
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Scioscia | + 1678 word(s) | 1678 | 2021-02-01 08:37:36 | | | |
| 2 | Dean Liu | Meta information modification | 1678 | 2021-02-08 03:37:11 | | |
Video Upload Options
The uterosacral ligaments (USLs) are extraperitoneal structures that extend backward from the posterior surface of the cervix and upper vagina to the second-to-fourth sacral vertebrae, forming the lateral boundaries of the rectouterine and rectovaginal spaces. They are composed mainly of connective tissue along with vessels and splanchnic nerve fibers.
1. Introduction
The uterosacral ligaments (USLs) are extraperitoneal structures that extend backward from the posterior surface of the cervix and upper vagina to the second-to-fourth sacral vertebrae, forming the lateral boundaries of the rectouterine and rectovaginal spaces. They are composed mainly of connective tissue along with vessels and splanchnic nerve fibers[1]. The USLs are surrounded by the paracervical and parametrial tissues, which are fibrous and fatty connective tissues rich with vessels and nerves. These structures can be clinically evaluated by a combined rectovaginal examination and by ultrasound, and they may be the site of diseases such as endometriosis and the later stages of cervical cancer.
Endometriosis is a common, chronic, and debilitating gynecological condition that affects between 5% and 15% of women of reproductive age[2][3]. It is characterized by the growth of tissue that looks and acts like endometrial tissue, exhibiting the same response to hormonal changes; however, this abnormal tissue growth occurs outside the uterus, usually on other organs inside the pelvis and in the abdominal cavity. These growths, called endometriosis implants, lead to local inflammatory reactions that, in turn, promote a fibrotic reaction and the formation of adhesions, which prevent organs from sliding along each other normally and can result in an altered normal pelvic anatomy[4][5][6]. This cascade of events often causes menstrual and/or chronic pelvic pain, infertility, or malfunction of the affected abdominopelvic organs[2].
Endometriosis of the uterosacral ligament (USL) is a leading cause of deep dyspareunia and pelvic pain[7][8], and large endometrial nodules may also involve the parametrium and the ureter, resulting in a more complex surgery [4][9]. The posterior pelvic compartment, and the USL specifically, have been reported to be among the most common locations for endometriosis lesions[10][11]. In a study of patients with all stages of endometriosis, Chapron et al.[11] reported a prevalence of USL endometriosis of about 69%. In 83% of cases, USL endometriosis was found as an isolated lesion (study population 241, number of lesions 344)[11]. We previously studied a large cohort of patients with ASRM-stage IV endometriosis (study population 1548; number of lesions 10,466)[10][12] and reported a USL endometriosis prevalence of about 52%, with a parametrial/paracervical involvement in 25% of cases.
Cervical cancer arises from the cervix and may invade adjacent structures, such as the vagina and the paracervical tissue, as it progresses. From the International Federation of Gynecology and Obstetrics (FIGO) stage IIB onwards, the cancer may spread further to the USLs and to more distant organs[13]. The estimated incidence of cervical cancer is of 15.7 per 100,000 women, although incidence varies widely among countries, with a generally high prevalence of early-stage cervical cancer due to cervical screening programs[14]. Extracervical cancerous lesions require a more aggressive treatment and are associated with higher case-fatality rates[14][15].
2. The Uterosacral Ligament
The uterosacral ligaments are retroperitoneal structures that extend posteriorly from the uterine cervix to sacrum, defining the lateral boundaries of the rectouterine and rectovaginal spaces (Figure 1). From an anatomical perspective, the USL can be divided into three parts: cervical, intermediate, and sacral sections[1]. The cervical section is composed of dense connective tissue containing small blood vessels and small branches of the inferior hypogastric plexus (IHP).
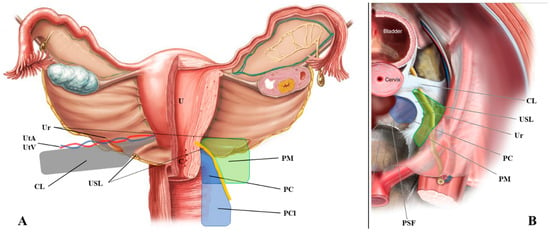
Figure 1. Anatomical definition of the uterosacral ligament, parametrium, paracervix, and paracolpium. (A) shows the frontal and (B) the upper view of the pelvic posterior compartment. Abbreviations: C, cervix; CL, cardinal ligament; PC, paracervix; PCl, paracolpium; PM, parametrium; PSF, presacral fascia; R, rectum; U, uterus; Ur, ureter; USL, uterosacral ligament; UtA, uterine artery; UtV, uterine vein.
