs, SMCs, and ECs (3:1:1 ratio) in a fibrin hydrogel and maintained them in culture on a rocking platform. After 7 days of culture, the constructs showed improved electromechanical coupling, calcium-handling, and force generation [78].
Synthetic hydrogels have received comparatively less attention for CTE than those of natural origin. Ma et al. used PEG to create cardiac microchambers (100 to 300 µm in height) that induced spatial organization of hESCs and hiPSCs and directed their cardiac differentiation [80][81]. In addition, hybrid hydrogels have a great potential for CTE as they can mimic biological properties of the ECM and, at the same time, be tuned to suit the mechanical properties expected or desired for cardiac constructs [82]. Despite their great potential, much research is still needed to ascertain the specific advantages that synthetic and hybrid hydrogels may have over commonly used natural hydrogels in the context of CTE. At any rate, irrespective of the type of hydrogel used, current 3D cardiac grafts are constrained in maximum thickness by the ~300 µm limit of oxygen diffusion in passive culture systems and, therefore, while ideal for miniature structures with some tissue-like functionalities, they may not be suited for applications that require fully capturing the high complexity of the native heart tissue structure.
4. Cardiac Spheroids (and‘Organoids’)
Spheroids are scaffold-free 3D cell constructs that rely on cell aggregation or self-organization and simulate aspects of the native cell microenvironment [83]. Cardiac spheroids can be constructed with CMs [84][85] and also include other cardiac resident cells such as FBs [86] or ECs [87]. Different percentages of various cell types have been tested for the generation of cardiac spheroids [86][88]. Spheroid-based systems are attractive to scientists for studying heterocellular interactions and drug effects because they only need low cell numbers to be formed. On the other hand, the absence of functional architecture limits the physiological analyses of the cells, like force generation and electrical conduction. Nevertheless, using the spheroid-based systems to deliver CMs into the damaged region of the heart has been reported. For instance, intramyocardial injection of cardiac spheroids in mice resulted in higher engraftment rates and improved electrical coupling with host myocardium, compared to single cell injection, which reveals potential for future clinical applications [89][90]. Moreover, several research groups are working on the generation of thicker functional structures using multicellular spheroids for further clinical testing. Noguchi et al. created a scaffold-free 3D tissue construct based on self-organization of 1 × 104 spheroids. The individual spheroids were, in turn, obtained by combining 3 cell sources: NRCMs, hECs and hFBs in a 7:1.5: 1.5 ratio. ECT constructs generated in this way remained adherent and presented signs of vascularization seven days after transplantation onto the heart of nude rats [91]. In a related approach, scientists created a biomaterial-free cardiac tissue by 3D printing multicellular cardiac spheroids that displayed spontaneous beating and ventricular-like action potentials, which were engrafted as well into the rat heart tissue [92]. A final example of this approach is the study by Kim et al., who generated elongated 3D heterocellular microtissues by fusing together multicellular cardiac spheroids containing CMs and cardiac FBs. The authors demonstrated that such microtissues formed an electrical syncytium after seven hours in culture [93].
Similar to spheroids, organoids are scaffold-free 3D cell constructs that simulate aspects of the native environmental conditions. However, a critically important characteristic of organoids that sets them apart from spheroids, is that organoids contain organ-specific cell types that self-organize in a way that is architecturally similar to that of the native organ [94][95][96]. While some researchers may use the terms organoids and spheroids interchangeably, there are important differences between them. Both spheroids and organoids can be generated from PSCs, PSC derivatives, or tissue-specific stem/progenitor cells. When using PSCs, the technique of organoid formation is inspired in the embryoid body system [94], 3D aggregates of PSCs where cells undergo specification and differentiation into cell derivatives of the three main embryo germ layers [95]. In contrast, spheroids are much simpler than organoids in terms of the cell types that conform them, do not self-organize into organ-like patterns or structures, and depend to a lesser extent on ECM properties and composition [96]. Even though several published reports describe the generation of human “cardiac organoids”, these rely on direct cardiac differentiation of hiPSC-derived embryoid bodies [97] or aggregation of cardiac cell types (CMs, cardiac FBs and cardiac ECs) [98][99][100][101][102], which actually represent typical examples of spheroids [94][95]. We believe that the use of the term “cardiac organoids” in this context is misleading since the structures generated in those studies lacked the organ-like complexity characteristic of true organoids. Very recently, Lee et al. have described the generation of bona fide cardiac organoids in the mouse system that showed atrium- and ventricular-like structures highly reminiscent of the native embryonic heart. For this purpose, the authors induced sequential morphological changes in PSC-derived cells by including a laminin-entactin complex in the ECM and FGF-4 in serum-free medium [103]. Perhaps the application of similar approaches to hiPSC derivatives could lead to the generation of true human cardiac organoids containing relevant organ-specific cell types with the capacity to self-organize in organ-like structures.
5. Heart-on-a-Chip
Microfluidic cell culture technologies enable researchers to create in vitro cell microenvironments that mimic organ-level physiology [104]. The term ‘organ-on-a-chip’ is generally applied to a microphysiological system, including the slides or plates that are connected to microfluidic devices to control perfusion of culture medium and exposure of defined stimuli [105]. Heart-on-a-chip technology refers specifically to microphysiological systems mimicking the function of heart tissue. In vivo-like cardiac cell culture systems could lead to a better understanding of (1) cardiac cell physiology; (2) cardiotoxicity of drugs intended for human used; (3) personalized treatments for CVD patients; and (3) mechanisms of heart regeneration [106][107][108]. In physiological conditions, the heart tissue is in direct contact with body fluids such as blood and lymph that exert physical forces (shear stress) on the cells. This continuous flow stimulation determines the cardiac cells structure, phenotype, intra- and extracellular interactions [109]. In vivo-like cardiac cell culture systems try to replicate these conditions in vitro. Thus, the heart-on-a-chip system provides suitable conditions to imitate biochemical, mechanical, and physical signals characteristic of heart tissue [110][111][112]. For example, it was shown that continuous perfusion enhances cell proliferation and parallel alignment of cells compared to static conditions [113]. In addition to perfusion, integrating mechanical and electrical stimulation into heart-on-a-chip devices also improves the maturation state of CMs [114][115][116][117], one of the key features for successful modeling of cardiac diseases [118]. Moreover, heart-on-a-chip systems are amenable to parallelization and thus to be used in high-throughput assays for drug screening and cardiotoxicity testing [108][119]. Particularly, the possibility of using hPSC-CMs brings an additional level of personalization to heart-on-a-chip systems [75][114][87][120][121][122][123]. For example, the Radisic laboratory developed a powerful platform, dubbed AngioChip, that integrated tissue engineering and organ-on-a-chip technologies to produce vascularized polymer-based microfluidic cardiac scaffolds. Such a platform can be used to generate both in vitro heart tissue models and in vivo implants for potential clinical application [122].
6. 3D Bioprinting
3D bioprinting is one of the latest additions to the tissue engineering toolbox, and one that could be used to create complex and large vascularized tissues [124][125]. Several methods of 3D bioprinting have been used in the context of CTE, including cell-laden hydrogel 3D structures [126], inkjet bioprinting [127], laser-assisted bioprinting [128], and extrusion-based bioprinting [129]. Biomaterials used in 3D bioprinting are based on piezo-resistive, high-conductance, and biocompatible soft materials. Gaetani et al. bioprinted a 2 × 2 cm ECT construct using human cardiac progenitor cells and alginate matrices, which was maintained for 2 weeks in vitro [31] or transplanted onto rat infarcted hearts where it led to cell engraftment [130]. Generation of 3D-bioprinted vascularized heart tissues using mouse iPSC-CMs with human ECs in a PEG/fibrin hydrogel has been recently reported showing improved connectivity to the host vasculature after subcutaneous transplantation in mice [131]. Despite the early stage of development, 3D bioprinting is a very promising technology for recapitulating the complex structure of heart tissue and already shows enormous potential in CTE. In a recent study, Noor et al. succeeded in bioprinting thick (2 mm) 3D vascularized and perfused ECT constructs that had high cell viability using an extrusion-based bioprinter. As bioinks, the authors used an ECM-based hydrogel derived from human decellularized omentum containing hiPSC-CMs, and gelatin containing iPSC-derived ECs and FBs. Computerized tomography of a patient’s heart was used to reproduce in vitro the structure and orientation of the blood vessels into the tissue. Bioprinted ECT constructs were transplanted into the omentum of rats and analyzed after seven days, when elongated and well-aligned CMs with massive striations were observed [56]. This study demonstrated that the possibility of using fully personalized materials makes 3D bioprinting technology very promising for clinical application by reducing the risk of immune rejection after transplantation. However, the system is still limited and further analyses should be performed to evaluate if heart tissue bioprinted in this manner could sustain normal blood pressure levels after transplantation [132].
3D bioprinting can also be combined with microfluidic systems to provide superior organ-level response with greater prediction of drug-induced capability [133]. On the other hand, recent advances in nanomaterial technology present an attractive platform for the creation of ECT constructs for biomedical applications. Electrospinning technology allows creating nanofibers with controlled dimensions and further development of 3D structures [134]. In addition, the nanofibrous structure provides appropriate conditions for pluripotent cells to anchor, migrate and differentiate [135]. Increasing attention is being given to these types of structures due to their distinct mechanical properties, high porosity and potential to induce formation of aligned tissues that can be successfully implanted to the heart [136].
