
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José S. Câmara | + 3363 word(s) | 3363 | 2021-02-01 04:09:17 | | | |
| 2 | Peter Tang | -3 word(s) | 3360 | 2021-02-08 11:17:41 | | |
Video Upload Options
Polyphenols as one of the most abundant classes of bioactive compounds present in plant-based foods emerge as a promising approach for the development of efficacious preventive agents against NCDs with reduced side effects.
1. Introduction
Non-communicable diseases (NCDs), mainly cardiovascular diseases (CVDs), cancer, chronic respiratory diseases, neurodegenerative diseases (NDs), and diabetes, represent a severe burden worldwide. According to the most recent data, NCDs caused over 70% of the 57 million deaths worldwide in 2016 [1]. The major risk factors contributing to this scenario have been identified as the combination of an unhealthy diet, poor physical activity, and alcohol and smoking abuse. Diet, in particular, is considered a leading risk factor for illness, death, and disability and it is estimated that one in five deaths are associated with a poor diet [2]. For this reason, dietary intervention holds great potential in the prevention, mitigation, or even treatment of the most prevalent NCDs. In fact, epidemiological evidence shows that the prevalence of CVDs, cancer, diabetes, and neurodegenerative diseases is lower in populations with diets involving higher ingestion of bioactive compounds, namely the Mediterranean diet [3][4].
2. Food Bioactive Compounds from Triterpenes to Polyphenols
Food bioactive compounds (FBCs) refer to all compounds, which are mostly without nutritional value and naturally present in food that exert a certain bioactive effect on the human body [5][6][7]. Often, such biological activity is acknowledged in compounds that have a positive effect, but this classification is very narrow because negative effects on an organism are also a form of bioactivity. This includes adverse effects such as toxicity, allergenicity, and mutagenicity, which are usually dependent on the dose and bioavailability of a given substance [5]. Nevertheless, in this review, FBCs will designate the dietary compounds that can elicit protective effects in our organism, therefore promoting its health and fitness. In this context, bioactive compounds present in popular beverages, such as tea, coffee, and wine, that constitute important sources of these compounds in the human diet will also be considered. FBCs encompass a wide range of compounds, mostly produced by plants as secondary metabolites, and used in different functions as competition, defence, attraction, and signalling [6][8] (Figure 1). Flavonoids, for instance, are protective agents against free radicals generated during photosynthesis. In turn, some terpenoids have been reported to attract pollinators or seed dispersers or inhibit competing plants and different alkaloids are used to repel herbivores and insects [6]. FBCs can be essentially found in fruits, vegetables, grains, and leaves and despite their importance for our health, there are no recommended daily intake values for the ingestion of most of the FBCs as it happens with proteins, lipids, or vitamins [5][9].
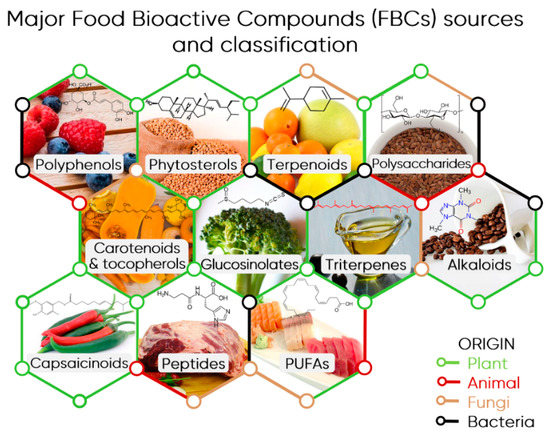
Figure 1. Major Food Bioactive Compounds (FBCs) sources and classification. An illustrative example of a source and compound is indicated for each class: polyphenols (chlorogenic acids in blueberry and raspberry fruits), phytosterols (stigmasterol in soybean), terpenoids (limonene in citrus fruits), polysaccharides (cellulose in flax seeds), carotenoids & tocopherols (β-carotene/vitamin A), glucosinolates (sulforaphane in broccoli), triterpenes (squalene from olive oil), alkaloids (caffeine in coffee beans), capsaicinoids (capsaicin in peppers), bioactive peptides (carnosine in red meat), and PUFAs (polyunsaturated fatty acids, docosahexaenoic acid—DHA, in different fishes).
3. Structure, Classification, and Biosynthesis of Polyphenols
3.1. Structure and Classification
Polyphenols constitute one of the most common and widespread groups of phytochemicals in the plant kingdom with more than 8000 phenolic structures currently known [10]. This heterogeneous group of compounds is chemically characterized by an aromatic ring with at least one hydroxyl group and their structure can vary from simple molecules, like phenolic acids, to highly polymerized compounds, such as tannins [11][12].