3. The Parametrium
The parametrium is the fibrous and fatty connective tissue that surrounds the uterus. It is bordered laterally by the internal iliac vessels, medially by the uterus, superiorly by the peritoneum, and inferiorly by the ureter. This tissue contains the uterine artery and the superficial uterine vein[17].
4. The Paracervix
The paracervix is the fibrous and fatty tissue that lies beneath the parametrium (Figure 1)[18]. Like the parametrium, it is bordered laterally by the internal iliac vessels, but its medial border is the upper two-thirds of the vaginal wall and the insertion of the USL. It is the upper part of the paracolpium that contains important functional nerves and vessels. The upper limit of the paracervix is the ureter and the lower limit is the levator ani muscle and the presacral fascia. The paracervix contains the inferior vesical artery, the vaginal artery, the proximal part of the deep uterine vein, the pelvic splanchnic nerves, the distal part of the hypogastric nerve, and the IHP.
5. What Ultrasound Should Investigate and Detect
Ultrasound visualization of the USL can be obtained using a transvaginal approach[19]. These images can be used to assess the presence of disease and determine if there is any parametrial involvement. For this procedure, the ultrasound probe is placed in the posterior vaginal fornix in longitudinal axis and then rotated laterally of about 45°, showing the hyperechoic USL. Disease (either endometriosis or cancer) appears as a hypoechoic lesion that alters the regular (fibrotic) pattern of the ligament. Parametrial involvement may be seen as an extension (same echogenicity) of the primary lesion more distant from the probe.
The cervix should appear on one side of the image to ensure that the edge of the ligament that is closest to the cervix can be assessed. The first important consideration during ultrasound of the USL is to not push the probe upwards while it is correctly placed. This method allows for clear visualization of not only the USL, but also the parametrium (distal to the probe) and, most importantly, the paracervix (proximal to the probe). As shown in Figure 2, we placed a 5 Ti-CronTM polyester suture (a heavy braided suture for orthopedics, diameter 0.7 mm) along a physiological USL during surgery to confirm the ultrasound visualization of the ligament. In this way, we could simultaneously visualize the vaginal wall, the paracervical tissue, the USL, and the parametrium. All structures could be visualized from the probe to more distant regions (Supplementary Materials Video S1).
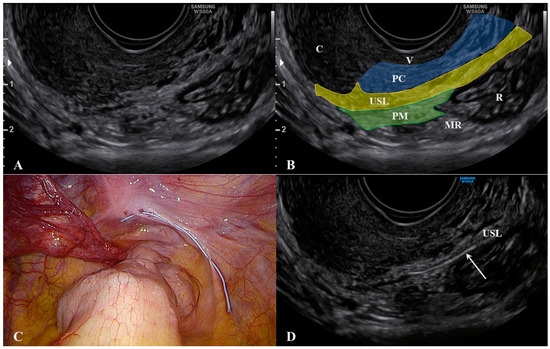
Figure 2. Ultrasound visualization of the uterosacral ligament, paracervix, and parametrium. Figure (A) shows the normal appearance and explanations are reported in figure (B). A polyester suture was placed on a physiological USL during laparoscopy (figure (C) and identified via ultrasound (arrow) in figure (D). Abbreviations: C, cervix; MR, mesorectum; PC, paracervix; PM, parametrium; R, rectum; USL, uterosacral ligament; V, vagina wall.
Another important consideration is that, during the ultrasound, strongly pushing the probe to make the USL closer to the vagina may result in a displacement (rotation) of the ligament, which can mask the actual extent of the lesion (Figure 3 and Supplementary materials Video S2).