7. Other Structures
So far, we have described the variety of approaches undertaken for the generation of ECT constructs that reproduce increasingly complex features of the human myocardial tissue. However, several groups have pursued approaches intended to model whole heart chambers. In an early attempt in 2007, scientists created a “pouch-like” single ventricle using a mixture of hydrogel composed of type I collagen and Matrigel and NRCMs, which was cultured in an agarose casting mold with the dimensions of a rat ventricle. After formation, it was transplanted onto the rat heart showing limited functional integration [137]. One year later, Lee et al. created a “cardiac organoid chamber” by seeding NRCMs mixed with a collagen/Matrigel hydrogel around a balloon-like shaped mold with a thickness of 0.65 mm and sizes of 4.5 to 9 mm for the unloaded outer diameter, equivalent to the sizes of embryonic rat hearts at 9.5–11 days of development. The authors succeeded in the creation of a heart ventricle but with a moderate contractile capacity [138]. In subsequent work, the same group used a similar method to produce a functional human cardiac chamber using hESC-CMs embedded in collagen-based hydrogel. They proved that such technology could facilitate drug discovery as it provides the capacity to measure clinically meaningful parameters of the heart like ejection fraction and developed pressure, as well as electrophysiological properties [139]. The Parker laboratory has also recently generated tissue-engineered ventricles by using nanofibrous scaffolds composed of PCL and gelatin seeded with either NRCMs or hiPSC-CMs on a rotating ellipsoidal collector. The authors showed that cells aligned following the fiber orientation, thus recapitulating the orientation of the native myocardium, although the contractile function was much smaller than those of the native rat/human ventricles most likely owing to the small thickness of the chamber [140]. Notably, the Feinberg group printed human cardiac ventricles in 2019 using the freeform embedding of suspended hydrogels (FRESH) method. In this case, type I collagen was used as a support material to create an ellipsoidal shell that was filled with hESC-CMs in a fibrinogen suspension. These engineered tissues displayed synchronized functional activity [141]. Finally, Kupfer et al. have very recently generated a 3D bioprinted chambered muscle pump using an optimized bioink formulation of ECM proteins that allowed hiPSC proliferation prior to differentiation. In this way, 3D bioprinted hiPSCs underwent cell expansion and differentiated into CMs in situ, yielding contiguous muscle walls of up to 500 μm in thickness. The resulting human chambered organoids showed macroscale contractions and continuous action potential propagation and were responsive to drugs and to external pacing [142].
References
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344.
- Yamato, M.; Okano, T. Cell sheet engineering. Mater. Today 2004, 7, 42–47, doi:10.1016/S1369-7021(04)00234-2.
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell Sheet Engineering: Recreating Tissues without Biodegradable Scaffolds. Biomaterials 2005, 26, 6415–6422.
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Cell Sheet Engineering for Myocardial Tissue Reconstruction. Biomaterials 2003, 24, 2309–2316, doi:10.1016/s0142-9612(03)00110-8.
- Sekine, H.; Shimizu, T.; Dobashi, I.; Matsuura, K.; Hagiwara, N.; Takahashi, M.; Kobayashi, E.; Yamato, M.; Okano, T. Cardi-ac Cell Sheet Transplantation Improves Damaged Heart Function via Superior Cell Survival in Comparison with Dissociat-ed Cell Injection. Tissue Eng. Part A 2011, 17, 2973–2980, doi:10.1089/ten.tea.2010.0659.
- Kopeček, J.; Yang, J. Smart Self-Assembled Hybrid Hydrogel Biomaterials. Angew. Chem. Int. Ed. 2012, 51, 7396–7417.
- Camci-Unal, G.; Annabi, N.; Dokmeci, M.R.; Liao, R.; Khademhosseini, A. Hydrogels for Cardiac Tissue Engineering. NPG Asia Mater. 2014, 6, e99–e99, doi:10.1038/am.2014.19.
- Sekine, H.; Shimizu, T.; Sakaguchi, K.; Dobashi, I.; Wada, M.; Yamato, M.; Kobayashi, E.; Umezu, M.; Okano, T. In Vitro Fab-rication of Functional Three-Dimensional Tissues with Perfusable Blood Vessels. Nat. Commun. 2013, 4, 1399, doi:10.1038/ncomms2406.
- Kawatou, M.; Masumoto, H.; Fukushima, H.; Morinaga, G.; Sakata, R.; Ashihara, T.; Yamashita, J.K. Modelling Torsade de Pointes Arrhythmias In Vitro in 3D Human iPS Cell-Engineered Heart Tissue. Nat. Commun. 2017, 8, 1078, doi:10.1038/s41467-017-01125-y.
- Sawa, Y.; Yoshikawa, Y.; Toda, K.; Fukushima, S.; Yamazaki, K.; Ono, M.; Sakata, Y.; Hagiwara, N.; Kinugawa, K.; Miyaga-wa, S. Safety and Efficacy of Autologous Skeletal Myoblast Sheets (TCD-51073) for the Treatment of Severe Chronic Heart Failure Due to Ischemic Heart Disease. Circ. J. 2015, 79, 991–999, doi:10.1253/circj.CJ-15-0243.
- Yui, Y. Concerns on a New Therapy for Severe Heart Failure Using Cell Sheets with Skeletal Muscle or Myocardial Cells from iPS Cells in Japan. NPJ Regen Med 2018, 3, 7, doi:10.1038/s41536-018-0047-2.
- Pomeroy, J.E.; Helfer, A.; Bursac, N. Biomaterializing the Promise of Cardiac Tissue Engineering. Biotechnol. Adv. 2019, doi:10.1016/j.biotechadv.2019.02.009.
- Shadrin, I.Y.; Allen, B.W.; Qian, Y.; Jackman, C.P.; Carlson, A.L.; Juhas, M.E.; Bursac, N. Cardiopatch Platform Enables Matu-ration and Scale-Up of Human Pluripotent Stem Cell-Derived Engineered Heart Tissues. Nat. Commun. 2017, 8, 1825, doi:10.1038/s41467-017-01946-x.
- Radisic, M.; Park, H.; Shing, H.; Consi, T.; Schoen, F.J.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Functional Assembly of Engineered Myocardium by Electrical Stimulation of Cardiac Myocytes Cultured on Scaffolds. Proc. Natl. Acad. Sci. USA. 2004, 101, 18129–18134, doi:10.1073/pnas.0407817101.
- Tandon, N.; Cannizzaro, C.; Chao, P.-H.G.; Maidhof, R.; Marsano, A.; Au, H.T.H.; Radisic, M.; Vunjak-Novakovic, G. Electri-cal Stimulation Systems for Cardiac Tissue Engineering. Nat. Protoc. 2009, 4, 155–173, doi:10.1038/nprot.2008.183.
- Barash, Y.; Dvir, T.; Tandeitnik, P.; Ruvinov, E.; Guterman, H.; Cohen, S. Electric Field Stimulation Integrated into Perfusion Bioreactor for Cardiac Tissue Engineering. Tissue Eng. Part C Methods 2010, 16, 1417–1426, doi:10.1089/ten.TEC.2010.0068.
- Maidhof, R.; Tandon, N.; Lee, E.J.; Luo, J.; Duan, Y.; Yeager, K.; Konofagou, E.; Vunjak-Novakovic, G. Biomimetic Perfusion and Electrical Stimulation Applied in Concert Improved the Assembly of Engineered Cardiac Tissue. J. Tissue Eng. Regen. Med. 2012, 6, e12–23, doi:10.1002/term.525.
- Bursac, N.; Papadaki, M.; Cohen, R.J.; Schoen, F.J.; Eisenberg, S.R.; Carrier, R.; Vunjak-Novakovic, G.; Freed, L.E. Cardiac Muscle Tissue Engineering: Toward an In Vitro Model for Electrophysiological Studies. Am. J. Physiol. Heart Circul. Physiol. 1999, 277, H433–H444.
- Radisic, M.; Park, H.; Martens, T.P.; Salazar-Lazaro, J.E.; Geng, W.; Wang, Y.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Pre-treatment of Synthetic Elastomeric Scaffolds by Cardiac Fibroblasts Improves Engineered Heart Tissue. J. Biomed. Mater. Res. A 2008, 86, 713–724, doi:10.1002/jbm.a.31578.
- Zandonella, C. Tissue Engineering: The Beat Goes On. Nature 2003, 421, 884–886, doi:10.1038/421884a.
- Sultana, N.; Hassan, M.I.; Lim, M.M. Scaffolding Biomaterials. In Composite Synthetic Scaffolds for Tissue Engineering and Regenerative Medicine; Sultana, N., Hassan, M.I., Lim, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–11 ISBN 9783319097558.
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber Assembly by Rotary Jet-Spinning. Nano Lett. 2010, 10, 2257–2261.
- Kenar, H.; Kose, G.T.; Toner, M.; Kaplan, D.L.; Hasirci, V. A 3D Aligned Microfibrous Myocardial Tissue Construct Cultured Under Transient Perfusion. Biomaterials 2011, 32, 5320–5329, doi:10.1016/j.biomaterials.2011.04.025.
- Papadaki, M.; Bursac, N.; Langer, R.; Merok, J.; Vunjak-Novakovic, G.; Freed, L.E. Tissue Engineering of Functional Cardiac Muscle: Molecular, Structural, and Electrophysiological Studies. Am. J. Physiol. Heart Circul. Physiol. 2001, 280, H168–H178.
- Bursac, N.; Loo, Y.; Leong, K.; Tung, L. Novel Anisotropic Engineered Cardiac Tissues: Studies of Electrical Propagation. Biochem. Biophys. Res. Commun. 2007, 361, 847–853, doi:10.1016/j.bbrc.2007.07.138.
- Carrier, R.L.; Rupnick, M.; Langer, R.; Schoen, F.J.; Freed, L.E.; Vunjak-Novakovic, G. Perfusion Improves Tissue Architec-ture of Engineered Cardiac Muscle. Tissue Eng. 2002, 8, 175–188, doi:10.1089/107632702753724950.
- Radisic, M.; Yang, L.; Boublik, J.; Cohen, R.J.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Medium Perfusion Enables En-gineering of Compact and Contractile Cardiac Tissue. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H507–16, doi:10.1152/ajpheart.00171.2003.
- Radisic, M.; Marsano, A.; Maidhof, R.; Wang, Y.; Vunjak-Novakovic, G. Cardiac Tissue Engineering Using Perfusion Bioreac-tor Systems. Nat. Protoc. 2008, 3, 719–738, doi:10.1038/nprot.2008.40.
- Shachar, M.; Benishti, N.; Cohen, S. Effects of Mechanical Stimulation Induced by Compression and Medium Perfusion on Cardiac Tissue Engineering. Biotechnol. Prog. 2012, 28, 1551–1559, doi:10.1002/btpr.1633.