There are several ways to classify polyphenols including their origin, biological function, and chemical structure [10][13]. This last one is probably the most adopted classification and divides polyphenols into two main groups: flavonoids and non-flavonoids (Figure 2) [11].
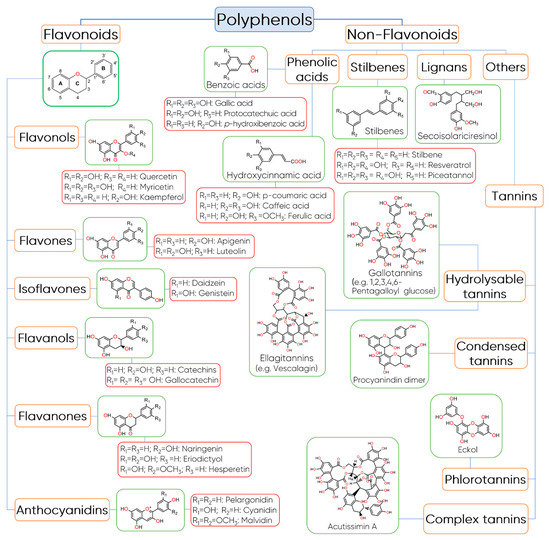
Figure 2. Classification and structure of polyphenols.
Flavonoids are very abundant in plant-based foods, such as fruits, vegetables, chocolate, tea, wine, among others, and to date, several hundred different flavonoids have been described and the number continues to increase [14][15][16]. In general, these compounds share a common basic structure of diphenylpropanes (C6-C3-C6), which consists of two benzene rings (Rings A and B) connected by a 3-carbon bridge, which usually forms an oxygenated heterocycle (Ring C) [17]. Based on the hydroxylation pattern and variations in the heterocyclic ring, flavonoids can be divided into six major subclasses including flavonols, flavones, isoflavones, flavanols, anthocyanidins, and flavanones [11].
Flavonols are mainly characterized by the presence of unsaturation in the heterocyclic ring, between the C2 and C3 carbons, a hydroxyl group in position 3, and by the presence of a ketone group in C4 [18]. Quercetin and kaempferol are the predominant phenolic compounds in this class followed by myricetin, isorhamnetin, fisetin, morin, and their glycoside derivatives [19]. Flavones differ from flavonols in terms of the absence of the 3-hydroxyl group in Ring C and the main components of this group are apigenin and luteolin. They are mainly present in their glycoside forms [11].
Flavanols or flavan-3-ols are another important class of flavonoids that are characterized by the presence of a saturated heterocyclic ring and a hydroxyl group in C3 [18]. Flavanols exist in both the monomer form (catechins) and the polymer form (proanthocyanidins, which are traditionally considered to be condensed tannins) [10][13]. Catechin and epicatechin are the predominant flavanols in fruits, while epigallocatechin, gallocatechin, and epigallocatechin gallate are commonly found in seeds of leguminous plants, grapes, and in tea [20][21].
Flavanones have a saturated heterocyclic ring, like flavanols, but with a ketone group in C4 and no hydroxyl group in C3. These flavonoids constitute a minority group in food, despite the fact they appear at high concentrations in citrus and tomatoes, and in certain aromatic plants [13][22]. The main aglycones are eriodictyol found in lemons, hesperedin in oranges, and naringenin in grapefruit [22].
Isoflavonoids, such as isoflavones, differ from the other classes of flavonoids because the Ring B is attached to C3 in Ring C instead of C2. Isoflavones have structural similarities to estrogens and they are occasionally referred to as “phytoestrogens” [23]. These flavonoids are found almost exclusively in leguminous plants, in which daidzein and genistein are the main isoflavones in soy [24].
Anthocyanidins are another important group of flavonoids that are responsible for the red, blue, and purple colours of the majority of flowers, fruits, vegetables, and certain varieties of grains, such as black rice [25]. In terms of chemical structure, anthocyanidins are polymethoxy or polyhydroxy derivatives of the flavylium cation, containing a C15 backbone structure, and the most commonly found are cyanidin, delphinidin, pelargonidin, peonidin, petunidin, and malvidin [26]. When these flavonoids are found in their glycosidic form, i.e., linked to a sugar moiety, they are called anthocyanins [18]. Commonly, the sugars bonded to anthocyanidins are monosaccharides (galactose, glucose, arabinose, and rhamnose) and di- or tri-saccharides formed by the combination of the four monosaccharides [27]. The main differences between anthocyanins and anthocyanidins results from (i) the number, the location, and the nature of sugars bonded to the molecule; (ii) the number and type of aromatic or aliphatic acids linked to the sugar; (iii) the position and number of hydroxyl groups; and (iv) the degree of methylation of these groups [28].
