| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Olga Garaschuk | + 4291 word(s) | 4291 | 2021-01-25 12:18:41 | | | |
| 2 | Rita Xu | -1692 word(s) | 2599 | 2021-02-04 02:59:03 | | |
Video Upload Options
Microglia, the innate immune cells of the brain, are commonly perceived as resident macrophages of the central nervous system (CNS). This definition, however, requires further specification, as under healthy homeostatic conditions, neither morphological nor functional properties of microglia mirror those of classical macrophages.
1. Introduction
The process of normal aging is accompanied by alterations in brain architecture. After the age of 50, brain weight decreases 2–3% per decade. As a consequence, brain weight in individuals of 80 years and older is typically 10% lower compared to that of young adults [1][2]. Although aging generally is not considered as a disease, it is a major risk factor for cognitive decline and various neurodegenerative diseases like Alzheimer’s disease (AD) or Parkinson’s disease (PD). Apart from brain atrophy, the senescent brain is considered to reside in a state of low-grade inflammation, a condition known as “inflammaging” [3]. This inflammatory state is likely caused by the ongoing stimulation of the immune system by internal or external factors, for example, long-lasting exposure to viral infection, presence of displaced or misfolded proteins of endogenous origin, cellular debris, etc. [4]. In addition, the brain is especially prone to accumulation of reactive oxygen species and oxidative stress because of its high energy demand and metabolic rate [2][5][6][7].
The aging-associated change in the inflammatory state of the brain is sensed by microglia, its major immune cells. Not surprisingly, these cells undergo the most prominent changes during the aging process. Microglia represent the first line of brain defense against endogenous and exogenous danger signals. They originate from erythromyeloid progenitors in the yolk sac, invade the brain during embryogenesis [8], and form a long-lived, autonomous, self-renewing population of cells [9]. Microglial cells exhibit a unique gene expression profile, which differentiates them from other CNS cells as well as other resident tissue macrophages. This unique identity is defined by their ontogeny as well as the influence of their specific environment in the CNS [10]. To fulfill their function as immune cells, microglia are equipped with a large repertoire of surface receptors enabling them to sense alterations in their environment [11]; e.g., expression of pattern recognition receptors enables them to detect danger-associated molecular patterns (DAMPs), like ATP or heat shock proteins, or pathogen-associated molecular patterns (PAMPs), like LPS [12][13][14].
As any disturbance of the brain environment profoundly affects the morphology and function of microglia, the morphological and functional phenotype of these cells changes along with the aging process. In the last years, it has become increasingly clear that the microglial population, present under specific conditions, is not homogeneous, but rather characterized by the presence of different phenotypes with unique properties [15][16][17]. Consistently, in the aging brain, senescent and dysfunctional as well as hyper-responsive and primed microglial phenotypes have been described.
Interestingly, the occurrence of the different microglial phenotypes seems to be sex-specific, with aging differently affecting the properties of microglia in males and females. In this context, it is important to note that gender is a predictor of susceptibility to several age-associated brain disorders. AD, for instance, has a higher (1.6–3:1) prevalence in women compared to men, whereas PD has a higher (3.5:1) prevalence in men compared to women [18]. Moreover, it seems that developmental disorders emerging early in life, e.g., schizophrenia or autism, are more common in males [19].
2. Functional Signature of Young Adult Microglia
2.1. Morphology and Surveillance
Microglia account for approximately 10% of all cells in the brain [21]. Although the microglial density remains largely constant throughout life, a certain degree of renewal and rearrangement has been reported [22][23]. Microglia in the young adult brain have a typical morphology, characterized by small somata and long, ramified processes. Microglial processes define a territorial domain of a single microglial cell, covering an area with a diameter of approximately 50 µm in the mouse as well as in the human brain [24]. Under physiological conditions, there is generally little overlap between territorial domains of microglia. Moreover, upon a relatively rare event of cell division within the healthy brain parenchyma, two sister cells show clear repulsion behavior, actively moving apart during the first three days after birth and reaching ~40 µm soma-to-soma distance by day 4 [23][25].