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95, doi:10.1016/S1369-7021(11)70058-X.
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.G.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P.G. Cardiac Tissue Engineer-ing Using Tissue Printing Technology and Human Cardiac Progenitor Cells. Biomaterials 2012, 33, 1782–1790, doi:10.1016/j.biomaterials.2011.11.003.
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for Cardiac Regeneration: From Seaweed to Clini-cal Trials. Glob Cardiol Sci Pract 2016, 2016, e201604, doi:10.21542/gcsp.2016.4.
- Fleischer, S.; Shapira, A.; Feiner, R.; Dvir, T. Modular Assembly of Thick Multifunctional Cardiac Patches. Proc. Natl. Acad. Sci. USA. 2017, 114, 1898–1903, doi:10.1073/pnas.1615728114.
- Fleischer, S.; Shapira, A.; Regev, O.; Nseir, N.; Zussman, E.; Dvir, T. Albumin Fiber Scaffolds for Engineering Functional Cardiac Tissues. Biotechnol. Bioeng. 2014, 111, 1246–1257, doi:10.1002/bit.25185.
- Bian, W.; Jackman, C.P.; Bursac, N. Controlling the Structural and Functional Anisotropy of Engineered Cardiac Tissues. Biofabrication 2014, 6, 024109.
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal Stem Cell-Based Tissue Regeneration is Governed by Recipient T Lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011, 17, 1594–1601, doi:10.1038/nm.2542.
- Zhang, D.; Shadrin, I.Y.; Lam, J.; Xian, H.-Q.; Snodgrass, H.R.; Bursac, N. Tissue-engineered Cardiac Patch for Advanced Functional Maturation of Human ESC-Derived Cardiomyocytes. Biomaterials 2013, 34, 5813–5820, doi:10.1016/j.biomaterials.2013.04.026.
- Radisic, M.; Euloth, M.; Yang, L.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. High-Density Seeding of Myocyte Cells for Cardiac Tissue Engineering. Biotechnol. Bioeng. 2003, 82, 403–414, Doi:10.1002/Bit.10594.
- Valls-Margarit, M.; Iglesias-García, O.; Di Guglielmo, C.; Sarlabous, L.; Tadevosyan, K.; Paoli, R.; Comelles, J.; Blanco-Almazán, D.; Jiménez-Delgado, S.; Castillo-Fernández, O.; et al. Engineered Macroscale Cardiac Constructs Elicit Human Myocardial Tissue-like Functionality. Stem Cell Rep. 2019, 13, 207–220, doi:10.1016/j.stemcr.2019.05.024.
- Yu, Y.; Alkhawaji, A.; Ding, Y.; Mei, J. Decellularized Scaffolds in Regenerative Medicine. Oncotarget 2016, 7, 58671–58683.
- Hodgson, M.J.; Knutson, C.C.; Momtahan, N.; Cook, A.D. Extracellular Matrix from Whole Porcine Heart Decellularization for Cardiac Tissue Engineering. Methods Mol. Biol. 2017, 95–102, doi:10.1007/7651_2017_31.
- Fontana, G.; Gershlak, J.; Adamski, M.; Lee, J.-S.; Matsumoto, S.; Le, H.D.; Binder, B.; Wirth, J.; Gaudette, G.; Murphy, W.L. Biofunctionalized Plants as Diverse Biomaterials for Human Cell Culture. Adv. Healthc. Mater. 2017, 6, doi:10.1002/adhm.201601225.
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLos ONE 2014, 9, e97835, doi:10.1371/journal.pone.0097835.
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of Subcutaneously Implanted Plant-Derived Cellulose Biomaterials. PLos ONE 2016, 11, e0157894, doi:10.1371/journal.pone.0157894.
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.K.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing Kingdoms: Using Decellularized Plants as Perfusable Tissue Engineering Scaffolds. Biomaterials 2017, 125, 13–22.
- Entcheva, E.; Bien, H.; Yin, L.; Chung, C.-Y.; Farrell, M.; Kostov, Y. Functional Cardiac Cell Constructs on Cellulose-Based Scaffolding. Biomaterials 2004, 25, 5753–5762, doi:10.1016/j.biomaterials.2004.01.024.
- Wippermann, J.; Schumann, D.; Klemm, D.; Albes, J.M.; Wittwer, T.; Strauch, J.; Wahlers, T. Preliminary Results of Small Arterial Substitutes Performed with a New Cylindrical Biomaterial Composed of Bacterial Cellulose. Thorac. Cardiovasc. Surg. 2008, 56, 592–596.
- Oberwallner, B.; Brodarac, A.; Anić, P.; Šarić, T.; Wassilew, K.; Neef, K.; Choi, Y.-H.; Stamm, C. Human Cardiac Extracellular Matrix Supports Myocardial Lineage Commitment of Pluripotent Stem Cells. Eur. J. Cardiothorac. Surg. 2015, 47, 416–25; dis-cussion 425, doi:10.1093/ejcts/ezu163.
- Kc, P.; Hong, Y.; Zhang, G. Cardiac Tissue-Derived Extracellular Matrix Scaffolds for Myocardial Repair: Advantages and Challenges. Regen Biomater 2019, 6, 185–199, doi:10.1093/rb/rbz017.
- Oberwallner, B.; Brodarac, A.; Choi, Y.-H.; Saric, T.; Anić, P.; Morawietz, L.; Stamm, C. Preparation of Cardiac Extracellular Matrix Scaffolds by Decellularization of Human Myocardium. J. Biomed. Mater. Res. A 2014, 102, 3263–3272, doi:10.1002/jbma.35000.
- Garreta, E.; de Oñate, L.; Fernández-Santos, M.E.; Oria, R.; Tarantino, C.; Climent, A.M.; Marco, A.; Samitier, M.; Martínez, E.; Valls-Margarit, M.; et al. Myocardial Commitment From Human Pluripotent Stem Cells: Rapid Production Of Human Heart Grafts. Biomaterials 2016, 98, 64–78, doi:10.1016/j.biomaterials.2016.04.003.
- Sánchez, P.L.; Fernández-Santos, M.E.; Costanza, S.; Climent, A.M.; Moscoso, I.; Gonzalez-Nicolas, M.A.; Sanz-Ruiz, R.; Rodríguez, H.; Kren, S.M.; Garrido, G.; et al. Acellular Human Heart Matrix: A Critical Step Toward Whole Heart Grafts. Bi-omaterials 2015, 61, 279–289, doi:10.1016/j.biomaterials.2015.04.056.
- Guyette, J.P.; Charest, J.M.; Mills, R.W.; Jank, B.J.; Moser, P.T.; Gilpin, S.E.; Gershlak, J.R.; Okamoto, T.; Gonzalez, G.; Milan, D.J.; et al. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118, 56–72, doi:10.1161/CIRCRESAHA.115.306874.
- Pedron, S.; Van Lierop, S.; Horstman, P.; Penterman, R.; Broer, D.J.; Peeters, E. Stimuli Responsive Delivery Vehicles for Cardiac Microtissue Transplantation. Adv. Funct. Mater. 2011, 21, 1624–1630, doi:10.1002/adfm.201002708.
- Birla, R.K.; Borschel, G.H.; Dennis, R.G.; Brown, D.L. Myocardial Engineering in Vivo: Formation and Characterization of Contractile, Vascularized Three-Dimensional Cardiac Tissue. Tissue Eng. 2005, 11, 803–813.
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Rajabi-Zeleti, S.; Annabi, N.; Aghdami, N.; Baharvand, H. Stem Cells and Injectable Hydrogels: Synergistic Therapeutics in Myocardial Repair. Biotechnol. Adv. 2016, 34, 362–379, doi:10.1016/j.biotechadv.2016.03.003.
- Riegler, J.; Tiburcy, M.; Ebert, A.; Tzatzalos, E.; Raaz, U.; Abilez, O.J.; Shen, Q.; Kooreman, N.G.; Neofytou, E.; Chen, V.C.; et al. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ. Res. 2015, 117, 720–730, doi:10.1161/CIRCRESAHA.115.306985.
- Weinberger, F.; Breckwoldt, K.; Pecha, S.; Kelly, A.; Geertz, B.; Starbatty, J.; Yorgan, T.; Cheng, K.-H.; Lessmann, K.; Stolen, T.; et al. Cardiac Repair in Guinea Pigs with Human Engineered Heart Tissue from Induced Pluripotent Stem Cells. Sci. Transl. Med. 2016, 8, 363ra148, doi:10.1126/scitranslmed.aaf8781.
- Zimmermann, W.-H.; Melnychenko, I.; Wasmeier, G.; Didié, M.; Naito, H.; Nixdorff, U.; Hess, A.; Budinsky, L.; Brune, K.; Michaelis, B.; et al. Engineered Heart Tissue Grafts Improve Systolic and Diastolic Function in Infarcted Rat Hearts. Nat. Med. 2006, 12, 452–458, doi:10.1038/nm1394.
- Hansen, A.; Eder, A.; Bönstrup, M.; Flato, M.; Mewe, M.; Schaaf, S.; Aksehirlioglu, B.; Schwörer, A.; Uebeler, J.; Eschenhagen, T. Development of a Drug Screening Platform Based on Engineered Heart Tissue. Circ. Res. 2010, 107, 35–44.
- Tulloch, N.L.; Muskheli, V.; Razumova, M.V.; Korte, F.S.; Regnier, M.; Hauch, K.D.; Pabon, L.; Reinecke, H.; Murry, C.E. Growth of Engineered Human Myocardium with Mechanical Loading and Vascular Coculture. Circ. Res. 2011, 109, 47–59, doi:10.1161/CIRCRESAHA.110.237206.
- Nunes, S.S.; Miklas, J.W.; Liu, J.; Aschar-Sobbi, R.; Xiao, Y.; Zhang, B.; Jiang, J.; Massé, S.; Gagliardi, M.; Hsieh, A.; et al. Bio-wire: a Platform for Maturation of Human Pluripotent Stem Cell–Derived Cardiomyocytes. Nat. Methods 2013, 10, 781–787.