Regarding non-flavonoids compounds, this group includes phenolic acids, stilbenes, lignans, and tannins. Phenolic acids are compounds characterized by a phenol ring that contain at least one carboxylic acid functionality [29]. In general, they are derived from two main phenolic compounds, namely benzoic acid and cinnamic acid. These compounds occur predominantly as hydroxybenzoic and hydroxycinnamic acids and may be found either in their free or conjugated forms [30]. The hydroxybenzoic acids are the simplest phenolic acids found in nature and contain seven carbon atoms (C6-C1), while hydroxycinnamic acids are characterized by a C6-C3 chain and are rarely found in their free form in plants [13]. Typically, they exist in the form of esters of hydroxy acids, i.e., quinic, shikimic, and tartaric, besides they are derivatives of sugars [18]. Caffeic, p-coumaric, ferulic, and sinapic acids are some representative examples of hydroxycinnamic derivatives, while vanillic, gallic, and syringic acids belong to hydroxybenzoic acids.
Stilbenes are a group of polyphenols derived from the phenylpropanoid pathway, which are characterized by two phenyl rings connected by a two-carbon methylene bridge (C6-C2-C6) [31]. Among stilbenes, resveratrol is probably the most prominent compound and one of the most studied due to its potent biological activities [32]. It has been found in several foods including grapes, peanuts, mulberries, and red wine [32].
Lignans is a group of natural non-flavonoids phytoestrogens structurally characterized by the combination of two phenylpropanoid units connected at the β and β' carbon atoms [33]. They have a wide occurrence in nature and the main sources of dietary lignans are whole-grain cereals, oilseeds, fruit, vegetables, and some beverages, such as tea, coffee, and wine [11]. These compounds may occur in the form of aglycones and glycosides and the most predominant dietary lignans are secoisolariciresinol, matairesinol, medioresinol, pinoresinol, lariciresinol, and syringaresinol [11].
Tannins are another large group of complex biomolecules of phenolic nature synthesized by a wide diversity of plants [34]. Based on their chemical structure, tannins are divided into four main categories: hydrolysable tannins, which are further subdivided into gallotannins and ellagitannins, condensed tannins, phlorotannins, and complex tannins. Gallotannins are tannins constituted by galloyl units or their meta-depsidic derivatives that are linked to diverse polyol-, catechin-, or triterpenoid units, while ellagitannins are tannins with at least two C–C coupled galloyl units with no glycosidically-bonded catechin unit [35]. Condensed tannins, also called proanthocyanindins, are oligomers or polymers composed of flavan-3-ol nuclei [36]. The most common basic units of condensed tannins are (+)-catechin, (+)-gallocatechin, (−)-epicatechin, (−)-epigallocatechin, and (−)-epigallocatechin gallate [36].
Phlorotannins, of which their common structural base is the phloroglucinol, are a class of tannins found in brown algae (Phaeophyta) [34]. Examples of phlorotannins identified in marine brown algae are phloroglucinol, eckol, fucodiphloroethol G, phlorofucofuroeckol A, 7-phloroeckol, dieckol, and 6,6-bieckol [37].
Complex tannins are a particular group of tannins in which a catechin unit is linked glycosidically to an ellagitannin or a gallotannin unit [35]. As the name implies, the structure of these compounds can be very complex, as in the case of Acutissimin A and eugenigrandin A, in which a gallocatechin or catechin, respectively, are complexed through a carbon–carbon bond to an ellagitannin unit [34].
3.2. Biosynthesis
The biosynthesis of polyphenols involves a complex network of routes that include the metabolism pathways of shikimic acid and malonic acid [38]. While the first one produces the most plant phenolic compounds, the second pathway (malonic acid) is an important source of phenolics in bacteria and fungi but is less significant in higher plants [38]. The shikimate pathway converts simple carbohydrates derived from the pentose phosphate pathway and glycolysis into the aromatic amino acids tyrosine and phenylalanine, which are the precursor compounds of the phenylpropanoids (Figure 3) [39]. In most vascular plants, phenylalanine is the primary substrate for phenylpropanoid synthesis, while tyrosine is used to a lesser extent in some plants [39].
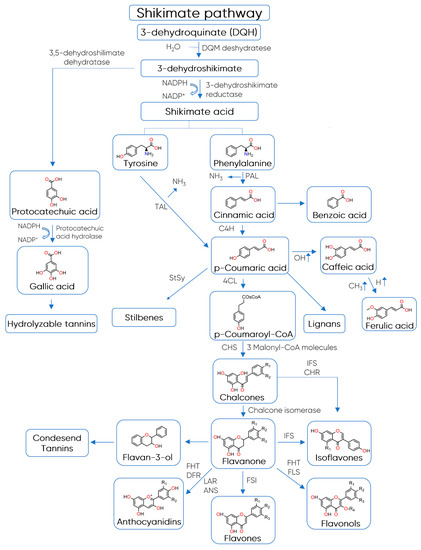
Figure 3. Biosynthesis of polyphenols. Abbreviations: 4CL—4-coumaryl:CoA ligase, ANS—anthocyanidin synthase, C4H—acid-4-hydroxylase, CHR—chalcone reductase, CHS—chalcone synthase, DFR—dihydroflavanone reductase, FHT—flavanone hydroxytransferase, FLS—flavonol synthase, FSI—flavanone synthase, IFS—isoflavanone synthase, LAR—leucoanthocyanidin synthase, PAL—phenylalanine ammonia lyase, StSy—stilbene synthase, TAL—tyrosine ammonia lyase (adapted from Fowler and Koffas [40] and de Araújo et al. [41]).
