| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesca Orso | + 1281 word(s) | 1281 | 2021-01-13 06:47:55 | | | |
| 2 | Nicole Yin | Meta information modification | 1281 | 2021-02-02 09:31:45 | | |
Video Upload Options
The totality of normal cells, blood vessels and molecules embedding the tumor cells and sustaining their growth, characterized by a continuous bidirectional reshaping.
1. Introduction
Uncontrolled proliferation, cell death resistance, replicative immortality, growth suppressor, evasion, angiogenesis induction, invasion and metastasis activation are all considered hallmarks of cancer[1]. Eleven years after the first edition of The Hallmarks of Cancer, metabolic energy reprogramming and immune escape avoidance mechanism have been highlighted and added as the emerging new hallmarks of cancer together with tumor-promoting inflammation, genome instability and mutations[2]. This underlines how our current understanding of cancer biology has shifted from a “cancer cell centric” perspective to a more inclusive concept which places cancer cells in a complex and interconnected network composed by the extracellular matrix (ECM), immune and stromal cells to which we generally refer as tumor microenvironment (TME)[3]. TME can collaborate with cancer cells, thus promoting tumor progression and metastatization as well as resistance to therapy[4].
2. Composition
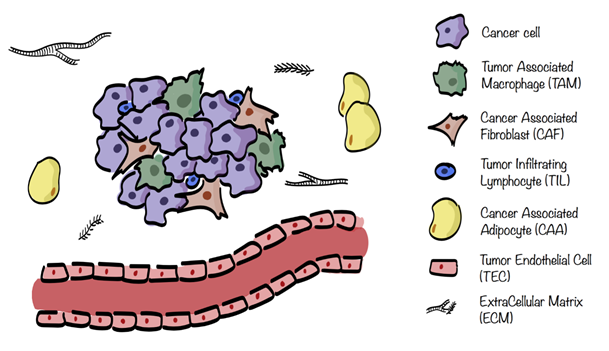
The different stromal cells of the TME are cancer associated fibroblasts (CAFs), tumor endothelial cells (TECs), mesenchymal stem cells (MSCs), cancer associated adipocytes (CAAs), and infiltrating immune cells (ICs), including tumor associated macrophages (TAMs), natural killers (NKs), neutrophils, dendritic cells (DCs), and tumor infiltrating lymphocytes (TILs), see Figure 1. These cells are characterized by specific metabolic traits and pathways which get modulated, depending on the surrounding situation[2][5].
Figure 1. Tumor microenvironment (TME) composition. TME surrounding cancer cells consists of extracellular matrix (ECM) in which are embedded several stromal cells such as cancer associated fibroblasts (CAFs), tumor endothelial cells (TECs), cancer associated adipocytes (CAAs), and infiltrating immune cells (ICs), including tumor associated macrophages (TAMs) and tumor infiltrating lymphocytes (TILs).
3. Tumor Microenvironment Metabolism
Cancer progression is sustained by the contribution of several non-malignant cells that are shaped and, which, in turn, influence cancer cell behavior and TME metabolic landscape[2][6]. For example, following the interaction with CAFs, cancer cells improve their invasiveness, their ability to intra- or extravasate as well as their stemness potential. All these processes create a stiff fibrotic matrix where the development of blood vessels is impaired, leading to inefficient delivery of nutrients and clearance of waste products of cellular metabolism, together with the formation of hypoxic areas[7]. This hypoxic response causes an enhanced glycolytic activity in the tumor cells with an increased lactate deposition. Cancer cells crosstalk metabolically with stromal cells within the TME and, in 2009, a metabolic coupling between CAFs and surrounding cancer cells was proposed and named “reverse Warburg effect”. While in the classical Warburg effect cancer cells display aerobic glycolysis in order to sustain their proliferation, in the “reverse Warburg effect” the cells of the TME are highly glycolytic whereas cancer cells are not. More in detail, cancer cells induce aerobic glycolysis in surrounding CAFs which start to release pyruvate and lactate used directly by cancer cells to fuel their TriCarboxylic Acid (TCA) cycle and OXPHOS[8]. This reprogramming is due to an increased expression of GLUT-1 and monocarboxylate transporter-4 (MCT-4) involved in glucose uptake and lactate release, respectively[9]. The increased release of lactate in the microenvironment contributes to its acidification, which, in turn, leads to activation of the matrix metalloprotease-9 (MMP-9) and of the epithelial-to-mesenchymal transition (EMT) program, promoting tumor progression[10][11]. However, the ‘reverse Warburg effect’ model does not apply to all CAF-cancer cell interactions. In fact, pancreatic and ovarian CAFs consume lactate and display low glycolytic activity[12][13]. Moreover, cancer associated stromal cells also reshape their metabolic landscape forcing the production of ketone bodies and glutamine and activating the mitophagy[14]. All these molecules could be exploited through OXPHOS, thus reprogramming cancer cells towards a respiratory metabolism. Furthermore, the