- Jackman, C.P.; Carlson, A.L.; Bursac, N. Dynamic Culture Yields Engineered Myocardium with Near-Adult Functional Out-put. Biomaterials 2016, 111, 66–79, doi:10.1016/j.biomaterials.2016.09.024.
- Nakane, T.; Masumoto, H.; Tinney, J.P.; Yuan, F.; Kowalski, W.J.; Ye, F.; LeBlanc, A.J.; Sakata, R.; Yamashita, J.K.; Keller, B.B. Impact of Cell Composition and Geometry on Human Induced Pluripotent Stem Cells-Derived Engineered Cardiac Tissue. Sci. Rep. 2017, 7, 45641, doi:10.1038/srep45641.
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.-L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined Engineered Human Myocardium with Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 2017, 135, 1832–1847, doi:10.1161/CIRCULATIONAHA.116.024145.
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Author Correction: Advanced Maturation of Human Cardiac Tissue Grown from Pluripotent Stem Cells. Na-ture 2019, 572, E16–E17, doi:10.1038/s41586-019-1415-9.
- Black, L.D., 3rd; Meyers, J.D.; Weinbaum, J.S.; Shvelidze, Y.A.; Tranquillo, R.T. Cell-Induced Alignment Augments Twitch Force in Fibrin Gel-Based Engineered Myocardium via Gap Junction Modification. Tissue Eng. Part A 2009, 15, 3099–3108, doi:10.1089/ten.TEA.2008.0502.
- Hansen, K.J.; Laflamme, M.A.; Gaudette, G.R. Development of a Contractile Cardiac Fiber from Pluripotent Stem Cell De-rived Cardiomyocytes. Front Cardiovasc Med 2018, 5, 52, doi:10.3389/fcvm.2018.00052.
- Naito, H.; Melnychenko, I.; Didié, M.; Schneiderbanger, K.; Schubert, P.; Rosenkranz, S.; Eschenhagen, T.; Zimmermann, W.-H. Optimizing Engineered Heart Tissue for Therapeutic Applications as Surrogate Heart Muscle. Circulation 2006, 114, I72–8, doi:10.1161/CIRCULATIONAHA.105.001560.
- Zimmermann, W.-H.; Didié, M.; Wasmeier, G.H.; Nixdorff, U.; Hess, A.; Melnychenko, I.; Boy, O.; Neuhuber, W.L.; Weyand, M.; Eschenhagen, T. Cardiac Grafting of Engineered Heart Tissue in Syngenic Rats. Circulation 2002, 106, I151–I157.
- Kensah, G.; Gruh, I.; Viering, J.; Schumann, H.; Dahlmann, J.; Meyer, H.; Skvorc, D.; Bär, A.; Akhyari, P.; Heisterkamp, A.; et al. A Novel Miniaturized Multimodal Bioreactor for Continuous In Situ Assessment of Bioartificial Cardiac Tissue During Stimulation and Maturation. Tissue Eng. Part C Methods 2011, 17, 463–473, doi:10.1089/ten.TEC.2010.0405.
- Schaaf, S.; Shibamiya, A.; Mewe, M.; Eder, A.; Stöhr, A.; Hirt, M.N.; Rau, T.; Zimmermann, W.-H.; Conradi, L.; Eschenhagen, T.; et al. Human Engineered Heart Tissue as a Versatile Tool in Basic Research and Preclinical Toxicology. PLos ONE 2011, 6, e26397, doi:10.1371/journal.pone.0026397.
- Thavandiran, N.; Dubois, N.; Mikryukov, A.; Massé, S.; Beca, B.; Simmons, C.A.; Deshpande, V.S.; McGarry, J.P.; Chen, C.S.; Nanthakumar, K.; et al. Design and Formulation of Functional Pluripotent Stem Cell-Derived Cardiac Microtissues. Proc. Natl. Acad. Sci. USA. 2013, 110, E4698–707, doi:10.1073/pnas.1311120110.
- Turnbull, I.C.; Karakikes, I.; Serrao, G.W.; Backeris, P.; Lee, J.-J.; Xie, C.; Senyei, G.; Gordon, R.E.; Li, R.A.; Akar, F.G.; et al. Advancing Functional Engineered Cardiac Tissues Toward a Preclinical Model of Human Myocardium. FASEB J. 2014, 28, 644–654, doi:10.1096/fj.13-228007.
- Mannhardt, I.; Breckwoldt, K.; Letuffe-Brenière, D.; Schaaf, S.; Schulz, H.; Neuber, C.; Benzin, A.; Werner, T.; Eder, A.; Schul-ze, T.; et al. Human Engineered Heart Tissue: Analysis of Contractile Force. Stem Cell Reports 2016, 7, 29–42, doi:10.1016/j.stemcr.2016.04.011.
- Ruan, J.-L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Me-chanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567, doi:10.1161/CIRCULATIONAHA.114.014998.
- Lemoine, M.D.; Mannhardt, I.; Breckwoldt, K.; Prondzynski, M.; Flenner, F.; Ulmer, B.; Hirt, M.N.; Neuber, C.; Horváth, A.; Kloth, B.; et al. Human iPSC-Derived Cardiomyocytes Cultured in 3D Engineered Heart Tissue Show Physiological Up-stroke Velocity and Sodium Current Density. Sci. Rep. 2017, 7, 5464, doi:10.1038/s41598-017-05600-w.
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered from Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery from Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730, doi:10.1161/CIRCULATIONAHA.117.030785.
- Xiao, Y.; Zhang, B.; Liu, H.; Miklas, J.W.; Gagliardi, M.; Pahnke, A.; Thavandiran, N.; Sun, Y.; Simmons, C.; Keller, G.; et al. Microfabricated Perfusable Cardiac Biowire: a Platform that Mimics Native Cardiac Bundle. Lab Chip 2014, 14, 869–882, doi:10.1039/c3lc51123e.
- Ma, Z.; Koo, S.; Finnegan, M.A.; Loskill, P.; Huebsch, N.; Marks, N.C.; Conklin, B.R.; Grigoropoulos, C.P.; Healy, K.E. Three-Dimensional Filamentous Human Diseased Cardiac Tissue Model. Biomaterials 2014, 35, 1367–1377, doi:10.1016/j.biomaterials.2013.10.052.
- Ma, Z.; Wang, J.; Loskill, P.; Huebsch, N.; Koo, S.; Svedlund, F.L.; Marks, N.C.; Hua, E.W.; Grigoropoulos, C.P.; Conklin, B.R.; et al. Self-Organizing Human Cardiac Microchambers Mediated by Geometric Confinement. Nat. Commun. 2015, 6, 7413, doi:10.1038/ncomms8413.
- Lau, H.K.; Kiick, K.L. Opportunities for Multicomponent Hybrid Hydrogels in Biomedical Applications. Biomacromolecules 2015, 16, 28–42.
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid Culture as a Tool for Creating 3D Complex Tissues. Trends Biotechnol. 2013, 31, 108–115.
- Beauchamp, P.; Moritz, W.; Kelm, J.M.; Ullrich, N.D.; Agarkova, I.; Anson, B.D.; Suter, T.M.; Zuppinger, C. Development and Characterization of a Scaffold-Free 3D Spheroid Model of Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes. Tissue Eng. Part C Methods 2015, 21, 852–861, doi:10.1089/ten.TEC.2014.0376.
- Nguyen, D.C.; Hookway, T.A.; Wu, Q.; Jha, R.; Preininger, M.K.; Chen, X.; Easley, C.A.; Spearman, P.; Deshpande, S.R.; Ma-her, K.; et al. Microscale Generation of Cardiospheres Promotes Robust Enrichment of Cardiomyocytes Derived from Hu-man Pluripotent Stem Cells. Stem Cell Reports 2014, 3, 260–268, doi:10.1016/j.stemcr.2014.06.002.
- Kofron, C.M.; Kim, T.Y.; King, M.E.; Xie, A.; Feng, F.; Park, E.; Qu, Z.; -R. Choi, B.; Mende, U. Gq-Activated Fibroblasts In-duce Cardiomyocyte Action Potential Prolongation and Automaticity in a Three-Dimensional Microtissue Environment. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H810–H827.
- Giacomelli, E.; Bellin, M.; Sala, L.; van Meer, B.J.; Tertoolen, L.G.J.; Orlova, V.V.; Mummery, C.L. Three-Dimensional Cardi-ac Microtissues Composed of Cardiomyocytes and Endothelial Cells Co-Differentiated from Human Pluripotent Stem Cells. Development 2017, 144, 1008–1017, doi:10.1242/dev.143438.
- Yan, Y.; Bejoy, J.; Xia, J.; Griffin, K.; Guan, J.; Li, Y. Cell Population Balance of Cardiovascular Spheroids Derived from Hu-man Induced Pluripotent Stem Cells. Sci. Rep. 2019, 9, 1295, doi:10.1038/s41598-018-37686-1.
- Oltolina, F.; Zamperone, A.; Colangelo, D.; Gregoletto, L.; Reano, S.; Pietronave, S.; Merlin, S.; Talmon, M.; Novelli, E.; Di-ena, M.; et al. Human Cardiac Progenitor Spheroids Exhibit Enhanced Engraftment Potential. PLos ONE 2015, doi:10.1371/journal.pone.0141632.
- Mattapally, S.; Zhu, W.; Fast, V.G.; Gao, L.; Worley, C.; Kannappan, R.; Borovjagin, A.V.; Zhang, J. Spheroids of Cardiomyo-cytes Derived from Human-Induced Pluripotent Stem Cells Improve Recovery from Myocardial Injury in Mice. Am. J. Phys-iol. Heart Circul. Physiol. 2018, 315, H327–H339.
- Noguchi, R.; Nakayama, K.; Itoh, M.; Kamohara, K.; Furukawa, K.; Oyama, J.-I.; Node, K.; Morita, S. Development of a Three-Dimensional Pre-Vascularized Scaffold-Free Contractile Cardiac Patch for Treating Heart Disease. J. Heart Lung Transplant. 2016, 35, 137–145, doi:10.1016/j.healun.2015.06.001.