First seminal in vivo studies in anesthetized mice have shown that the fine microglial processes are extremely motile, continuously moving to interact with other cell types (neurons, astrocytes) or blood vessels [26][27]. This apparently random process movement enables them to monitor the current state of their immediate environment; a function which is known as surveillance. Surveillance is also important for the microglial contribution to proper wiring of the neuronal network during development [28] and the refinement of synaptic connectivity in the adult brain [29][30][31]. Therefore, microglia are thought to have an important role in the maintenance of the proper function and integrity of the neuronal circuitry. Mechanistically, the surveillance function of microglia depends on their membrane potential, which is under control of a K+-channel THIK-1 (TWIK-related halothane-inhibited K+-channel), which is tonically active under basal conditions [32].
The frequency of contacts between microglial processes and neurons is dependent on neuronal activity. Manipulations leading to reduced activity of neurons (e.g., sensory deprivation, reduction in body temperature, or application of the voltage-gated Na+-channel blocker tetrodotoxin) reduced the contact frequency between microglial processes and synapses [29]. Microglial processes can be recruited upon stimulation of glutamatergic receptors. During application of exogenous glutamate or NMDA (N-Methyl-D-aspartate), microglial processes extend towards neurons; and this recruitment is dependent on activation of neuronal NMDA receptors and accompanying Ca2+ influx, followed by ATP release from neurons and purinergic P2Y12 receptor-mediated stimulation on microglia [22][32]. Interestingly, a recent in vivo study has shown that a hyper- as well as a hypoactive state of the neural network can increase process outgrowth [34], indicating that any abnormal neuronal activity, irrespective of the direction of this abnormality, increases microglial process dynamics. Consistently, the dynamics of microglial surveillance is also changed by different kinds of anesthesia, known to modify neuronal activity [35][36]. During wakefulness, noradrenergic tone and activation of microglial β2 adrenoreceptors reduce the ramification and the surveillance territory of microglia as well as the surveillance speed of their processes [35][36]. The increased surveillance and more pronounced ramified morphology in the anesthetized brain seems to be a consequence of suppressed neuronal activity [35]. Importantly, however, also under awake conditions increases and decreases in baseline neuronal activity (i.e., shifts in the homeostatic set point) cause an increase in microglial arborization and process surveillance [35].
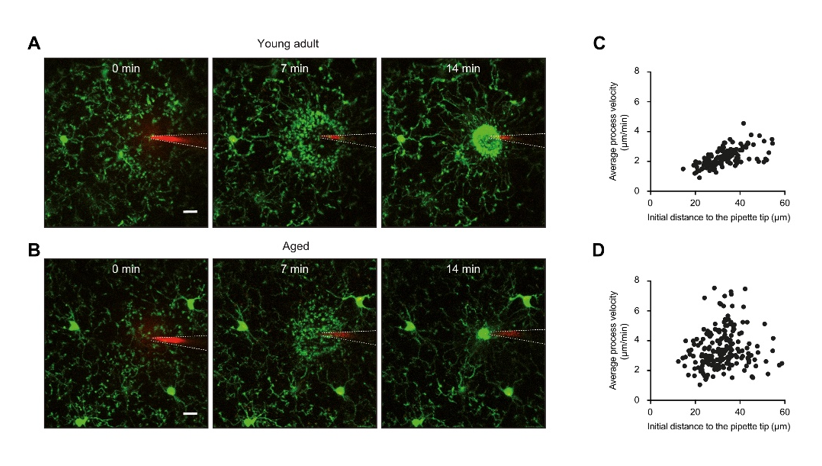
The multidirectional basal movement during resting conditions is rapidly changed towards directed movement upon injury or damage [26][37][38]. By doing so, microglial processes build up a spherical containment around the area affected by the insult, aiming at the removal of danger signals and cell debris and protection of the surrounding tissue. The built-up of a spherical containment (Figure 1A) requires a high degree of coordination between the individual processes of different microglial cells. Indeed, it turned out that the movement speed of the individual process is directly proportional to its initial distance from the DAMP-containing (insult) area [39][40] (Figure 1C). In this way, processes located further away move faster and quickly join up with the processes located closer to the insult area. Together, these processes are forming a symmetric and tight insulation compartment. In contrast to the mechanisms of basal process motility, THIK-1 is not important for directed process movement towards injury [32]. Instead, it relies on the activation of microglial P2Y12 receptors, mediated by ATP release from damaged cells [26][41]. Similar to the basal process movement, the microglial response towards (laser-induced) injury depends on the arousal state of the animal and is stronger in the anesthetized mice [35][36]. In addition, the velocity of process movement towards laser-induced damage is higher under isoflurane anesthesia, as compared to the awake condition [42].