production of pro-inflammatory cytokines by stromal cells can push cancer cells towards an OXPHOS phenotype. The key regulator of the pro-inflammatory stimuli is the NF-kB transcription factor, involved in the control of energy balance and metabolic adaptation to glucose starvation, by upregulating mitochondrial function[15]. Acidosis of interstitial space develops as an intrinsic consequence of the Warburg effect. Indeed, in normal conditions, the extracellular pH is around 7.4 and its levels decrease to a range of 6.7–7.1 in cancer[16]. The highly glycolytic activity of tumor cells results in both lowering of intracellular pH and lactate accumulation, which, in turn, leads to the inhibition of glycolysis itself. In order to avoid such inhibition, cancer cells upregulate MCTs and Na+H+ exchangers (NHE) or H+ ATPase pumps, devoted to lactate and protons export, respectively[17]. Moreover, the acidification of the TME reduces the response of the immune system against the tumor[18][19] and it is detrimental for drug delivery and efficacy[20][21]. This happens because at least one pH-titratable group is present in common drugs which are usually weak bases. As a result, at lower pH, drugs can be protonated, thus affecting their possibility to permeate through the cell membrane[16]. For all these reasons, acidic TME targeting started to be considered for tumor therapy. In this line, small-molecule inhibitors, nanotheranostic systems responsive to pH to deliver chemotherapeutic drugs specifically to acidic microenvironment and pH-sensitive biomaterials were developed[22][23][24]. An example is represented by mesoporous organosilica particles (MONs) able to deliver doxorubicin to low pH compartments[23]. In parallel, acidosis can mediate also the ‘immune escape’. It was demonstrated that T cells exposed to acidic environment show an increased threshold of activation and an enhanced expression of negative regulatory signals, namely CTLA4 and IFNɣR2[25].
ICs display a strong interplay between immune response and metabolism. Following activation, immune cells start to proliferate and to produce effector molecules such as cytokines and cytotoxic granules. In order to fuel the higher biomass and the increased energy demands, activated immune cells rewire—and reprogram their metabolic pathways[26]. Interestingly, several studies have shown that limiting levels of glucose impair T cell effector activity which relies on glycolysis and, therefore, on glucose availability[27][28]. Even if the pivotal function of cell metabolism is to provide energy and substrates, it is important to underline that it also plays an essential role in instructing the phenotype and the differentiation of ICs influencing cancer outcome and therapy resistance[5][26]. Therefore, a good understanding of the metabolic processes of ICs and how they are regulated is essential to harness anti-immunity response and improve cancer survival. Besides glucose, also fatty acids and amino acids, such as glutamine, are importantly involved in immune cell fate and response[29][30].
Overall, the metabolic reprogramming of TME is strongly affected by hypoxia, which leads to an impairment of OXPHOS, an enhancement of glycolytic activity and production of mitochondrial reactive oxygen species (ROS). In particular, oxygen deprivation pushes cancer cells towards a more glycolytic phenotype in a hypoxia-inducible factor-1α (HIF-1α)-dependent manner. In turn, HIF-1α activity relies on redox, oxygen levels, PI3K/mTOR/Akt, and c-MYC-related pathways involved in tumor cell metabolism, growth and survival[9][31][32][33]. HIF-1α, in fact, induces the expression of pyruvate dehydrogenase Kinase 1 (PDK1), leading to decreased activity of the pyruvate dehydrogenase complex (PDC) with a consequent reduction of oxygen consumption and enhanced glycolysis[34][35]. Moreover, HIF-1α increases glucose uptake and causes a more efficient glycolytic breakdown due to the induction of GLUT-1[36] and GLUT-3 expression[37]. The metabolic crosstalk among tumor and stromal cells is finely regulated and extracellular vesicles (EVs) are key messengers in this communication. The analysis of EVs involved in metabolic rewiring revealed the presence of proteins, lipids, different RNA species and metabolites. Among the various RNAs present in EVs, miRs exert a central role in the metabolic crosstalk by directly suppressing metabolic enzymes or transporters as well as by controlling important regulators of metabolic processes such as HIF-1α, PI3K/mTOR/Akt, and MYC pathways. In fact, miRs are recently emerging as regulators of the metabolic reprogramming in ICs and CAFs[38][39].