- Ong, C.S.; Fukunishi, T.; Zhang, H.; Huang, C.Y.; Nashed, A.; Blazeski, A.; DiSilvestre, D.; Vricella, L.; Conte, J.; Tung, L.; et al. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017, 7, 4566, doi:10.1038/s41598-017-05018-4.
- Kim, T.Y.; Kofron, C.M.; King, M.E.; Markes, A.R.; Okundaye, A.O.; Qu, Z.; Mende, U.; Choi, B.-R. Directed Fusion of Cardiac Spheroids into Larger Heterocellular Microtissues Enables Investigation of Cardiac Action Potential Propagation via Car-diac Fibroblasts. PLos ONE 2018, 13, e0196714.
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125.
- Evans, M. Discovering Pluripotency: 30 Years of Mouse Embryonic Stem Cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 680–686.
- Knight, E.; Przyborski, S. Advances in 3D Cell Culture Technologies Enabling Tissue-Like Structures to Be Created In Vitro. J. Anat. 2015, 227, 746–756.
- Shkumatov, A.; Baek, K.; Kong, H. Matrix Rigidity-Modulated Cardiovascular Organoid Formation from Embryoid Bodies. PLos ONE 2014, 9, e94764, doi:10.1371/journal.pone.0094764.
- Mills, R.J.; Parker, B.L.; Quaife-Ryan, G.A.; Voges, H.K.; Needham, E.J.; Bornot, A.; Ding, M.; Andersson, H.; Polla, M.; El-liott, D.A.; et al. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-Proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell 2019, 24, 895–907.e6, doi:10.1016/j.stem.2019.03.009.
- Hoang, P.; Wang, J.; Conklin, B.R.; Healy, K.E.; Ma, Z. Generation of Spatial-Patterned Early-Developing Cardiac Organoids Using Human Pluripotent Stem Cells. Nat. Protoc. 2018, 13, 723–737, doi:10.1038/nprot.2018.006.
- Varzideh, F.; Pahlavan, S.; Ansari, H.; Halvaei, M.; Kostin, S.; Feiz, M.-S.; Latifi, H.; Aghdami, N.; Braun, T.; Baharvand, H. Human Cardiomyocytes Undergo Enhanced Maturation in Embryonic Stem Cell-Derived Organoid Transplants. Biomateri-als 2019, 192, 537–550, doi:10.1016/j.biomaterials.2018.11.033.
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human Car-diac Organoids for the Modelling of Myocardial Infarction and Drug Cardiotoxicity. Nat. Biomed. Eng 2020, 4, 446–462, doi:10.1038/s41551-020-0539-4.
- Richards, D.J.; Coyle, R.C.; Tan, Y.; Jia, J.; Wong, K.; Toomer, K.; Menick, D.R.; Mei, Y. Inspiration from Heart Development: Biomimetic Development of Functional Human Cardiac Organoids. Biomaterials 2017, 142, 112–123, doi:10.1016/j.biomaterials.2017.07.021.
- Lee, J.; Sutani, A.; Kaneko, R.; Takeuchi, J.; Sasano, T.; Kohda, T.; Ihara, K.; Takahashi, K.; Yamazoe, M.; Morio, T.; et al. In Vitro Generation of Functional Murine Heart Organoids via FGF4 and Extracellular Matrix. Nat. Commun. 2020, 11, 4283, doi:10.1038/s41467-020-18031-5.
- Huh, D.; Torisawa, Y.-S.; Hamilton, G.A.; Kim, H.J.; Ingber, D.E. Microengineered Physiological Biomimicry: Organs-on-Chips. Lab Chip 2012, 12, 2156–2164, doi:10.1039/c2lc40089h.
- Ewart, L.; Dehne, E.-M.; Fabre, K.; Gibbs, S.; Hickman, J.; Hornberg, E.; Ingelman-Sundberg, M.; Jang, K.-J.; Jones, D.R.; Lauschke, V.M.; et al. Application of Microphysiological Systems to Enhance Safety Assessment in Drug Discovery. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 65–82.
- Zhang, X.; Wang, Q.; Gablaski, B.; Zhang, X.; Lucchesi, P.; Zhao, Y. A Microdevice for Studying Intercellular Electromechani-cal Transduction in Adult Cardiac Myocytes. Lab Chip 2013, 13, 3090–3097, doi:10.1039/c3lc50414j.
- Jastrzebska, E.; Tomecka, E.; Jesion, I. Heart-on-a-Chip Based on Stem Cell Biology. Biosens. Bioelectron. 2016, 75, 67–81, doi:10.1016/j.bios.2015.08.012.
- Ribas, J.; Sadeghi, H.; Manbachi, A.; Leijten, J.; Brinegar, K.; Zhang, Y.S.; Ferreira, L.; Khademhosseini, A. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl In Vitro Toxicol 2016, 2, 82–96, doi:10.1089/aivt.2016.0002.
- Kujala, V.J.; Pasqualini, F.S.; Goss, J.A.; Nawroth, J.C.; Parker, K.K. Laminar Ventricular Myocardium on a Microelectrode Array-Based Chip. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 3534–3543, doi:10.1039/C6TB00324A.
- Chen, Y.; Chan, H.N.; Michael, S.A.; Shen, Y.; Chen, Y.; Tian, Q.; Huang, L.; Wu, H. A Microfluidic Circulatory System Inte-grated with Capillary-Assisted Pressure Sensors. Lab Chip 2017, 17, 653–662, doi:10.1039/c6lc01427e.
- Ghiaseddin, A.; Pouri, H.; Soleimani, M.; Vasheghani-Farahani, E.; Ahmadi Tafti, H.; Hashemi-Najafabadi, S. Cell Laden Hydrogel Construct on-a-Chip for Mimicry of Cardiac Tissue in-Vitro Study. Biochem. Biophys. Res. Commun. 2017, 484, 225–230, doi:10.1016/j.bbrc.2017.01.029.
- Simmons, C.S.; Petzold, B.C.; Pruitt, B.L. Microsystems for Biomimetic Stimulation of Cardiac Cells. Lab Chip 2012, 12, 3235–3248, doi:10.1039/c2lc40308k.
- Kobuszewska, A.; Tomecka, E.; Zukowski, K.; Jastrzebska, E.; Chudy, M.; Dybko, A.; Renaud, P.; Brzozka, Z. Heart-on-a-Chip: An Investigation of the Influence of Static and Perfusion Conditions on Cardiac (H9C2) Cell Proliferation, Morpholo-gy, and Alignment. SLAS Technol 2017, 22, 536–546, doi:10.1177/2472630317705610.
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019, 176, 913–927.e18, doi:10.1016/j.cell.2018.11.042.
- Nguyen, M.-D.; Tinney, J.P.; Ye, F.; Elnakib, A.A.; Yuan, F.; El-Baz, A.; Sethu, P.; Keller, B.B.; Giridharan, G.A. Effects of Phys-iologic Mechanical Stimulation on Embryonic Chick Cardiomyocytes Using a Microfluidic Cardiac Cell Culture Model. Anal. Chem. 2015, 87, 2107–2113, doi:10.1021/ac503716z.
- Marsano, A.; Conficconi, C.; Lemme, M.; Occhetta, P.; Gaudiello, E.; Votta, E.; Cerino, G.; Redaelli, A.; Rasponi, M. Beating Heart on a Chip: A Novel Microfluidic Platform to Generate Functional 3D Cardiac Microtissues. Lab Chip 2016, 16, 599–610, doi:10.1039/c5lc01356a.
- Grosberg, A.; Alford, P.W.; McCain, M.L.; Parker, K.K. Ensembles of Engineered Cardiac Tissues for Physiological and Pharmacological Study: Heart on a Chip. Lab Chip 2011, 11, 4165–4173, doi:10.1039/c1lc20557a.
- Tu, C.; Chao, B.S.; Wu, J.C. Strategies for Improving the Maturity of Human Induced Pluripotent Stem Cell-Derived Cardi-omyocytes. Circ. Res. 2018, 123, 512–514, doi:10.1161/CIRCRESAHA.118.313472.
- Zhang, Y.S.; Aleman, J.; Arneri, A.; Bersini, S.; Piraino, F.; Shin, S.R.; Dokmeci, M.R.; Khademhosseini, A. From Cardiac Tis-sue Engineering to Heart-on-a-Chip: Beating Challenges. Biomed. Mater. 2015, 10, 034006, doi:10.1088/1748-6041/10/3/034006.
- Chu, X.; Bleasby, K.; Evers, R. Species Differences in Drug Transporters and Implications for Translating Preclinical Find-ings to Humans. Expert Opin. Drug Metab. Toxicol. 2013, 9, 237–252, doi:10.1517/17425255.2013.741589.
- Hirt, M.N.; Boeddinghaus, J.; Mitchell, A.; Schaaf, S.; Börnchen, C.; Müller, C.; Schulz, H.; Hubner, N.; Stenzig, J.; Stoehr, A.; et al. Functional Improvement and Maturation of Rat and Human Engineered Heart Tissue by Chronic Electrical Stimula-tion. J. Mol. Cell. Cardiol. 2014, 74, 151–161, doi:10.1016/j.yjmcc.2014.05.009.
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Massé, S.; Kim, J.; Reis, L.; et al. Biodegradable Scaffold with Built-In Vasculature for Organ-on-a-Chip Engineering and Direct Surgical Anastomosis.
- Nat. Mater. 2016, 15, 669–678, doi:10.1038/nmat4570.
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P.; et al. Human iPSC-Based Cardiac Microphysiological System for Drug Screening Applications. Sci. Rep. 2015, 5, 8883, doi:10.1038/srep08883.
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convo-luted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845.
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-Dimensional Bioprinting of Thick Vascularized Tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184, doi:10.1073/pnas.1521342113.
- Dhariwala, B.; Hunt, E.; Boland, T. Rapid Prototyping of Tissue-Engineering Constructs, Using Photopolymerizable Hydro-gels and Stereolithography. Tissue Eng. 2004, 10, 1316–1322, doi:10.1089/ten.2004.10.1316.
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of Inkjet Printing to Tissue Engineering. Biotechnol. J. 2006, 1, 910–917, doi:10.1002/biot.200600081.