Figure 1. Microglial process extension towards an ATP source is less coordinated in the aged brain. (A,B) Maximum intensity projection images (80–100 μm below the cortical surface, 2 μm step size), obtained in vivo in the cortex of young adult (A) and aged (B) CX3CR1GFP/+ mice. Images show the extension of processes of GFP-positive microglia (green) towards a pipette containing 5 mM ATP and 200 µM of red fluorescent dye Alexa594 at three different time points (see time stamp) after ATP application. White dashed lines accentuate the locations of the pipette. (C,D) Scatter plots showing the relationship between the initial distance of the microglial process to the tip of the ATP-containing pipette and its average movement velocity in young adult (C) and aged (D) mice. Panels (A,B) are reproduced from ref. [58] and panels (C,D) are reproduced from ref. [39].
In summary, microglia in the young adult brain have a unique morphology characterized by small somata and long, elaborate processes, which are dynamic structures continuously surveying their environment in an apparently random manner. Upon damage or injury, processes switch to the directed movement towards the insult, relying upon a high degree of coordination between individual processes of different cells.
2.2. Ca2+ Signaling
Intracellular Ca2+ signaling is actively involved into both sensor and effector functions of microglia [34][43][44][45]. Due to technical reasons, however, it has long been challenging to study microglial Ca2+ signaling in vivo. Labeling individual microglial cells by means of single-cell electroporation, our group was the first to study Ca2+ signals in microglia residing in the intact brain [37]. The results of this study showed that under healthy homeostatic conditions, somatic Ca2+ signaling in microglia is infrequent, in contrast to that of other CNS cells, like neurons and astrocytes. However, microglia vividly respond with large Ca2+ transients to local neuronal damage in their immediate vicinity. These damage-induced Ca2+ transients were mediated by activation of metabotropic purinergic receptors, which are highly expressed in microglia [46], as well as Ca2+ release from the intracellular Ca2+ stores [37]. Subsequent studies substantiated our hypothesis that abundant somatic Ca2+ transients in microglia reflect pathological alterations in tissue homeostasis, e.g., aging [39][40], laser-induced focal brain injury [47], amyloid pathology [48], or peripheral inflammation [44][47]. Interestingly, the majority of Ca2+ transients evoked by laser-induced brain injury occurred in the microglial processes only [47]. This finding raised the suspicion that microglial Ca2+ signals might exist in several flavors: (i) The ones involving the entire cell (see Figure 1D in ref. [37]), as well as (ii) those restricted to subcellular microdomains, similar to what is known for astrocytes [49]. The use of a ratiometric genetically-encoded Ca2+ indicator Twitch-2B in cortical microglia [50] allowed monitoring not only transient, but also sustained alterations of the intracellular free Ca2+ concentration ([Ca2+]i). The results showed that in young adult mice under homeostatic conditions in vivo basal [Ca2+]i in microglia is low, but increases significantly after laser-induced tissue damage, acute tissue slicing, or cell culturing [50], thus underscoring the ability of microglia to respond to the disturbance of tissue homeostasis not only with transient, but also with sustained changes in [Ca2+]i.
Change in the activity state of the surrounding neural network represents one of such disturbances of tissue homeostasis. Consistently, both an increase (caused, for example, by kainate-induced status epilepticus or activation of excitatory Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)) and a decrease (induced by isoflurane anesthesia or inhibitory DREADDs) in neuronal activity increased intracellular Ca2+ signaling in microglia [34]. The latter Ca2+ signals also compartmentalized to microglial processes, similar to the laser damage-induced Ca2+ signals described above [47]. Increased incidence of Ca2+ transients in microglial processes was directly associated with process extension and outgrowth of new processes. Of note, both somatic and process-restricted Ca2+ signaling in microglia increased after kainate-induced status epilepticus, indicating that stronger stimuli are needed to activate somatic Ca2+ signaling in microglia.