The key reaction that connects primary to secondary phenolic metabolism is catalysed by the enzyme phenylalanine ammonia lyase (PAL), where L-phenylalanine is deaminated to produce trans-cinnamic acid, leading to the C6–C3 structures [38]. The final intermediate 4-coumaroyl-CoA and three molecules of malonyl-CoA are then condensed to produce naringenin chalcone by the enzyme chalcone synthase (CHS) [10]. Chalcone is isomerized by chalcone flavanone isomerase into a flavanone, which is the basic skeleton to all flavonoid classes [10].
4. Polyphenols and Health
A substantial body of evidence that has accumulated over the past decades indicates that phytochemicals, including polyphenols, terpenoids, alkaloids, and sulphur-holding compounds, can have positive outcomes on human health by virtue of their biological activities, such as antioxidant, antimicrobial, and anti-inflammatory activities. Amidst phytocomponents, polyphenols are the most investigated worldwide. Contemporary research has demonstrated that the mechanism of action implied in the protective effects of polyphenols occurs via cellular signalling pathways and it is not directly triggered by epigenetic actions in the context of physiological and pathological limitations. Besides, recent studies have evidenced that the polyphenol ingestion of more than 1 g/day is correlated with diminished onset and progress of chronic ailments related to oxidative stress (Figure 4), especially CVDs and NDs, age-related pathologies, type 2 diabetes mellitus, as well as several forms of cancer [42][43].
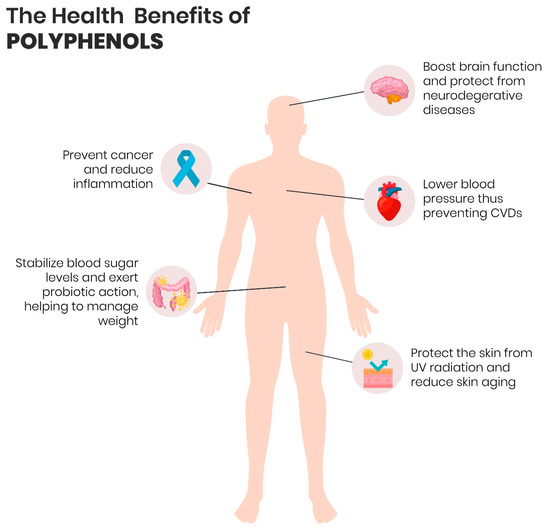
Figure 4. The potential benefits of dietary consumption of polyphenols for human health.
4.1. Cancer
Cancer is a disease induced by the abnormal and uncontrolled growth of cells that can interfere with the normal functions of the host. Worldwide, one in six deaths is caused by cancer, which corresponds to 30% of all premature deaths in adults (30–69 years). Furthermore, the number of cancer deaths is expected to double in the current century [44]. It is established that cancer development may be influenced by hereditary factors and/or lifestyle. In fact, the pathways that lead to cell mutagenesis remain poorly elucidated. However, it is known that the components of diet can exert a protective action against carcinogenesis [44][45]. Polyphenols are natural compounds present in many plants that according to literature, influence in the prevention of malignancy neoplasia [44][45]. The formation of reactive oxygen species (ROS) is reputable to produce several negative outcomes in the organism, such as inflammatory and chronic diseases. Under normal conditions, ROS production is balanced by cellular antioxidant activity; however, it can be affected by immune responses against extracellular pathogens and inflammation, which can lead to overproduction of ROS, resulting in an imbalance between pro-oxidant and antioxidant systems. The excess of ROS may be a factor that increases cell proliferation through mutations in DNA, leading to the development of carcinomas [45][46]. Thus, the antioxidant activity of the polyphenols can favour the normal functioning of cells. Also, some compounds, called anti-cancer, act on genome stability, and increase the cellular antioxidant protection by induction of antioxidant enzymes [45][47]. Some polyphenols, such as resveratrol, quercetin, apigenin, curcumin, among others, act in the upregulation of p53 protein expression in several cancer cell lines, through phosphorylation, acetylation, and reduction of oxidative stress, which can lead to cell cycle arrest, DNA repair, and finally, to apoptosis of malignant cells. Interestingly, the increasing p53 expression can overcome chemoresistance of cancer cells by reduction of mutation p53 [46]. Some authors verified that low dose concentrations of polyphenols have decreased cell cancer viability in in vitro models. For instance, the flavones hispidulin and luteolin showed high cytotoxic actions against human leukaemia cells (MV4-11 cell line), being the induced cell apoptosis achieved at lower concentrations, with IC50 values of 8.2 and 3.1 μM, respectively [48]. Overall, in in vivo studies, these bioactive compounds act mainly by inhibiting tumour growth and reducing their size. Additionally, some flavonoids have displayed inhibitory effects against cancer metastasis, as in the case of anthocyanins and resveratrol, which prevented melanoma lung metastasis in mouse models [49][50].