- Yan, J.; Huang, Y.; Chrisey, D.B. Laser-Assisted Printing of Alginate Long Tubes and Annular Constructs. Biofabrication 2013, 5, 015002, doi:10.1088/1758-5082/5/1/015002.
- Beyer, S.T.; Bsoul, A.; Ahmadi, A.; Walus, K. 3D Alginate Constructs for Tissue Engineering Printed Using a Coaxial Flow Focusing Microfluidic Device. 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sen-sors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, 2013, pp. 1206–1209, doi: 10.1109/Transducers.2013.6626990.
- Gaetani, R.; Feyen, D.A.M.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.F.M.; Sluijter, J.P.G. Epicardial Application of Cardiac Progenitor Cells in a 3D-Printed Gelatin/Hyaluronic Acid Patch Preserves Cardiac Function after Myocardial Infarction. Biomaterials 2015, 61, 339–348, doi:10.1016/j.biomaterials.2015.05.005.
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344, doi:10.1002/advs.201900344.
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D.; et al. A Multi-Cellular 3D Bioprinting Approach for Vascularized Heart Tissue Engineering Based on HUVECs and iPSC-Derived Cardiomyocytes. Sci. Rep. 2018, 8, 13532, doi:10.1038/s41598-018-31848-x.
- Lee, H.; Cho, D.-W. One-Step Fabrication of an Organ-on-a-Chip with Spatial Heterogeneity Using a 3D Bioprinting Tech-nology. Lab Chip 2016, 16, 2618–2625, doi:10.1039/c6lc00450d.
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347, doi:10.1016/j.biotechadv.2010.01.004.
- Huang, S.; Yang, Y.; Yang, Q.; Zhao, Q.; Ye, X. Engineered Circulatory Scaffolds for Building Cardiac Tissue. J. Thorac. Dis. 2018, 10, S2312–S2328, doi:10.21037/jtd.2017.12.92.
- Castilho, M.; Hochleitner, G.; Wilson, W.; van Rietbergen, B.; Dalton, P.D.; Groll, J.; Malda, J.; Ito, K. Mechanical Behavior of a Soft Hydrogel Reinforced with Three-Dimensional Printed Microfibre Scaffolds. Sci. Rep. 2018, 8, 1245, doi:10.1038/s41598-018-19502-y.
- Yildirim, Y.; Naito, H.; Didie, M.; Karikkineth, B.C.; Biermann, D.; Eschenhagen, T.; -H. Zimmermann, W. Development of a Biological Ventricular Assist Device: Preliminary Data from a Small Animal Model. Circulation 2007, 116, I16–I23.
- Lee, E.J.; Kim, D.E.; Azeloglu, E.U.; Costa, K.D. Engineered Cardiac Organoid Chambers: Toward a Functional Biological Model Ventricle. Tissue Eng. Part A 2008, 14, 215–225.
- Li, R.A.; Keung, W.; Cashman, T.J.; Backeris, P.C.; Johnson, B.V.; Bardot, E.S.; Wong, A.O.T.; Chan, P.K.W.; Chan, C.W.Y.; Costa, K.D. Bioengineering an Electro-Mechanically Functional Miniature Ventricular Heart Chamber from Human Plu-ripotent Stem Cells. Biomaterials 2018, 163, 116–127, doi:10.1016/j.biomaterials.2018.02.024.
- MacQueen, L.A.; Sheehy, S.P.; Chantre, C.O.; Zimmerman, J.F.; Pasqualini, F.S.; Liu, X.; Goss, J.A.; Campbell, P.H.; Gonzalez, G.M.; Park, S.-J.; et al. A Tissue-Engineered Scale Model of the Heart Ventricle. Nat. Biomed. Eng. 2018, 2, 930–941.
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487, doi:10.1126/science.aav9051.
- Kopeček, J.; Yang, J. Smart Self-Assembled Hybrid Hydrogel Biomaterials. Angew. Chem. Int. Ed. 2012, 51, 7396–7417.
- Camci-Unal, G.; Annabi, N.; Dokmeci, M.R.; Liao, R.; Khademhosseini, A. Hydrogels for Cardiac Tissue Engineering. NPG Asia Mater. 2014, 6, e99–e99, doi:10.1038/am.2014.19.
- Black, L.D., 3rd; Meyers, J.D.; Weinbaum, J.S.; Shvelidze, Y.A.; Tranquillo, R.T. Cell-Induced Alignment Augments Twitch Force in Fibrin Gel-Based Engineered Myocardium via Gap Junction Modification. Tissue Eng. Part A 2009, 15, 3099–3108, doi:10.1089/ten.TEA.2008.0502.
- Hansen, K.J.; Laflamme, M.A.; Gaudette, G.R. Development of a Contractile Cardiac Fiber from Pluripotent Stem Cell De-rived Cardiomyocytes. Front Cardiovasc Med 2018, 5, 52, doi:10.3389/fcvm.2018.00052.
- Naito, H.; Melnychenko, I.; Didié, M.; Schneiderbanger, K.; Schubert, P.; Rosenkranz, S.; Eschenhagen, T.; Zimmermann, W.-H. Optimizing Engineered Heart Tissue for Therapeutic Applications as Surrogate Heart Muscle. Circulation 2006, 114, I72–8, doi:10.1161/CIRCULATIONAHA.105.001560.
- Lau, H.K.; Kiick, K.L. Opportunities for Multicomponent Hybrid Hydrogels in Biomedical Applications. Biomacromolecules 2015, 16, 28–42.
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid Culture as a Tool for Creating 3D Complex Tissues. Trends Biotechnol. 2013, 31, 108–115.
- Beauchamp, P.; Moritz, W.; Kelm, J.M.; Ullrich, N.D.; Agarkova, I.; Anson, B.D.; Suter, T.M.; Zuppinger, C. Development and Characterization of a Scaffold-Free 3D Spheroid Model of Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes. Tissue Eng. Part C Methods 2015, 21, 852–861, doi:10.1089/ten.TEC.2014.0376.
- Nguyen, D.C.; Hookway, T.A.; Wu, Q.; Jha, R.; Preininger, M.K.; Chen, X.; Easley, C.A.; Spearman, P.; Deshpande, S.R.; Ma-her, K.; et al. Microscale Generation of Cardiospheres Promotes Robust Enrichment of Cardiomyocytes Derived from Hu-man Pluripotent Stem Cells. Stem Cell Reports 2014, 3, 260–268, doi:10.1016/j.stemcr.2014.06.002.
- Kofron, C.M.; Kim, T.Y.; King, M.E.; Xie, A.; Feng, F.; Park, E.; Qu, Z.; -R. Choi, B.; Mende, U. Gq-Activated Fibroblasts In-duce Cardiomyocyte Action Potential Prolongation and Automaticity in a Three-Dimensional Microtissue Environment. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H810–H827.
- Yan, Y.; Bejoy, J.; Xia, J.; Griffin, K.; Guan, J.; Li, Y. Cell Population Balance of Cardiovascular Spheroids Derived from Hu-man Induced Pluripotent Stem Cells. Sci. Rep. 2019, 9, 1295, doi:10.1038/s41598-018-37686-1.
- Polonchuk, L.; Chabria, M.; Badi, L.; Hoflack, J.-C.; Figtree, G.; Davies, M.J.; Gentile, C. Cardiac Spheroids as Promising In Vitro Models to Study the Human Heart Microenvironment. Sci. Rep. 2017, 7, 7005.
- Oltolina, F.; Zamperone, A.; Colangelo, D.; Gregoletto, L.; Reano, S.; Pietronave, S.; Merlin, S.; Talmon, M.; Novelli, E.; Di-ena, M.; et al. Human Cardiac Progenitor Spheroids Exhibit Enhanced Engraftment Potential. PLos ONE 2015, doi:10.1371/journal.pone.0141632.
- Mattapally, S.; Zhu, W.; Fast, V.G.; Gao, L.; Worley, C.; Kannappan, R.; Borovjagin, A.V.; Zhang, J. Spheroids of Cardiomyo-cytes Derived from Human-Induced Pluripotent Stem Cells Improve Recovery from Myocardial Injury in Mice. Am. J. Phys-iol. Heart Circul. Physiol. 2018, 315, H327–H339.
- Noguchi, R.; Nakayama, K.; Itoh, M.; Kamohara, K.; Furukawa, K.; Oyama, J.-I.; Node, K.; Morita, S. Development of a Three-Dimensional Pre-Vascularized Scaffold-Free Contractile Cardiac Patch for Treating Heart Disease. J. Heart Lung Transplant. 2016, 35, 137–145, doi:10.1016/j.healun.2015.06.001.
- Ong, C.S.; Fukunishi, T.; Zhang, H.; Huang, C.Y.; Nashed, A.; Blazeski, A.; DiSilvestre, D.; Vricella, L.; Conte, J.; Tung, L.; et al. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017, 7, 4566, doi:10.1038/s41598-017-05018-4.
- Kim, T.Y.; Kofron, C.M.; King, M.E.; Markes, A.R.; Okundaye, A.O.; Qu, Z.; Mende, U.; Choi, B.-R. Directed Fusion of Cardiac Spheroids into Larger Heterocellular Microtissues Enables Investigation of Cardiac Action Potential Propagation via Car-diac Fibroblasts. PLos ONE 2018, 13, e0196714.
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125.
- Evans, M. Discovering Pluripotency: 30 Years of Mouse Embryonic Stem Cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 680–686.
- Knight, E.; Przyborski, S. Advances in 3D Cell Culture Technologies Enabling Tissue-Like Structures to Be Created In Vitro. J. Anat. 2015, 227, 746–756.
- Shkumatov, A.; Baek, K.; Kong, H. Matrix Rigidity-Modulated Cardiovascular Organoid Formation from Embryoid Bodies. PLos ONE 2014, 9, e94764, doi:10.1371/journal.pone.0094764.
- Mills, R.J.; Parker, B.L.; Quaife-Ryan, G.A.; Voges, H.K.; Needham, E.J.; Bornot, A.; Ding, M.; Andersson, H.; Polla, M.; El-liott, D.A.; et al. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-Proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell 2019, 24, 895–907.e6, doi:10.1016/j.stem.2019.03.009.