References
- Douglas Hanahan; Robert A Weinberg; The Hallmarks of Cancer. Cell 2000, 100, 57-70, 10.1016/s0092-8674(00)81683-9.
- Douglas Hanahan; Robert A. Weinberg; Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646-674, 10.1016/j.cell.2011.02.013.
- Florian R. Greten; Sergei I. Grivennikov; Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27-41, 10.1016/j.immuni.2019.06.025.
- Kenneth C. Valkenburg; Amber E. De Groot; Kenneth J. Pienta; Targeting the tumour stroma to improve cancer therapy. Nature Reviews Clinical Oncology 2018, 15, 366-381, 10.1038/s41571-018-0007-1.
- Carla Riera-Domingo; Annette Audigé; Sara Granja; Wan-Chen Cheng; Ping-Chih Ho; Fátima Baltazar; Christian Stockmann; Massimiliano Mazzone; Immunity, Hypoxia, and Metabolism–the Ménage à Trois of Cancer: Implications for Immunotherapy. Physiological Reviews 2020, 100, 1-102, 10.1152/physrev.00018.2019.
- Andrea Morandi; E. Giannoni; Paola Chiarugi; Nutrient Exploitation within the Tumor–Stroma Metabolic Crosstalk. Trends in Cancer 2016, 2, 736-746, 10.1016/j.trecan.2016.11.001.
- L. R. Rudmik; A.M. Magliocco; Molecular mechanisms of hepatic metastasis in colorectal cancer. Journal of Surgical Oncology 2005, 92, 347-359, 10.1002/jso.20393.
- Stephanos Pavlides; Diana Whitaker-Menezes; Remedios Castello-Cros; Neal Flomenberg; Agnieszka K. Witkiewicz; Philippe G. Frank; Mathew C. Casimiro; Chenguang Wang; Paolo Fortina; Sankar Addya; et al.Richard G. PestellUbaldo E. Martinez-OutschoornFederica SotgiaMichael P. Lisanti The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984-4001, 10.4161/cc.8.23.10238.
- Tania Fiaschi; Alberto Marini; Elisa Giannoni; Maria Letizia Taddei; Paolo Gandellini; Alina De Donatis; Michele Lanciotti; Sergio Serni; Paolo Cirri; Paola Chiarugi; et al. Reciprocal Metabolic Reprogramming through Lactate Shuttle Coordinately Influences Tumor-Stroma Interplay. Cancer Research 2012, 72, 5130-5140, 10.1158/0008-5472.can-12-1949.
- Santi, A.; Caselli, A.; Paoli, P.; Corti, D.; Camici, G.; Pieraccini, G.; Taddei, M.L.; Serni, S.; Chiarugi, P.; Cirri, P. The effects of CA IX catalysis products within tumor microenvironment. Cell Commun. Signal. 2013, 11, 81.
- Fiaschi, T.; Giannoni, E.; Taddei, M.L.; Cirri, P.; Marini, A.; Pintus, G.; Nativi, C.; Richichi, B.; Scozzafava, A.; Carta, F.; et al. Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial-mesenchymal transition in prostate carcinoma cells. Cell Cycle 2013, 12, 1791–1801.
- Sousa, C.M.; Biancur, D.E.; Wang, X.; Halbrook, C.J.; Sherman, M.H.; Zhang, L.; Kremer, D.; Hwang, R.F.; Witkiewicz, A.K.; Ying, H.; et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 2016, 536, 479–483.
- Yang, L.; Achreja, A.; Yeung, T.L.; Mangala, L.S.; Jiang, D.; Han, C.; Baddour, J.; Marini, J.C.; Ni, J.; Nakahara, R.; et al. Targeting Stromal Glutamine Synthetase in Tumors Disrupts Tumor Microenvironment-Regulated Cancer Cell Growth. Cell Metab. 2016, 24, 685–700.
- Ubaldo E. Martinez-Outschoorn; Zhao Lin; Diana Whitaker-Menezes; Anthony Howell; Michael P. Lisanti; Federica Sotgia; Ketone bodies and two-compartment tumor metabolism: Stromal ketone production fuels mitochondrial biogenesis in epithelial cancer cells. Cell Cycle 2012, 11, 3956-3963, 10.4161/cc.22136.
- Mauro, C.; Leow, S.C.; Anso, E.; Rocha, S.; Thotakura, A.K.; Tornatore, L.; Moretti, M.; De Smaele, E.; Beg, A.A.; Tergaonkar, V.; et al. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat. Cell Biol. 2011, 13, 1272–1279.
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677.
- Counillon, L.; Bouret, Y.; Marchiq, I.; Pouyssegur, J. Na(+)/H(+) antiporter (NHE1) and lactate/H(+) symporters (MCTs) in pH homeostasis and cancer metabolism. Biochim. Biophys. Acta 2016, 1863, 2465–2480.