Polyphenols have shown antiproliferative activities on multidrug-resistant cells. This is the case for alpinumisoflavone, which in low concentrations (IC50 value of 5.41 μM), inhibited the growth of doxorubicin-resistant leukaemia cells (CEM/ADR5000 cell line) [51]. Similarly, quercetin has the ability to reverse the resistance of lung cancer cells to paclitaxel drug, inhibiting Akt and depolarization of mitochondrial membrane potential, while exerting synergistic effects with the cancer-drug, increasing its therapeutic action [52]. Luteolin has also presented a synergetic effect in lung cancer treatment with tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) [53]. Moreover, the combined use of polyphenols with chemotherapy can be interesting to mitigate the side effects of treatment, such as reported in the work of Tajaldini et al. [54], where they verified that the treatment against oesophageal cancer in mice using doxorubicin combined with naringin or orange-peel extract inhibited tumour growth, successfully increased apoptotic cell death, and in contrast to the chemotherapy used alone, resulted in lower systemic toxicity, decreased oxidative stress, and body weight maintenance.
4.2. Cardiovascular Diseases (CVDs)
CVDs are the main causes of death worldwide. The incidence of these disorders increases with non-modifiable factors, e.g., age, gender, and hereditary conditions; however, modified factors also have an important influence in the prevention or development of CVDs, such as cholesterol levels, obesity, hypertension, type 2 diabetes mellitus control, diet, smoking, stress, and other conditions [47][55]. Some polyphenols have been extensively investigated due to their cardioprotective actions, namely vasodilator and antiplatelet potentials, as well as their ability to reduce both blood pressure and LDL-cholesterol. Hypercholesterolemia is a condition that significantly increases the risk of CVDs, mainly atherosclerosis [56]. Numerous studies have verified the positive effects of polyphenols ingestion on blood cholesterol levels. In clinical trial studies, daily anthocyanin supplementation (320 mg/24 days) played a significant role in the reduction of total cholesterol (TC) and LDL-cholesterol (LDL-C) [56]; oral administration of catechins from green tea (1315 mg/day/12 months) was efficient to reduce LDL-C in postmenopausal women without altering high-density lipoprotein cholesterol (HDL-C) concentrations [57].
In diabetic patients, treatment with polyphenols also proved to be effective in controlling the parameters of the disease. Resveratrol treatment (800 mg/day/2 months) has shown promising results in type 2 diabetes patients: it not only improved antioxidant activity in the blood, but also reduced both body weight and blood pressure [58]. In a diabetic-mouse model, this stilbene administration lowered blood glucose, plasma triglyceride levels, and inflammation factors [59].
Some authors report the protective effects of curcumin in patients with CVDs. This curcuminoid has high anti-inflammatory and antioxidant activities that act on the factors of transcription regulation, which in turn make them act against the development and progression of CVDs, such as atherosclerosis, myocardial infarction, stroke, cardiac hypertrophy, and aortic aneurysm [55].
Overweight and obesity are conditions associated with several CVDs. In some cases, an imbalance in the individual's intestinal flora may be a highly contributing factor to these conditions. Regarding this, in vivo studies have shown that the oral administration of polyphenols, namely catechins, anthocyanins, and procyanidins, simulate the growth of prebiotics microorganisms, e.g., Lactobacillus, Bifidobacterium, Akkermansia, Roseburia, and Faecalibacterium spp, which lead to a reduction of fat mass, liver steatosis, body weight gain, and also to the regularization of glucose metabolism [60].
