| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takeshi Hatanaka | + 3760 word(s) | 3760 | 2021-01-11 05:01:43 | | | |
| 2 | Vivi Li | -1 word(s) | 3759 | 2021-02-01 05:24:01 | | | | |
| 3 | Conner Chen | Meta information modification | 3759 | 2021-10-12 05:37:39 | | |
Video Upload Options
Lenvatinib, which is an oral multikinase inhibitor, showed non-inferiority to the sorafenib in terms of overall survival (OS) and a higher objective response rate (ORR) and better progression-free survival (PFS) in patients with hepatocellular carcinoma (HCC). A good liver function and Barcelona Clinic Liver Cancer (BCLC) intermediate stage were key factors in achieving therapeutic efficacy. The management of adverse events plays an important role in continuing lenvatinib treatment. While sequential therapies contributed to prolonging overall survival, effective molecular targeted agents for administration after lenvatinib have not been established. Repeated transcatheter arterial chemoembolization (TACE) was associated with a decline in the liver function and poor therapeutic response in BCLC intermediate patients. Recently, the Asia-Pacific Primary Liver Cancer Expert (APPLE) Consensus Statement proposed the criteria for TACE unsuitability. Upfront systemic therapy may be better for BCLC intermediate stage HCC patients with a high tumor burden, while selective TACE will be recommended to obtain a curative response in patients with a low tumor burden. This entry introduce the therapeutic response, management of adverse events, post-progression treatment after lenvatinib and treatment strategy for BCLC intermediate stage HCC.
2. Therapeutic Response of Lenvatinib
1.1. The Objective Response Rate of Lenvatinib
Many real-world studies have reported the short-term therapeutic response of lenvatinib, resulting in an ORR of 29.9–53.5%, as assessed by mRECIST [1][2][3][4][5][6][7][8], and 14–25%, as assessed by RECIST ver.1.1 [3][6][9][10]. These findings seemed to be in agreement with the results of phase 2 and 3 studies (Table 1). To date, many researchers have also investigated the ORR as a predictive factor in the clinical setting. Ueshima et al. [1] reported that the high ORR was found in patients with albumin-bilirubin (ALBI) grade 1 at baseline. We reported that BCLC intermediate stage was a significant predictive factor that was associated with the ORR in a multivariate analysis [2], which agreed with the results regarding the ORRs in the analysis of a Japanese subpopulation in the REFLECT trial [11]. A high ORR was found in patients with a good PS [5], Child-Pugh class A [5], and Child-Pugh score 5 (CP-5A) [7]. Some of the researchers also reported that the high relative dose intensity (RDI) at four weeks (30 days) [7][12][13] or eight weeks (60 days) [3][5][14][15] was relevant to therapeutic response of lenvatinib, including the PFS and OS (Table 2). The cut-off values of the RDI were similar, ranging from 66% to 75%. A high RDI was associated with a good liver function at baseline [3][12][13][14][15], low tumor stage [3][14], and an initial full dose of lenvatinib [3][14], which indicated that the liver function and early tumor stage would play an important role in obtaining a high RDI. Interestingly, low body weight [12] or BMI [3] were relevant to low RDI. While the reason for this is unclear, it might be related to the high incidence of adverse events (AEs) in patients with low BMI values [8]. In contrast, a retrospective study showed that the delivered dose intensity/body surface area ratio (DBR) was better a predictive factor than the RDI [15]. Because only one retrospective study has compared the RDI and DBR, further research was conducted in order to assess the usefulness of DBR. Some researchers reported the utility of contrast-enhanced ultrasonography at day 7 [16], AFP change at two weeks [17], and radiological imaging at two weeks [18] in order to predict the ORR of lenvatinib early, although the numbers of subjects were limited in these studies. These findings suggest that it is preferable to start lenvatinib at a full dose for patients with ALBI grade 1 and BCLC intermediate stage. When possible, an early tumor assessment was also preferable.