- Calcinotto, A.; Filipazzi, P.; Grioni, M.; Iero, M.; De Milito, A.; Ricupito, A.; Cova, A.; Canese, R.; Jachetti, E.; Rossetti, M.; et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012, 72, 2746–2756.
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007, 109, 3812–3819.
- Baltazar, F.; Pinheiro, C.; Morais-Santos, F.; Azevedo-Silva, J.; Queiros, O.; Preto, A.; Casal, M. Monocarboxylate transporters as targets and mediators in cancer therapy response. Histol. Histopathol. 2014, 29, 1511–1524.
- Peppicelli, S.; Bianchini, F.; Calorini, L. Extracellular acidity, a “reappreciated” trait of tumor environment driving malignancy: Perspectives in diagnosis and therapy. Cancer Metastasis Rev. 2014, 33, 823–832.
- Zhong, S.; Jeong, J.H.; Chen, Z.; Chen, Z.; Luo, J.L. Targeting Tumor Microenvironment by Small-Molecule Inhibitors. Transl. Oncol. 2020, 13, 57–69.
- Zhang, Y.; Dang, M.; Tian, Y.; Zhu, Y.; Liu, W.; Tian, W.; Su, Y.; Ni, Q.; Xu, C.; Lu, N.; et al. Tumor Acidic Microenvironment Targeted Drug Delivery Based on pHLIP-Modified Mesoporous Organosilica Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 30543–30552.
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167.
- Bosticardo, M.; Ariotti, S.; Losana, G.; Bernabei, P.; Forni, G.; Novelli, F. Biased activation of human T lymphocytes due to low extracellular pH is antagonized by B7/CD28 costimulation. Eur. J. Immunol. 2001, 31, 2829–2838.
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic Instruction of Immunity. Cell 2017, 169, 570–586.
- Ho, P.C.; Bihuniak, J.D.; Macintyre, A.N.; Staron, M.; Liu, X.; Amezquita, R.; Tsui, Y.C.; Cui, G.; Micevic, G.; Perales, J.C.; et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell 2015, 162, 1217–1228.
- Kamphorst, A.O.; Araki, K.; Ahmed, R. Beyond adjuvants: Immunomodulation strategies to enhance T cell immunity. Vaccine 2015, 33 (Suppl. 2), B21–B28.
- Mazzone, M.; Menga, A.; Castegna, A. Metabolism and TAM functions-it takes two to tango. FEBS J. 2018, 285, 700–716.
- Chapman, N.M.; Boothby, M.R.; Chi, H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 2019.
- Viola, A.; Munari, F.; Sanchez-Rodriguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462.
- MacIver, N.J.; Michalek, R.D.; Rathmell, J.C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 2013, 31, 259–283.
- Giannoni, E.; Bianchini, F.; Calorini, L.; Chiarugi, P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid. Redox Signal. 2011, 14, 2361–2371.
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185.
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197.
- Changhu Chen; Nabendu Pore; Alireza Behrooz; Faramarz Ismail-Beigi; Amit Maity; Regulation of glut1 mRNA by Hypoxia-inducible Factor-1. Journal of Biological Chemistry 2001, 276, 9519-9525, 10.1074/jbc.m010144200.
- Imari Mimura; Masaomi Nangaku; Yasuharu Kanki; Shuichi Tsutsumi; Tsuyoshi Inoue; Takahide Kohro; Shogo Yamamoto; Takanori Fujita; Teppei Shimamura; Jun-Ichi Suehiro; et al.Akashi TaguchiMika KobayashiKyoko TanimuraTakeshi InagakiToshiya TanakaTakao HamakuboJuro SakaiHiroyuki AburataniTatsuhiko KodamaYouichiro Wada Dynamic Change of Chromatin Conformation in Response to Hypoxia Enhances the Expression of GLUT3 (SLC2A3) by Cooperative Interaction of Hypoxia-Inducible Factor 1 and KDM3A. Molecular and Cellular Biology 2012, 32, 3018-3032, 10.1128/mcb.06643-11.
- Yao, Q.; Song, Z.; Wang, B.; Zhang, J.A. Emerging roles of microRNAs in the metabolic control of immune cells. Cancer Lett. 2018, 433, 10–17.
- Wang, Z.; Tan, Y.; Yu, W.; Zheng, S.; Zhang, S.; Sun, L.; Ding, K. Small role with big impact: miRNAs as communicators in the cross-talk between cancer-associated fibroblasts and cancer cells. Int. J. Biol. Sci. 2017, 13, 339–348.