In summary, the in vivo data obtained so far show that in the young adult brain the incidence of both somatic and process-restricted microglial Ca2+ signals under homeostatic conditions is low. However, it readily increases even in response to relatively mild disturbance of tissue homeostasis, as, for example, anesthesia or activation of excitatory/inhibitory DREADDs in the surrounding neurons, with process-restricted Ca2+ signals having lower and somatic Ca2+ signals having higher activation thresholds.
2.3. Gender-Specific Differences
The incidence and disease progression of psychiatric and neurological disorders with an inflammatory component differs between male and female individuals and the X chromosome is known for its high concentration of immune-related genes [51], pointing to the possible sex-specific operation of the brain’s immune system. Consistently, microglia express receptors for sex hormones and are, therefore, differently modulated by hormone status in males and females [51]. Moreover, microglial density was shown to differ in adult female and male mice. However, the literature results are not entirely consistent. Whereas in one study, microglial cell numbers were higher in the hippocampus of 3–24 months old female mice [52], a more recent study suggests that the density of microglia in young adult mice is higher in the hippocampus and cortex of male compared to female mice [53]. In yet another study, microglial density was similar in males and females in the somatosensory cortex of 2 months old mice [54].
Male and female microglia seem to react differently to brain damage, depending on the type of insult. Upon stab wound injury in the cortex/corpus callosum, a higher microglial cell density around the injury site was found in males. Of note, these microglia had a non-reactive and neuroprotective phenotype, consistent with a less severe stab wound-induced reduction in neuronal density in male compared to female mice [55]. In contrast, during ischemia, the damaged area was larger in males compared to females, and female microglia implanted into the brains of males had a protective effect [19]. Therefore, it seems that microglia react to the disturbance or damage of brain parenchyma in a sex- and injury-specific manner.
Adult female individuals have a higher susceptibility to autoimmune and inflammatory diseases [56], and a sex-specific comparison of transcriptomes of mouse microglia revealed that female microglia have a higher expression of genes, associated with inflammatory and immune responses (Figure S2 in ref. [54]). This points to a more immune-activated state of female microglia. At the same time, a detailed characterization of sex-specific properties of cortical microglia [53] revealed larger soma size and a higher expression of MHC proteins as well as some types of purinergic receptors in male microglia. In addition, higher baseline inward and outward conductances of the cell membrane and a stronger response to ATP were observed in male compared to female microglia [53]. Consistently, the functional in vivo studies from our group also document higher alertness of microglia in young adult male compared to female mice. Thus, under basal conditions the fraction of microglia showing spontaneous Ca2+ transients was significantly higher in male compared to female mice [40]. To substantiate the differences between young adult male and female mice, we analyzed a previously published RNAseq data set [57]. In this data set, the genes associated with inflammation were significantly upregulated in young male compared with female mice. The Ca2+ signaling-related pathways in general, as well as biological processes summarized under gene ontology annotations “release of sequestered calcium into cytosol,” “calcium-mediated signaling using intracellular calcium source,” or “positive regulation of cytosolic calcium ion concentration” were all upregulated in young adult male compared to female mice, but the difference observed did not reach the level of statistical significance [40]. These data are in good agreement with a study showing a higher transcriptional activation of NF-kB, a factor playing a key role in regulating the immune response, in male microglia [19]. When comparing transcription profiles of young adult male and female microglia, this study revealed that 79% of genes which were involved in NF-kB-mediated immune or inflammatory responses were more expressed in male mice [19]. Together, the literature data reveal a clear sex-specific signature of young adult microglia. Although these data are not entirely consistent, male microglia seem to be slightly more activated under homeostatic conditions. Microglia’s reactions to insults, however, might turn out to be damage- and brain region-specific.
References
- Drachman, D.A. Aging of the brain, entropy, and Alzheimer disease. Neurology 2006, 67, 1340–1352, doi:10.1212/01.wnl.0000240127.89601.83.
- von Bernhardi, R.; Tichauer, J.E.; Eugenin, J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. Neurochem. 2010, 112, 1099–1114, doi:10.1111/j.1471-4159.2009.06537.x.