Table 1. The therapeutic response to lenvatinib treatment.
| Author/Ref. No. | Years | Country | No. of pts | ORR (%) | Median PFS (Months) | Median OS (Months) | Predictive Factors of ORR | Commentary | |
|---|---|---|---|---|---|---|---|---|---|
| mRECIST | RECIST ver.1.1 | ||||||||
| Kudo M./[19] | 2018 | Global | 478 | 40.6 | 18.8 | 7.3 | 13.6 | NA | REFLECT trial |
| Ueshima K./[1] | 2019 | Japan | 82 | 39.0 | NA | 7.6 | could not be reached | ALBI grade 1 | ALBI grade 1; ORR of 57.1%, median PFS of 18.9 months |
| Hatanaka T./[2] | 2020 | Japan | 94 | 30.4 | NA | 5.4 | NA | BCLC intermediate stage | BCLC intermediate stage; ORR of 47.6%, median PFS 8.0 months |
| Sasaki R./[3] | 2019 | Japan | 81 | 34.6 | 17.3 | NA | 11.6 | NA | The patients with the good ORR and those with high RDI had significantly good OS. |
| Sho T./[4] | 2020 | Japan | 105 | 53.5 | NA | 9.8 | NA | NA | |
| Ogushi K./[5] | 2020 | Japan | 181 | 30.4 | NA | NA | 369 (days) | CP-A, PS 0, RDI > 0.70 | CP-A; ORR of 36.5%. RDI > 0.70: ORR of 47.7%. CP-A and BCLC intermediate stage were the predictive factors affecting the OS. |
| Maruta S./[6] | 2020 | Japan | 152 | 40.8 | 15.8 | 5.1 | 13.3 | NA | |
| Ohki T./[7] | 2020 | Japan | 77 | 29.9 | NA | 5.6 | NA | CP-A, RDI > 70% at 30 days, AFP reduction | RDI > 70%; ORR of 45.2%, RDI ≤ 70%; ORR of 11.4%. The significant factors associated with the PFS were CP-5A, tumor size ≥ 40 mm among pretreatment factors. |
| Hiraoka A./[8] | 2019 | Japan | 152 | 38.7 | NA | NA | could not be reached | NA | mALBI 2b or 3 was unfavorable predictive factor relevant to the OS. |
| Wang D.X./[9] | 2020 | China | 54 | NA | 22.2 | 5.6 | could not be reached | could not be found in a multivariate analysis. | CP-A, BCLC intermediate stage and PVTT significantly affected the PFS. |
| Cheon J./[10] | 2020 | Korea | 92 | NA | 14.1 | 4.3 | 7.1 | NA | |
AFP; α-fetoprotein, ALBI; albumin-bilirubin grade, BCLC; Barcelona Clinic Liver Cancer, CP-A; Child-Pugh class A, mRECIST; modified Response Evaluation Criteria in Solid Tumors, NA; not available, No.; number, ORR; objective response rate, OS; overall survival, PFS; progression-free survival, pts; patients, PVTT; portal vein tumor thrombosis, RDI; relative dose intensity.
Table 2. Reports associated with dose intensity.