4.3. Neurodegenerative Diseases (NDDs)
The population's life expectancy has increased worldwide, which may determine the rise of the burden of age-related global diseases, mainly neurodegenerative diseases (NDDs), since the occurrence of such disorders is associated with age progression (prevalence tends to increase after 65 years). When the human central nervous system (CNS) stops functioning properly, neuron regeneration is inhibited, thus leading to death. A significant increase in neuronal loss happens in Alzheimer's disease (AD), Parkinson's disease (PD), and multiple sclerosis, among others. Although NDDs have been widely studied, so far, there is no cure for such deleterious illnesses. NDDs treatments aim to slow down the degenerative processes of neurons [61]. Oxidative stress has been addressed as a major contributor to the development and progression of neurodegeneration; thus, several studies have explored the use of polyphenols against NDDs. A diet rich in polyphenols can contribute to the neurological health of the elderly. For example, the daily ingestion of anthocyanin-rich cherry juice (200 mL/12 weeks) by older adults (>70 years) with mild to moderate dementia during 12 weeks was sufficient to significantly improve the verbal fluency, as well as short- and long-term memory [62]. In addition, the oral administration of polyphenols from white grape juice (20–40 mg/kg/day) decreased inflammatory and oxidative processes in autoimmune encephalomyelitis-induced mice, which is the most used model for multiple sclerosis in vivo [61]. AD is a chronic progressive neurodegenerative disorder resulting in disturbances of memory and cognitive function, which is associated with neuroinflammation and deposition of amyloid- beta (Aβ) in the brain. Extracts of Arabidopsis thaliana, rich in polyphenols, namely derivatives of the flavonols quercetin and kaempferol, have mitigated the neuroinflammation induced by Aβ through activation of the nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response. In an in vivo assay, the same extract efficiently restored the locomotor activity of AD Drosophila melanogaster flies expressing human Aβ1–42 [63]. Withal, polyphenols have displayed protective activity against the side effects of chemotherapy in neurological cells, a disorder known as chemobrain, which can affect > 75% of cancer patients [64]. According to Shi et al. [64], resveratrol administered to mice (100 mg/kg/day/3 weeks) exerted neuroprotective effects against chemobrain induced by cancer treatment with docetaxel, adriamycin, and cyclophosphamide (DAC).
References
- NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018, 392, 1072–1088, doi:10.1016/s0140-6736(18)31992-5.
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z., et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2019, 393, 1958–1972, doi:10.1016/s0140-6736(19)30041-8.
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, doi:10.3390/nu11092059.
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ 2008, 337, a1344, doi:10.1136/bmj.a1344.
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Muller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: definition and assessment of activity. Nutrition 2009, 25, 1202–1205, doi:10.1016/j.nut.2009.04.023.
- Bernhoft, A. A brief review on bioactive compounds in plants. Bioactive compounds in plants- benefits and risks for man and animals 2010, 11-17.
- Plumb, J.; Pigat, S.; Bompola, F.; Cushen, M.; Pinchen, H.; Norby, E.; Astley, S.; Lyons, J.; Kiely, M.; Finglas, P. eBASIS (Bioactive Substances in Food Information Systems) and Bioactive Intakes: Major Updates of the Bioactive Compound Composition and Beneficial Bioeffects Database and the Development of a Probabilistic Model to Assess Intakes in Europe. Nutrients 2017, 9, doi:10.3390/nu9040320.
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–633, doi:10.1104/pp.122.3.627.
- Astley, S.; Finglas, P. Nutrition and Health. In Reference Module in Food Science, Elsevier: 2016; doi:10.1016/B978-0-08-100596-5.03425-9.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243, doi:10.1002/ptr.6419.
- Kondratyuk, T.P.; Pezzuto, J.M. Natural Product Polyphenols of Relevance to Human Health. Pharm. Biol. 2004, 42, 46–63, doi:10.3109/13880200490893519.
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications, Galanakis, C.M., Ed. Woodhead Publishing: 2018; 10.1016/B978-0-12-813572-3.00001-4;pp. 3-44.
- Amawi, H.; Ashby, C.R.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: what’s limiting? Chinese Journal of Cancer 2017, 36, 50, doi:10.1186/s40880-017-0217-4.
- Gonçalves, J.; Mendes, B.; Silva, C.L.; Câmara, J.S. Development of a novel microextraction by packed sorbent-based approach followed by ultrahigh pressure liquid chromatography as a powerful technique for quantification phenolic constituents of biological interest in wines. J. Chromatogr. A 2012, 1229, 13–23, doi:10.1016/j.chroma.2012.01.023.
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Chemical Fingerprint of Free Polyphenols and Antioxidant Activity in Dietary Fruits and Vegetables Using a Non-Targeted Approach Based on QuEChERS Ultrasound-Assisted Extraction Combined with UHPLC-PDA. Antioxidants (Basel) 2020, 9, doi:10.3390/antiox9040305.
- Pietta, P.; Minoggio, M.; Bramati, L. Plant Polyphenols: Structure, Occurrence and Bioactivity. In Stud. Nat. Prod. Chem., Rahman, A.-u., Ed. Elsevier: 2003; Vol. 28, pp. 257-312.
- Oliveira, L.d.L.d.; Carvalho, M.V.d.; Melo, L. Health promoting and sensory properties of phenolic compounds in food. Revista Ceres 2014, 61, 764–779.