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. NY. Acad. Sci 2000, 908, 244–254, doi:10.1111/j.1749-6632.2000.tb06651.x.
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and Garb-aging. Trends Endocrinol. Metab. 2017, 28, 199–212, doi:10.1016/j.tem.2016.09.005.
- Garaschuk, O.; Semchyshyn, H.M.; Lushchak, V.I. Healthy brain aging: Interplay between reactive species, inflammation and energy supply. Ageing Res. Rev. 2018, 43, 26–45, doi:10.1016/j.arr.2018.02.003.
- Lucin, K.M.; Wyss-Coray, T. Immune activation in brain aging and neurodegeneration: Too much or too little? Neuron 2009, 64, 110–122, doi:10.1016/j.neuron.2009.08.039.
- von Bernhardi, R.; Eugenin-von Bernhardi, L.; Eugenin, J. Microglial cell dysregulation in brain aging and neurodegeneration. Aging Neurosci. 2015, 7, 124, doi:10.3389/fnagi.2015.00124.
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845, doi:10.1126/science.1194637.
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Neurosci. 2007, 10, 1538–1543, doi:10.1038/nn2014.
- Crotti, A.; Ransohoff, R.M. Microglial Physiology and Pathophysiology: Insights from Genome-wide Transcriptional Profiling. Immunity 2016, 44, 505–515, doi:10.1016/j.immuni.2016.02.013.
- Hickman, S.E.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.C.; Means, T.K.; El Khoury, J. The microglial sensome revealed by direct RNA sequencing. Neurosci. 2013, 16, 1896–1905, doi:10.1038/nn.3554.
- Brawek, B.; Garaschuk, O. Microglial calcium signaling in the adult, aged and diseased brain. Cell Calcium 2013, 53, 159–169, doi:10.1016/j.ceca.2012.12.003.
- Garaschuk, O.; Verkhratsky, A. Physiology of Microglia. Methods Mol. Biol. 2019, 2034, 27–40, doi:10.1007/978-1-4939-9658-2_3.
- Garaschuk, O. Age-related changes in microglial physiology: The role for healthy brain ageing and neurodegenerative disorders. Neuroforum 2017, 23, A182–A191.
- Stratoulias, V.; Venero, J.L.; Tremblay, M.E.; Joseph, B. Microglial subtypes: Diversity within the microglial community. EMBO J. 2019, 38, e101997, doi:10.15252/embj.2019101997.
- Masuda, T.; Sankowski, R.; Staszewski, O.; Prinz, M. Microglia Heterogeneity in the Single-Cell Era. Cell Rep. 2020, 30, 1271–1281, doi:10.1016/j.celrep.2020.01.010.
- Sankowski, R.; Bottcher, C.; Masuda, T.; Geirsdottir, L.; Sagar; Sindram, E.; Seredenina, T.; Muhs, A.; Scheiwe, C.; Shah, M.J.; et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Neurosci. 2019, 22, 2098–2110, doi:10.1038/s41593-019-0532-y.
- Villa, A.; Della Torre, S.; Maggi, A. Sexual differentiation of microglia. Neuroendocrinol. 2019, 52, 156–164, doi:10.1016/j.yfrne.2018.11.003.
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511, doi:10.1016/j.celrep.2018.05.048.
- Flurkey, K.; Currer, J.M.; Harrison, D.E. The mouse in aging research. In The Mouse in Biomedical Research, 2 ed.; Fox, J.G., Barthold, S., Davisson, M., Newcomer, C., Quimby, F., Smith, A., Eds.; American College Laboratory Animal Medicine (Elsevier): Burlington, MA, USA, 2007; pp. 6637–6672.
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170, doi:10.1016/0306-4522(90)90229-w.
- Eyo, U.B.; Peng, J.; Swiatkowski, P.; Mukherjee, A.; Bispo, A.; Wu, L.J. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. Neurosci. 2014, 34, 10528–10540, doi:10.1523/JNEUROSCI.0416-14.2014.
- Askew, K.; Li, K.; Olmos-Alonso, A.; Garcia-Moreno, F.; Liang, Y.; Richardson, P.; Tipton, T.; Chapman, M.A.; Riecken, K.; Beccari, S.; et al. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep. 2017, 18, 391–405, doi:10.1016/j.celrep.2016.12.041.