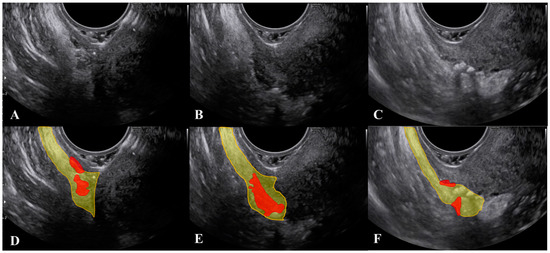
Figure 3. Modification of the visualization of lesions of the uterosacral ligament during the ultrasound when the probe is pushed. Figure 3 (D–F) represent the marked images respectively of figure (A–C). The uterosacral ligament is marked in yellow and lesions in red.
To obtain a correct visualization of both the USL and the paracervical and parametrial tissues, we suggest not only relying on the subjective feeling of the operator (i.e., not pushing), but also visualizing the small vessels within the lowest part of the USL using color Doppler (Figure 4 and Supplementary Materials Video S3). These thin vessels may be temporarily closed by pushing the probe. Ligament rotation and vessel disappearance are particularly evident for endometriosis lesions but are less pronounced in cases of cervical cancer spread, as the latter lesions are integral with the cervix (Figure 5) and the cervix is less easy to rotate.
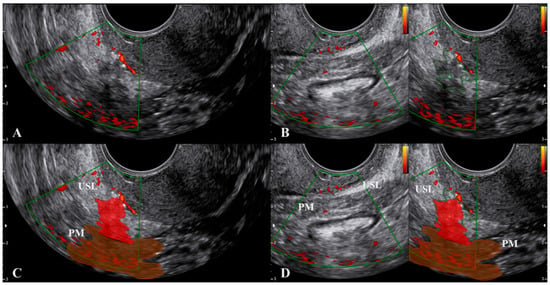
Figure 4. Visualization of the small vessels within the lowest part of the uterosacral ligament using color Doppler. Figure (C,D) are the marked images of figure (A,B), respectively. Endometriosis lesions of the uterosacral ligament and parametrium are in red. Abbreviations: PM, parametrium; USL, uterosacral ligament.
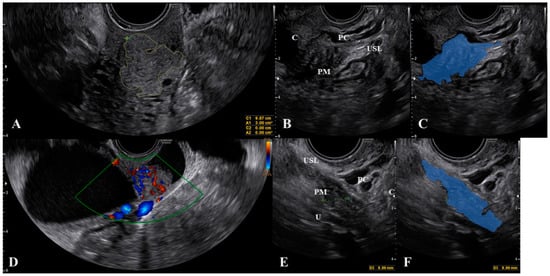
Figure 5. Cervical carcinoma (A,D) that spreads to the uterosacral ligaments (B) as marked in figure (C). Figure (E) demonstrate the uterosacral spread of the cancer as marked in figure (F). Abbreviations: C, cervix; PC, paracervix; PM, parametrium; U, ureter; USL, uterosacral ligament.
The diagnostic accuracy for endometriosis lesions evaluated using ultrasound has been reported as moderate (sensitivity 0.67, specificity 0.86)[20], which is similar to the accuracy of MRI (sensitivity 0.70, specificity 0.93)[21]. Alcazar et al.[22] assessed parametrial infiltration in cervical cancer and reported a similar diagnostic accuracy for ultrasound (sensitivity 0.78, specificity 0.96), which was also comparable to the performance of MRI. For many years, MRI was the diagnostic gold standard for assessing the extent of lesions palpated with a bimanual combined rectovaginal examination (Figure 6).
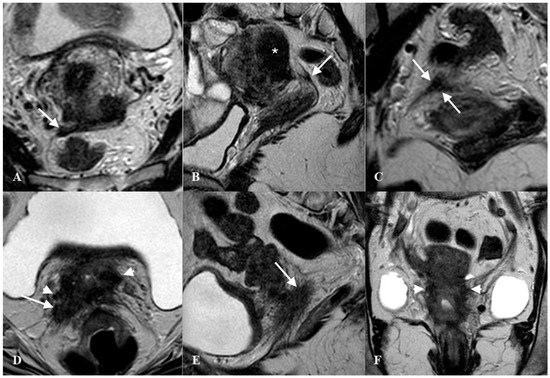
Figure 6. Thickened right uterosacral ligament in a woman with deep infiltrating endometriosis and adenomyosis. Axial HR T2w-Turbo Spin Echo (TSE) image (A), Sagittal HR T2w-TSE image (B), Coronal HR T2w-TSE image (C). The right uterosacral ligament shows a nodular thickening (arrows). Diffuse adenomyosis of posterior uterine wall (*). Advanced cervical cancer infiltrating right uterosacral ligament (arrow) and parametrium (arrowhead). Axial HR T2w-Turbo Spin Echo (TSE) image (D), Sagittal HR T2w-TSE image (E), Coronal HR T2w-TSE image (F).