- Alihosseini, F.; Sun, G. Antibacterial colorants for textiles. In Functional Textiles for Improved Performance, Protection and Health; Pan, N., Sun, G., Eds.; Woodhead Publishing: Duxford, UK, 2011; pp. 376–403.
- Arts, I.C.; van de Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food. Chem. 2000, 48, 1746–1751, doi:10.1021/jf000025h.
- Arts, I.C.; van De Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J. Agric. Food. Chem. 2000, 48, 1752–1757, doi:10.1021/jf000026+.
- Pérez-Chabela, M.L.; Hernández-Alcántara, A.M. Agroindustrial Coproducts as Sources of Novel Functional Ingredients. In Food Processing for Increased Quality and Consumption; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: 2018; pp. 219–250.
- Klejdus, B.; Vacek, J.; Benešová, L.; Kopecký, J.; Lapčík, O.; Kubáň, V. Rapid-resolution HPLC with spectrometric detection for the determination and identification of isoflavones in soy preparations and plant extracts. Anal. Bioanal. Chem. 2007, 389, 2277–2285, doi:10.1007/s00216-007-1606-3.
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076, doi:10.3390/molecules24061076.
- Francavilla, A.; Joye, I.J. Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients 2020, 12, 2922.
- Celli, G.B.; Tan, C.; Selig, M.J. Anthocyanidins and Anthocyanins. In Encyclopedia of Food Chemistry, Melton, L., Varelis, P., Shahidi, F., Eds. Elsevier: 2019; Vol. 3, pp. 218-223.
- Bureau, S.; Renard, C.M.G.C.; Reich, M.; Ginies, C.; Audergon, J.-M. Change in anthocyanin concentrations in red apricot fruits during ripening. LWT - Food Science and Technology 2009, 42, 372–377, doi:10.1016/j.lwt.2008.03.010.
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. Chapter 2 - Sweet Cherry Phenolic Compounds: Identification, Characterization, and Health Benefits. In Stud. Nat. Prod. Chem., Atta ur, R., Ed. Elsevier: 2018; Vol. 59, pp. 31-78.
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic Potential of Dietary Phenolic Acids. Advances in Pharmacological Sciences 2015, 2015, 823539, doi:10.1155/2015/823539.
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Research International 2013, 2013.
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155, doi:10.1016/j.plantsci.2009.05.012.
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxidative Medicine and Cellular Longevity 2016, 2016, 3128951, doi:10.1155/2016/3128951.
- Nikolić, I.; Savić-Gajić, I.; Tačić, A.; Savić, I. Classification and biological activity of phytoestrogens: A review. Advanced technologies 2017, 6, 96–106, doi:10.5937/savteh1702096n.
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 620, doi:10.3390/molecules25030614.
- Khanbabaee, K.; van Ree, T. Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18, 641–649, doi:10.1039/B101061L.
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Med. Chem. 2008, 8, 436–447, doi:10.2174/138955708784223486.
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224, doi:10.1016/j.procbio.2011.09.015.
- Saltveit, M.E. Synthesis and Metabolism of Phenolic Compounds. In Fruit and Vegetable Phytochemicals, 2017; 10.1002/9781119158042.ch5;pp. 115-124.
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development; 6th, Ed. Sinauer Associates: USA, 2014; pp. 700.
- Fowler, Z.L.; Koffas, M.A.G. Biosynthesis and biotechnological production of flavanones: current state and perspectives. Appl. Microbiol. Biotechnol. 2009, 83, 799–808, doi:10.1007/s00253-009-2039-z.
- de Araujo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535, doi:10.1016/j.foodchem.2020.127535.
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. New phytochemicals as potential human anti-aging compounds: Reality, promise, and challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 942–957, doi:10.1080/10408398.2016.1233860.
- Daglia, M. New advances in biotechnology of phytochemicals. Biotechnol. Adv. 2020, 38, 107445, doi:10.1016/j.biotechadv.2019.107445.
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J. Med. Chem. 2017, 60, 9413–9436, doi:10.1021/acs.jmedchem.6b01026.
- Thyagarajan, A.; Forino, A.S.; Konger, R.L.; Sahu, R.P. Dietary Polyphenols in Cancer Chemoprevention: Implications in Pancreatic Cancer. Antioxidants 2020, 9, 651, doi:10.3390/antiox9080651.
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: updates and future directions. Biotechnol. Adv. 2020, 38, 107385, doi:10.1016/j.biotechadv.2019.04.007.
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic Compounds as Nutraceuticals or Functional Food Ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806, doi:10.2174/1381612822666161227153906.