- Tvrdik, P.; Kalani, M.Y.S. In Vivo Imaging of Microglial Calcium Signaling in Brain Inflammation and Injury. J. Mol. Sci. 2017, 18, doi:10.3390/ijms18112366.
- Brawek, B.; Garaschuk, O. Monitoring in vivo function of cortical microglia. Cell Calcium 2017, 64, 109–117, doi:10.1016/j.ceca.2017.02.011.
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Neurosci. 2005, 8, 752–758, doi:10.1038/nn1472.
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318, doi:10.1126/science.1110647.
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458, doi:10.1126/science.1202529.
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. Neurosci. 2009, 29, 3974–3980, doi:10.1523/JNEUROSCI.4363-08.2009.
- Tremblay, M.E.; Lowery, R.L.; Majewska, A.K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010, 8, e1000527, doi:10.1371/journal.pbio.1000527.
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705, doi:10.1016/j.neuron.2012.03.026.
- Madry, C.; Kyrargyri, V.; Arancibia-Carcamo, I.L.; Jolivet, R.; Kohsaka, S.; Bryan, R.M.; Attwell, D. Microglial Ramification, Surveillance, and Interleukin-1beta Release Are Regulated by the Two-Pore Domain K(+) Channel THIK-1. Neuron 2018, 97, 299–312, doi:10.1016/j.neuron.2017.12.002.
- Dissing-Olesen, L.; LeDue, J.M.; Rungta, R.L.; Hefendehl, J.K.; Choi, H.B.; MacVicar, B.A. Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. Neurosci. 2014, 34, 10511–10527, doi:10.1523/JNEUROSCI.0405-14.2014.
- Umpierre, A.D.; Bystrom, L.L.; Ying, Y.; Liu, Y.U.; Worrell, G.; Wu, L.J. Microglial calcium signaling is attuned to neuronal activity in awake mice. Elife 2020, 9, doi:10.7554/eLife.56502.
- Liu, Y.U.; Ying, Y.; Li, Y.; Eyo, U.B.; Chen, T.; Zheng, J.; Umpierre, A.D.; Zhu, J.; Bosco, D.B.; Dong, H.; et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Neurosci. 2019, 22, 1771–1781, doi:10.1038/s41593-019-0511-3.
- Stowell, R.D.; Sipe, G.O.; Dawes, R.P.; Batchelor, H.N.; Lordy, K.A.; Whitelaw, B.S.; Stoessel, M.B.; Bidlack, J.M.; Brown, E.; Sur, M.; et al. Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Neurosci. 2019, 22, 1782–1792, doi:10.1038/s41593-019-0514-0.
- Eichhoff, G.; Brawek, B.; Garaschuk, O. Microglial calcium signal acts as a rapid sensor of single neuron damage in vivo. Biophys. Acta 2011, 1813, 1014–1024, doi:10.1016/j.bbamcr.2010.10.018.
- Schwendele, B.; Brawek, B.; Hermes, M.; Garaschuk, O. High-resolution in vivo imaging of microglia using a versatile nongenetically encoded marker. J. Immunol. 2012, 42, 2193–2196, doi:10.1002/eji.201242436.
- Olmedillas Del Moral, M.; Asavapanumas, N.; Uzcategui, N.L.; Garaschuk, O. Healthy Brain Aging Modifies Microglial Calcium Signaling In Vivo. Int. J. Mol. Sci. 2019, 20, 589, doi:10.3390/ijms20030589.
- Olmedillas Del Moral, M.; Frohlich, N.; Figarella, K.; Mojtahedi, N.; Garaschuk, O. Effect of Caloric Restriction on the in vivo Functional Properties of Aging Microglia. Immunol. 2020, 11, 750, doi:10.3389/fimmu.2020.00750.
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.B.; Julius, D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Neurosci. 2006, 9, 1512–1519, doi:10.1038/nn1805.
- Sun, W.; Suzuki, K.; Toptunov, D.; Stoyanov, S.; Yuzaki, M.; Khiroug, L.; Dityatev, A. In vivo Two-Photon Imaging of Anesthesia-Specific Alterations in Microglial Surveillance and Photodamage-Directed Motility in Mouse Cortex. Neurosci. 2019, 13, 421, doi:10.3389/fnins.2019.00421.