- Hoang, P.; Wang, J.; Conklin, B.R.; Healy, K.E.; Ma, Z. Generation of Spatial-Patterned Early-Developing Cardiac Organoids Using Human Pluripotent Stem Cells. Nat. Protoc. 2018, 13, 723–737, doi:10.1038/nprot.2018.006.
- Varzideh, F.; Pahlavan, S.; Ansari, H.; Halvaei, M.; Kostin, S.; Feiz, M.-S.; Latifi, H.; Aghdami, N.; Braun, T.; Baharvand, H. Human Cardiomyocytes Undergo Enhanced Maturation in Embryonic Stem Cell-Derived Organoid Transplants. Biomateri-als 2019, 192, 537–550, doi:10.1016/j.biomaterials.2018.11.033.
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human Car-diac Organoids for the Modelling of Myocardial Infarction and Drug Cardiotoxicity. Nat. Biomed. Eng 2020, 4, 446–462, doi:10.1038/s41551-020-0539-4.
- Richards, D.J.; Coyle, R.C.; Tan, Y.; Jia, J.; Wong, K.; Toomer, K.; Menick, D.R.; Mei, Y. Inspiration from Heart Development: Biomimetic Development of Functional Human Cardiac Organoids. Biomaterials 2017, 142, 112–123, doi:10.1016/j.biomaterials.2017.07.021.
- Lee, J.; Sutani, A.; Kaneko, R.; Takeuchi, J.; Sasano, T.; Kohda, T.; Ihara, K.; Takahashi, K.; Yamazoe, M.; Morio, T.; et al. In Vitro Generation of Functional Murine Heart Organoids via FGF4 and Extracellular Matrix. Nat. Commun. 2020, 11, 4283, doi:10.1038/s41467-020-18031-5.
- Huh, D.; Torisawa, Y.-S.; Hamilton, G.A.; Kim, H.J.; Ingber, D.E. Microengineered Physiological Biomimicry: Organs-on-Chips. Lab Chip 2012, 12, 2156–2164, doi:10.1039/c2lc40089h.
- Ewart, L.; Dehne, E.-M.; Fabre, K.; Gibbs, S.; Hickman, J.; Hornberg, E.; Ingelman-Sundberg, M.; Jang, K.-J.; Jones, D.R.; Lauschke, V.M.; et al. Application of Microphysiological Systems to Enhance Safety Assessment in Drug Discovery. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 65–82.
- Jastrzebska, E.; Tomecka, E.; Jesion, I. Heart-on-a-Chip Based on Stem Cell Biology. Biosens. Bioelectron. 2016, 75, 67–81, doi:10.1016/j.bios.2015.08.012.
- Ribas, J.; Sadeghi, H.; Manbachi, A.; Leijten, J.; Brinegar, K.; Zhang, Y.S.; Ferreira, L.; Khademhosseini, A. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl In Vitro Toxicol 2016, 2, 82–96, doi:10.1089/aivt.2016.0002.
- Kujala, V.J.; Pasqualini, F.S.; Goss, J.A.; Nawroth, J.C.; Parker, K.K. Laminar Ventricular Myocardium on a Microelectrode Array-Based Chip. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 3534–3543, doi:10.1039/C6TB00324A.
- Chen, Y.; Chan, H.N.; Michael, S.A.; Shen, Y.; Chen, Y.; Tian, Q.; Huang, L.; Wu, H. A Microfluidic Circulatory System Inte-grated with Capillary-Assisted Pressure Sensors. Lab Chip 2017, 17, 653–662, doi:10.1039/c6lc01427e.
- Ghiaseddin, A.; Pouri, H.; Soleimani, M.; Vasheghani-Farahani, E.; Ahmadi Tafti, H.; Hashemi-Najafabadi, S. Cell Laden Hydrogel Construct on-a-Chip for Mimicry of Cardiac Tissue in-Vitro Study. Biochem. Biophys. Res. Commun. 2017, 484, 225–230, doi:10.1016/j.bbrc.2017.01.029.
- Simmons, C.S.; Petzold, B.C.; Pruitt, B.L. Microsystems for Biomimetic Stimulation of Cardiac Cells. Lab Chip 2012, 12, 3235–3248, doi:10.1039/c2lc40308k.
- Kobuszewska, A.; Tomecka, E.; Zukowski, K.; Jastrzebska, E.; Chudy, M.; Dybko, A.; Renaud, P.; Brzozka, Z. Heart-on-a-Chip: An Investigation of the Influence of Static and Perfusion Conditions on Cardiac (H9C2) Cell Proliferation, Morpholo-gy, and Alignment. SLAS Technol 2017, 22, 536–546, doi:10.1177/2472630317705610.
- Nguyen, M.-D.; Tinney, J.P.; Ye, F.; Elnakib, A.A.; Yuan, F.; El-Baz, A.; Sethu, P.; Keller, B.B.; Giridharan, G.A. Effects of Phys-iologic Mechanical Stimulation on Embryonic Chick Cardiomyocytes Using a Microfluidic Cardiac Cell Culture Model. Anal. Chem. 2015, 87, 2107–2113, doi:10.1021/ac503716z.
- Marsano, A.; Conficconi, C.; Lemme, M.; Occhetta, P.; Gaudiello, E.; Votta, E.; Cerino, G.; Redaelli, A.; Rasponi, M. Beating Heart on a Chip: A Novel Microfluidic Platform to Generate Functional 3D Cardiac Microtissues. Lab Chip 2016, 16, 599–610, doi:10.1039/c5lc01356a.
- Grosberg, A.; Alford, P.W.; McCain, M.L.; Parker, K.K. Ensembles of Engineered Cardiac Tissues for Physiological and Pharmacological Study: Heart on a Chip. Lab Chip 2011, 11, 4165–4173, doi:10.1039/c1lc20557a.
- Tu, C.; Chao, B.S.; Wu, J.C. Strategies for Improving the Maturity of Human Induced Pluripotent Stem Cell-Derived Cardi-omyocytes. Circ. Res. 2018, 123, 512–514, doi:10.1161/CIRCRESAHA.118.313472.
- Zhang, Y.S.; Aleman, J.; Arneri, A.; Bersini, S.; Piraino, F.; Shin, S.R.; Dokmeci, M.R.; Khademhosseini, A. From Cardiac Tis-sue Engineering to Heart-on-a-Chip: Beating Challenges. Biomed. Mater. 2015, 10, 034006, doi:10.1088/1748-6041/10/3/034006.
- Chu, X.; Bleasby, K.; Evers, R. Species Differences in Drug Transporters and Implications for Translating Preclinical Find-ings to Humans. Expert Opin. Drug Metab. Toxicol. 2013, 9, 237–252, doi:10.1517/17425255.2013.741589.
- Hirt, M.N.; Boeddinghaus, J.; Mitchell, A.; Schaaf, S.; Börnchen, C.; Müller, C.; Schulz, H.; Hubner, N.; Stenzig, J.; Stoehr, A.; et al. Functional Improvement and Maturation of Rat and Human Engineered Heart Tissue by Chronic Electrical Stimula-tion. J. Mol. Cell. Cardiol. 2014, 74, 151–161, doi:10.1016/j.yjmcc.2014.05.009.
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Massé, S.; Kim, J.; Reis, L.; et al. Biodegradable Scaffold with Built-In Vasculature for Organ-on-a-Chip Engineering and Direct Surgical Anastomosis.
- Nat. Mater. 2016, 15, 669–678, doi:10.1038/nmat4570.
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P.; et al. Human iPSC-Based Cardiac Microphysiological System for Drug Screening Applications. Sci. Rep. 2015, 5, 8883, doi:10.1038/srep08883.
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convo-luted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845.
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-Dimensional Bioprinting of Thick Vascularized Tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184, doi:10.1073/pnas.1521342113.
- Dhariwala, B.; Hunt, E.; Boland, T. Rapid Prototyping of Tissue-Engineering Constructs, Using Photopolymerizable Hydro-gels and Stereolithography. Tissue Eng. 2004, 10, 1316–1322, doi:10.1089/ten.2004.10.1316.
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of Inkjet Printing to Tissue Engineering. Biotechnol. J. 2006, 1, 910–917, doi:10.1002/biot.200600081.
- Yan, J.; Huang, Y.; Chrisey, D.B. Laser-Assisted Printing of Alginate Long Tubes and Annular Constructs. Biofabrication 2013, 5, 015002, doi:10.1088/1758-5082/5/1/015002.
- Beyer, S.T.; Bsoul, A.; Ahmadi, A.; Walus, K. 3D Alginate Constructs for Tissue Engineering Printed Using a Coaxial Flow Focusing Microfluidic Device. 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sen-sors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, 2013, pp. 1206–1209, doi: 10.1109/Transducers.2013.6626990.
- Gaetani, R.; Feyen, D.A.M.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.F.M.; Sluijter, J.P.G. Epicardial Application of Cardiac Progenitor Cells in a 3D-Printed Gelatin/Hyaluronic Acid Patch Preserves Cardiac Function after Myocardial Infarction. Biomaterials 2015, 61, 339–348, doi:10.1016/j.biomaterials.2015.05.005.
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D.; et al. A Multi-Cellular 3D Bioprinting Approach for Vascularized Heart Tissue Engineering Based on HUVECs and iPSC-Derived Cardiomyocytes. Sci. Rep. 2018, 8, 13532, doi:10.1038/s41598-018-31848-x.
- Lee, H.; Cho, D.-W. One-Step Fabrication of an Organ-on-a-Chip with Spatial Heterogeneity Using a 3D Bioprinting Tech-nology. Lab Chip 2016, 16, 2618–2625, doi:10.1039/c6lc00450d.
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347, doi:10.1016/j.biotechadv.2010.01.004.
- Huang, S.; Yang, Y.; Yang, Q.; Zhao, Q.; Ye, X. Engineered Circulatory Scaffolds for Building Cardiac Tissue. J. Thorac. Dis. 2018, 10, S2312–S2328, doi:10.21037/jtd.2017.12.92.