- Chen, L.C.; Huang, H.L.; HuangFu, W.C.; Yen, S.C.; Ngo, S.T.; Wu, Y.W.; Lin, T.E.; Sung, T.Y.; Lien, S.T.; Tseng, H.J., et al. Biological Evaluation of Selected Flavonoids as Inhibitors of MNKs Targeting Acute Myeloid Leukemia. J. Nat. Prod. 2020, 83, 2967–2975, doi:10.1021/acs.jnatprod.0c00516.
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The therapeutic potential of resveratrol in a mouse model of melanoma lung metastasis. Int. Immunopharmacol. 2020, 88, 106905, doi:10.1016/j.intimp.2020.106905.
- Su, C.-C.; Wang, C.-J.; Huang, K.-H.; Lee, Y.-J.; Chan, W.-M.; Chang, Y.-C. Anthocyanins from Hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. Journal of Functional Foods 2018, 48, 614–631, doi:10.1016/j.jff.2018.07.032.
- Kuete, V.; Mbaveng, A.T.; Nono, E.C.; Simo, C.C.; Zeino, M.; Nkengfack, A.E.; Efferth, T. Cytotoxicity of seven naturally occurring phenolic compounds towards multi-factorial drug-resistant cancer cells. Phytomedicine 2016, 23, 856–863, doi:10.1016/j.phymed.2016.04.007.
- Wang, Y.; Yu, H.; Wang, S.; Gai, C.; Cui, X.; Xu, Z.; Li, W.; Zhang, W. Targeted delivery of quercetin by nanoparticles based on chitosan sensitizing paclitaxel-resistant lung cancer cells to paclitaxel. Materials Science and Engineering: C 2021, 119, 111442, doi:10.1016/j.msec.2020.111442.
- Yan, J.; Wang, Q.; Zheng, X.; Sun, H.; Zhou, Y.; Li, D.; Lin, Y.; Wang, X. Luteolin enhances TNF-related apoptosis-inducing ligand's anticancer activity in a lung cancer xenograft mouse model. Biochem. Biophys. Res. Commun. 2012, 417, 842–846, doi:10.1016/j.bbrc.2011.12.055.
- Tajaldini, M.; Samadi, F.; Khosravi, A.; Ghasemnejad, A.; Asadi, J. Protective and anticancer effects of orange peel extract and naringin in doxorubicin treated esophageal cancer stem cell xenograft tumor mouse model. Biomed. Pharmacother. 2020, 121, 109594, doi:10.1016/j.biopha.2019.109594.
- Li, H.; Sureda, A.; Devkota, H.P.; Pittala, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020, 38, 107343, doi:10.1016/j.biotechadv.2019.01.010.
- Zhang, X.; Zhu, Y.; Song, F.; Yao, Y.; Ya, F.; Li, D.; Ling, W.; Yang, Y. Effects of purified anthocyanin supplementation on platelet chemokines in hypocholesterolemic individuals: a randomized controlled trial. Nutr Metab (Lond) 2016, 13, 86, doi:10.1186/s12986-016-0146-2.
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: a randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682, doi:10.3945/ajcn.116.137075.
- Seyyedebrahimi, S.; Khodabandehloo, H.; Nasli Esfahani, E.; Meshkani, R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018, 55, 341–353, doi:10.1007/s00592-017-1098-3.
- Yan, F.; Sun, X.; Xu, C. Protective effects of resveratrol improve cardiovascular function in rats with diabetes. Experimental and Therapeutic Medicine 2017, 10.3892/etm.2017.5537, doi:10.3892/etm.2017.5537.
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. Journal of Functional Foods 2020, 74, 104169, doi:10.1016/j.jff.2020.104169.
- Giacoppo, S.; Galuppo, M.; Lombardo, G.E.; Ulaszewska, M.M.; Mattivi, F.; Bramanti, P.; Mazzon, E.; Navarra, M. Neuroprotective effects of a polyphenolic white grape juice extract in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia 2015, 103, 171–186, doi:10.1016/j.fitote.2015.04.003.
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341, doi:10.1007/s00394-015-1083-y.
- Mattioli, R.; Francioso, A.; d'Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.M.; Wessjohann, L.; Gazzino, R.; Costantino, P., et al. Anti-Inflammatory Activity of A Polyphenolic Extract from Arabidopsis thaliana in In Vitro and In Vivo Models of Alzheimer's Disease. Int J Mol Sci 2019, 20, doi:10.3390/ijms20030708.
- Shi, D.D.; Dong, C.M.; Ho, L.C.; Lam, C.T.W.; Zhou, X.D.; Wu, E.X.; Zhou, Z.J.; Wang, X.M.; Zhang, Z.J. Resveratrol, a natural polyphenol, prevents chemotherapy-induced cognitive impairment: Involvement of cytokine modulation and neuroprotection. Neurobiol. Dis. 2018, 114, 164–173, doi:10.1016/j.nbd.2018.03.006.