- Garaschuk, O. Imaging microcircuit function in healthy and diseased brain. Neurol. 2013, 242, 41–49, doi:10.1016/j.expneurol.2012.02.009.
- Riester, K.; Brawek, B.; Savitska, D.; Frohlich, N.; Zirdum, E.; Mojtahedi, N.; Heneka, M.T.; Garaschuk, O. In vivo characterization of functional states of cortical microglia during peripheral inflammation. Brain Behav. Immun. 2020, 87, 243–255, doi:10.1016/j.bbi.2019.12.007.
- Garaschuk, O. The role of NLRP3 inflammasome for microglial response to peripheral inflammation. Neural Regen Res. 2021, 16, 294–295, doi:10.4103/1673-5374.290889.
- Inoue, K. Purinergic systems in microglia. Cell Mol. Life Sci. 2008, 65, 3074–3080, doi:10.1007/s00018-008-8210-3.
- Pozner, A.; Xu, B.; Palumbos, S.; Gee, J.M.; Tvrdik, P.; Capecchi, M.R. Intracellular calcium dynamics in cortical microglia responding to focal laser injury in the PC::G5-tdT reporter mouse. Mol. Neurosci. 2015, 8, 12, doi:10.3389/fnmol.2015.00012.
- Brawek, B.; Schwendele, B.; Riester, K.; Kohsaka, S.; Lerdkrai, C.; Liang, Y.; Garaschuk, O. Impairment of in vivo calcium signaling in amyloid plaque-associated microglia. Acta Neuropathol. 2014, 127, 495–505, doi:10.1007/s00401-013-1242-2.
- Srinivasan, R.; Huang, B.S.; Venugopal, S.; Johnston, A.D.; Chai, H.; Zeng, H.; Golshani, P.; Khakh, B.S. Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Neurosci. 2015, 18, 708–717, doi:10.1038/nn.4001.
- Brawek, B.; Liang, Y.; Savitska, D.; Li, K.; Fomin-Thunemann, N.; Kovalchuk, Y.; Zirdum, E.; Jakobsson, J.; Garaschuk, O. A new approach for ratiometric in vivo calcium imaging of microglia. Rep. 2017, 7, 6030, doi:10.1038/s41598-017-05952-3.
- Nissen, J.C. Microglial Function across the Spectrum of Age and Gender. J. Mol. Sci. 2017, 18, 561, doi:10.3390/ijms18030561.
- Mouton, P.R.; Long, J.M.; Lei, D.L.; Howard, V.; Jucker, M.; Calhoun, M.E.; Ingram, D.K. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002, 956, 30–35, doi:10.1016/s0006-8993(02)03475-3.
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783, doi:10.1016/j.celrep.2018.08.001.
- Thion, M.S.; Low, D.; Silvin, A.; Chen, J.; Grisel, P.; Schulte-Schrepping, J.; Blecher, R.; Ulas, T.; Squarzoni, P.; Hoeffel, G.; et al. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell 2018, 172, 500–516 e516, doi:10.1016/j.cell.2017.11.042.
- Acaz-Fonseca, E.; Duran, J.C.; Carrero, P.; Garcia-Segura, L.M.; Arevalo, M.A. Sex differences in glia reactivity after cortical brain injury. Glia 2015, 63, 1966–1981, doi:10.1002/glia.22867.
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Rev. Immunol. 2016, 16, 626–638, doi:10.1038/nri.2016.90.
- Sala Frigerio, C.; Wolfs, L.; Fattorelli, N.; Thrupp, N.; Voytyuk, I.; Schmidt, I.; Mancuso, R.; Chen, W.T.; Woodbury, M.E.; Srivastava, G.; et al. The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Abeta Plaques. Cell Rep. 2019, 27, 1293–1306, doi:10.1016/j.celrep.2019.03.099.
- Brawek, B.; Skok M.; Garaschuk, O. Changing functional signatures of microglia along the axis of brain aging. Int. J. Mol. Sci. 2021, 22, 1091, doi: 10.3390/ijms22031091.