Recently, fusion imaging has gained interest for determining USL and parametrial involvement in endometriosis and cervical cancer spread[23][24][25]. Fusion imaging, also referred to as real-time virtual sonography, displays live ultrasound imagery and previously-acquired MRI images side-by-side on a single screen. This technique requires an average of 13 min to perform[24], but early work has shown that it may improve the detection rate of parametrial involvement[23][24]. Larger studies are required to confirm these pivotal findings. One of the main advantages of fusion imaging is the visualization of peripheral nerves (i.e., the hypogastric nerve). Peripheral nerves cannot be seen via ultrasound but are visible with an MRI; although MRI is multiplanar, it is more expensive, requires between 20 and 30 min to be completed, and does not allow a real-time evaluation. During fusion imaging, the MRI image reconstruction follows all ultrasound probe movements (real-time evaluation), making it possible to obtain oblique sections with a clear visualization of nerves[25].
References
- Vu, D.; Haylen, B.T.; Tse, K.; Farnsworth, A. Surgical Anatomy of the Uterosacral Ligament. Int. Urogynecol. J. 2010, 21, 1123–1128.
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256.
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799.
- Lima, R.; Abdalla-Ribeiro, H.; Nicola, A.L.; Eras, A.; Lobao, A.; Ribeiro, P.A. Endometriosis on the Uterosacral Ligament: A Marker of Ureteral Involvement. Fertil. Steril. 2017, 107, 1348–1354.
- Guerriero, S.; Condous, G.; Van den Bosch, T.; Valentin, L.; Leone, F.P.G.; Van Schoubroeck, D.; Exacoustos, C.; Installé, A.J.F.; Martins, W.P.; Abrao, M.S.; et al. Systematic Approach to Sonographic Evaluation of the Pelvis in Women with Suspected Endometriosis, Including Terms, Definitions and Measurements: A Consensus Opinion from the International Deep Endometriosis Analysis (IDEA) Group. Ultrasound Obstet. Gynecol. 2016, 48, 318–332.
- Fratelli, N.; Scioscia, M.; Bassi, E.; Musola, M.; Minelli, L.; Trivella, G. Transvaginal Sonography for Preoperative Assessment of Deep Endometriosis. J. Clin. Ultrasound 2013, 41, 69–75.
- Chopin, N.; Vieira, M.; Borghese, B.; Foulot, H.; Dousset, B.; Coste, J.; Mignon, A.; Fauconnier, A.; Chapron, C. Operative Management of Deeply Infiltrating Endometriosis: Results on Pelvic Pain Symptoms According to a Surgical Classification. J. Minim. Invasive Gynecol. 2005, 12, 106–112.
- Fauconnier, A.; Chapron, C.; Dubuisson, J.-B.; Vieira, M.; Dousset, B.; Bréart, G. Relation between Pain Symptoms and the Anatomic Location of Deep Infiltrating Endometriosis. Fertil. Steril. 2002, 78, 719–726.
- Mabrouk, M.; Raimondo, D.; Arena, A.; Iodice, R.; Altieri, M.; Sutherland, N.; Salucci, P.; Moro, E.; Seracchioli, R. Parametrial Endometriosis: The Occult Condition That Makes the Hard Harder. J. Minim. Invasive Gynecol. 2019, 26, 871–876.
- Scioscia, M.; Bruni, F.; Ceccaroni, M.; Steinkasserer, M.; Stepniewska, A.; Minelli, L. Distribution of Endometriotic Lesions in Endometriosis Stage IV Supports the Menstrual Reflux Theory and Requires Specific Preoperative Assessment and Therapy. Acta Obstet. Gynecol. Scand 2011, 90, 136–139.
- Chapron, C.; Fauconnier, A.; Vieira, M.; Barakat, H.; Dousset, B.; Pansini, V.; Vacher-Lavenu, M.C.; Dubuisson, J.B. Anatomical Distribution of Deeply Infiltrating Endometriosis: Surgical Implications and Proposition for a Classification. Hum. Reprod. 2003, 18, 157–161.