- Castilho, M.; Hochleitner, G.; Wilson, W.; van Rietbergen, B.; Dalton, P.D.; Groll, J.; Malda, J.; Ito, K. Mechanical Behavior of a Soft Hydrogel Reinforced with Three-Dimensional Printed Microfibre Scaffolds. Sci. Rep. 2018, 8, 1245, doi:10.1038/s41598-018-19502-y.
- Yildirim, Y.; Naito, H.; Didie, M.; Karikkineth, B.C.; Biermann, D.; Eschenhagen, T.; -H. Zimmermann, W. Development of a Biological Ventricular Assist Device: Preliminary Data from a Small Animal Model. Circulation 2007, 116, I16–I23.
- Lee, E.J.; Kim, D.E.; Azeloglu, E.U.; Costa, K.D. Engineered Cardiac Organoid Chambers: Toward a Functional Biological Model Ventricle. Tissue Eng. Part A 2008, 14, 215–225.
- Li, R.A.; Keung, W.; Cashman, T.J.; Backeris, P.C.; Johnson, B.V.; Bardot, E.S.; Wong, A.O.T.; Chan, P.K.W.; Chan, C.W.Y.; Costa, K.D. Bioengineering an Electro-Mechanically Functional Miniature Ventricular Heart Chamber from Human Plu-ripotent Stem Cells. Biomaterials 2018, 163, 116–127, doi:10.1016/j.biomaterials.2018.02.024.
- MacQueen, L.A.; Sheehy, S.P.; Chantre, C.O.; Zimmerman, J.F.; Pasqualini, F.S.; Liu, X.; Goss, J.A.; Campbell, P.H.; Gonzalez, G.M.; Park, S.-J.; et al. A Tissue-Engineered Scale Model of the Heart Ventricle. Nat. Biomed. Eng. 2018, 2, 930–941.
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487, doi:10.1126/science.aav9051.
- Kupfer, M.E.; Lin, W.-H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; Bhuiyan, D.; Lenz, M.; Ai, J.; Mahutga, R.R.; et al. In Situ Expansion, Differentiation and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Or-ganoid. Circ. Res. 2020, doi:10.1161/CIRCRESAHA.119.316155.
- Garoffolo, G.; Pesce, M. Mechanotransduction in the Cardiovascular System: From Developmental Origins to Homeostasis and Pathology. Cells 2019, 8, doi:10.3390/cells8121607.
- Fink, C.; Ergün, S.; Kralisch, D.; Remmers, U.; Weil, J.; Eschenhagen, T. Chronic Stretch of Engineered Heart Tissue Induces Hypertrophy and Functional Improvement. FASEB J. 2000, 14, 669–679, doi:10.1096/fasebj.14.5.669.
- Lasher, R.A.; Pahnke, A.Q.; Johnson, J.M.; Sachse, F.B.; Hitchcock, R.W. Electrical Stimulation Directs Engineered Cardiac Tissue to an Age-Matched Native Phenotype. J. Tissue Eng. 2012, 3, doi:10.1177/2041731412455354.
- Ruwhof, C. Mechanical Stress-Induced Cardiac Hypertrophy: Mechanisms and Signal Transduction Pathways. Cardiovasc. Res. 2000, 47, 23–37.
- Severs, N.J. The cardiac Muscle Cell. Bioessays 2000, 22, 188–199, doi:10.1002/(SICI)1521-1878(200002)22:2<188::AID-BIES10>3.0.CO;2-T.
- Lie-Venema, H.; van den Akker, N.M.S.; Bax, N.A.M.; Winter, E.M.; Maas, S.; Kekarainen, T.; Hoeben, R.C.; DeRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Origin, Fate, and Function of Epicardium-Derived Cells (EPDCs) in Normal and Abnormal Cardiac Development. Sci. World J. 2007, 7, 1777–1798, doi:10.1100/tsw.2007.294.
- Goette, A.; Lendeckel, U. Electrophysiological Effects of Angiotensin II. Part I: Signal Transduction and Basic Electrophysio-logical Mechanisms. Europace 2008, 10, 238–241, doi:10.1093/europace/eum283.
- Zhang, P.; Su, J.; Mende, U. Cross Talk between Cardiac Myocytes and Fibroblasts: From Multiscale Investigative Ap-proaches to Mechanisms and Functional Consequences. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1385–H1396, doi:10.1152/ajpheart.01167.2011.
- Brutsaert, D.L. Cardiac Endothelial-Myocardial Signaling: Its Role in Cardiac Growth, Contractile Performance, and Rhythmicity. Physiol. Rev. 2003, 83, 59–115, doi:10.1152/physrev.00017.2002.
- Iyer, R.K.; Odedra, D.; Chiu, L.L.Y.; Vunjak-Novakovic, G.; Radisic, M. Vascular Endothelial Growth Factor Secretion by Nonmyocytes Modulates Connexin-43 Levels in Cardiac Organoids. Tissue Eng. Part A 2012, 18, 1771–1783, doi:10.1089/ten.TEA.2011.0468.
- Garzoni, L.R.; Rossi, M.I.D.; de Barros, A.P.D.N.; Guarani, V.; Keramidas, M.; Balottin, L.B.L.; Adesse, D.; Takiya, C.M.; Man-so, P.P.; Otazú, I.B.; et al. Dissecting Coronary Angiogenesis: 3D Co-Culture of Cardiomyocytes with Endothelial or Mesen-chymal Cells. Exp. Cell Res. 2009, 315, 3406–3418, doi:10.1016/j.yexcr.2009.09.016.
- Liau, B.; Zhang, D.; Bursac, N. Functional Cardiac Tissue Engineering. Regen. Med. 2012, 7, 187–206, doi:10.2217/rme.11.122.
- Xue, T.; Cho, H.C.; Akar, F.G.; Tsang, S.-Y.; Jones, S.P.; Marbán, E.; Tomaselli, G.F.; Li, R.A. Functional Integration of Electri-cally Active Cardiac Derivatives from Genetically Engineered Human Embryonic Stem Cells with Quiescent Recipient Ven-tricular Cardiomyocytes: Insights into the Development of Cell-Based Pacemakers. Circulation 2005, 111, 11–20, doi:10.1161/01.CIR.0000151313.18547.A2.
- Ponard, J.G.C.; Kondratyev, A.A.; Kucera, J.P. Mechanisms of Intrinsic Beating Variability in Cardiac Cell Cultures and Model Pacemaker Networks. Biophys. J. 2007, 92, 3734–3752, doi:10.1529/biophysj.106.091892.
- Radisic, M.; Fast, V.G.; Sharifov, O.F.; Iyer, R.K.; Park, H.; Vunjak-Novakovic, G. Optical Mapping of Impulse Propagation in Engineered Cardiac Tissue. Tissue Eng. Part A 2009, 15, 851–860.
- Schaffer, P.; Ahammer, H.; Müller, W.; Koidl, B.; Windisch, H. Di-4-ANEPPS Causes Photodynamic Damage to Isolated Car-diomyocytes. Pflug. Arch. 1994, 426, 548–551, doi:10.1007/BF00378533.
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved Patch-Clamp Techniques for high-Resolution Cur-rent Recording from Cells and Cell-Free Membrane Patches. Pflug. Arch. 1981, 391, 85–100, doi:10.1007/BF00656997.
- Del Corsso, C.; Srinivas, M.; Urban-Maldonado, M.; Moreno, A.P.; Fort, A.G.; Fishman, G.I.; Spray, D.C. Transfection of Mammalian Cells with Connexins and Measurement of Voltage Sensitivity of Their Gap Junctions. Nat. Protoc. 2006, 1, 1799–1809, doi:10.1038/nprot.2006.266.
- Brown, K.T. Advanced Micropipette Techniques for Cell Physiology; Wiley: Hoboken, NJ, USA, 1986; ISBN 9780471909521.
- Kehat, I.; Gepstein, A.; Spira, A.; Itskovitz-Eldor, J.; Gepstein, L. High-Resolution Electrophysiological Assessment of Hu-man Embryonic Stem Cell-Derived Cardiomyocytes. Circ. Res. 2002, 91, 659–661.
- Reppel, M.; Pillekamp, F.; Brockmeier, K.; Matzkies, M.; Bekcioglu, A.; Lipke, T.; Nguemo, F.; Bonnemeier, H.; Hescheler, J. The Electrocardiogram of Human Embryonic Stem Cell-Derived Cardiomyocytes. J. Electrocardiol. 2005, 38, 166–170, doi:10.1016/j.jelectrocard.2005.06.029.
- Tung, L.; Zhang, Y. Optical Imaging of Arrhythmias in Tissue Culture. J. Electrocardiol. 2006, 39, S2–S6, doi:10.1016/j.jelectrocard.2006.04.010.
- Efimov, I.R.; Nikolski, V.P.; Salama, G. Optical Imaging of the Heart. Circ. Res. 2004, 95, 21–33, doi:10.1161/01.RES.0000130529.18016.35.
- Lieu, D.K.; Turnbull, I.C.; Costa, K.D.; Li, R.A. Engineered Human Pluripotent Stem Cell-Derived Cardiac Cells and Tissues for Electrophysiological Studies. Drug Discov. Today Dis. Models 2012, 9, e209–e217, doi:10.1016/j.ddmod.2012.06.002.
- Van Der Velden, J.; Klein, L.J.; Zaremba, R.; Boontje, N.M.; Huybregts, M.A.; Stooker, W.; Eijsman, L.; de Jong, J.W.; Visser, C.A.; Visser, F.C.; et al. Effects of Calcium, Inorganic Phosphate, and pH on Isometric Force in Single Skinned Cardiomyo-cytes from Donor and Failing Human Hearts. Circulation 2001, 104, 1140–1146, doi:10.1161/hc3501.095485.
- Karakikes, I.; Ameen, M.; Termglinchan, V.; Wu, J.C. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: In-sights into Molecular, Cellular, and Functional Phenotypes. Circ. Res. 2015, 117, 80–88.
- Hunter, P.J.; Pullan, A.J.; Smaill, B.H. Modeling Total Heart Function. Annu. Rev. Biomed. Eng. 2003, 5, 147–177, doi:10.1146/annurev.bioeng.5.040202.121537.