- Revised American Society for Reproductive Medicine Classification of Endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821.
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical Cancer. Lancet 2019, 393, 169–182.
- Landy, R.; Pesola, F.; Castañón, A.; Sasieni, P. Impact of Cervical Screening on Cervical Cancer Mortality: Estimation Using Stage-Specific Results from a Nested Case–Control Study. Br. J. Cancer 2016, 115, 1140–1146.
- Muallem, M.Z.; Diab, Y.; Sehouli, J.; Fujii, S. Nerve-Sparing Radical Hysterectomy: Steps to Standardize Surgical Technique. Int. J. Gynecol. Cancer 2019, 29, 1203–1208.
- Yabuki, Y.; Sasaki, H.; Hatakeyama, N.; Murakami, G. Discrepancies between Classic Anatomy and Modern Gynecologic Surgery on Pelvic Connective Tissue Structure: Harmonization of Those Concepts by Collaborative Cadaver Dissection. Am. J. Obstet. Gynecol. 2005, 193, 7–15.
- Ceccaroni, M.; Clarizia, R.; Roviglione, G.; Ruffo, G. Neuro-Anatomy of the Posterior Parametrium and Surgical Considerations for a Nerve-Sparing Approach in Radical Pelvic Surgery. Surg. Endosc. 2013, 27, 4386–4394.
- Ercoli, A.; Delmas, V.; Fanfani, F.; Gadonneix, P.; Ceccaroni, M.; Fagotti, A.; Mancuso, S.; Scambia, G. Terminologia Anatomica versus Unofficial Descriptions and Nomenclature of the Fasciae and Ligaments of the Female Pelvis: A Dissection-Based Comparative Study. Am. J. Obstet. Gynecol. 2005, 193, 1565–1573.
- Leonardi, M.; Martins, W.P.; Espada, M.; Arianayagam, M.; Condous, G. Proposed Technique to Visualize and Classify Uterosacral Ligament Deep Endometriosis with and without Infiltration into Parametrium or Torus Uterinus. Ultrasound Obstet. Gynecol. 2020, 55, 137–139.
- Guerriero, S.; Ajossa, S.; Minguez, J.A.; Jurado, M.; Mais, V.; Melis, G.B.; Alcazar, J.L. Accuracy of Transvaginal Ultrasound for Diagnosis of Deep Endometriosis in Uterosacral Ligaments, Rectovaginal Septum, Vagina and Bladder: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2015, 46, 534–545.
- Guerriero, S.; Saba, L.; Pascual, M.A.; Ajossa, S.; Rodriguez, I.; Mais, V.; Alcazar, J.L. Transvaginal Ultrasound vs Magnetic Resonance Imaging for Diagnosing Deep Infiltrating Endometriosis: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2018, 51, 586–595.
- Alcazar, J.L.; García, E.; Machuca, M.; Quintana, R.; Escrig, J.; Chacón, E.; Mínguez, J.A.; Chiva, L. Magnetic Resonance Imaging and Ultrasound for Assessing Parametrial Infiltration in Cervical Cancer. A Systematic Review and Meta-Analysis. Med. Ultrason. 2020, 22, 85–91.
- Millischer, A.-E.; Salomon, L.J.; Santulli, P.; Borghese, B.; Dousset, B.; Chapron, C. Fusion Imaging for Evaluation of Deep Infiltrating Endometriosis: Feasibility and Preliminary Results: Fusion Imaging of Endometriosis. Ultrasound Obstet. Gynecol. 2015, 46, 109–117.
- Moro, F.; Gui, B.; Arciuolo, D.; Bertoldo, V.; Borzi, R.; Romeo, P.; Petta, F.; Cambi, F.; Pasciuto, T.; Zannoni, G.F.; et al. Fusion Imaging of Ultrasound and MRI in the Assessment of Locally Advanced Cervical Cancer: A Prospective Study. Int. J. Gynecol. Cancer 2020, 30, 456–465.
- Scioscia, M.; Virgilio, B.A.; Scardapane, A.; Pontrelli, G. Fusion Imaging: A Novel Diagnostic Tool for Nerve-Sparing Surgery for Deep Infiltrating Endometriosis. J. Minim. Invasive Gynecol. 2020, 27, 246–247.