| Author/Ref. No. | Years | No. of pts | Assessment | Time | Cut-off Value | Associated Factors | Therapeutic Response | Commentary |
|---|---|---|---|---|---|---|---|---|
| Sasaki R./[3] | 2019 | 81 | RDI | 8 weeks | 67% | BMI, PS, BCLC stage, platelet count, PT, albumin, initial dose | OS | Median OS could not be reached during the observation. |
| Ogushi K./[5] | 2020 | 181 | RDI | 8 weeks | 70% | NA | ORR | Mean RDI gradually decreased from the start of lenvatinib to 2 months. |
| Ohki T./[7] | 2020 | 77 | RDI | 30 days | 70% | NA | ORR, PFS | RDI > 70%; median PFS of 9.3 months |
| Kirino S./[12] | 2020 | 60 | RDI | 4 weeks | 70% | Body weight, ALBI score, albumin, grade 3 or 4 adverse events | OS, time to discontinuation of treatment | Median OS could not be reached and median duration of lenvatinib was 9.5 months in patients with RDI > 70%. |
| Ono A./[13] | 2020 | 41 | RDI | 4 weeks | 70% | Platelet count, albumin, AST, AFP-L3, DCP, ALBI score, TKI experience | PFS | RDI > 70%; median PFS of 6.7 months. RDI in 3–4 weeks significantly reduced compared to 1–2 weeks. |
| Takahashi A./[14] | 2019 | 50 | RDI | 8 weeks | 75% | Child-Pugh class A, ALBI, EHS, initial dose | ORR, PFS | RDI > 75%; ORR of 68%, median PFS of 7.4 months |
| Eso Y./[15] | 2019 | 49 | DBR, RDI | 60 days | DBR of 238.9, RDI of 66% | BSA, mALBI grade 1 + 2a, BTR | ORR, PFS | DBR was calculated as the delivered DI divided by BSA. |
AFP-L3; Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein, ALBI score; albumin-bilirubin score, AST; aspartate transaminase, BCLC stage; Barcelona Clinic Liver Cancer stage, BMI; body mass index, BSA; body surface area, BTR; branched-chain amino acids tyrosine ratio, CONUT; Controlling Nutrition Status, DBR; the delivered dose intensity/body surface area ratio, DCP; des-carboxy prothrombin, DI; dose intensity, EHS; extrahepatic spread, NA; not available, No.; number, OS; overall survival, PFS; progression-free survival, PS; performance status, PT; prothrombin time, pts; patients, RDI; relative dose intensity, TKI; tyrosine kinase inhibitor.
1.2. The Progression-Free Survival and Overall Survival
PFS was defined as the time from randomization (in phase 3 study) or start of treatment (in real-world studies) to disease progression or death. The median PFS in lenvatinib-treated patients was reported to be 7.3 months (95% confidence interval (CI) 5.6–7.5) in a phase 3 study [19], while some of the retrospective studies reported the median PFS of 4.3–9.8 months [1][2][4][6][7][9][10]. Our retrospective study reported that the BCLC intermediate stage was significantly associated with PFS in a multivariate analysis (median PFS; 8.0 months in intermediate stage, 4.0 months in advanced stage) [2]. There were no differences in PFS between the treatment-naïve patients and patients who had received previous treatment. The PFS in patients with extrahepatic spread was shorter in comparison to the patients without extrahepatic spread [2]. Another retrospective study showed that CP-5A, tumor size ≥ 40 mm were significant pretreatment factors and that the incidence of thyroid dysfunction and appetite loss was associated with worse PFS [7]. The results of the present study indicated that a good liver function, small tumor size, and careful management of AEs during treatment contributed to prolonged PFS [7]. Ono et al. reported that an RDI of >70% at four weeks was correlated with PFS [13]. Takahashi et al. revealed that patients with an RDI of ≥75% at eight weeks showed better PFS in comparison to those with RDI < 75% [14]. These studies indicated that a maintained RDI was associated with longer PFS [13][14]. Because the liver function and early tumor stage would play an important role in obtaining a high RDI, as we mentioned above, the administration of full-dose lenvatinib to patients with BCLC intermediate stage (or low tumor burden) would be expected to result in good PFS. The management of AEs was crucial in obtaining good PFS (Table 1).
The median OS was 13.6 months (95% CI 12.1–14.9) in a phase 3 study [19]. The median OS was shown to be 7.1–13.3 months, according to some retrospective studies [3][5][6][10], and modified ALBI (mALBI) grade 2b or 3 [8], Child-Pugh class B [5], BCLC advanced stage [5][20], and elevated C-reactive protein [21] were found to be significant unfavorable factors. While the starting dose of sorafenib did not influence OS according to one retrospective study [22], the patients with the higher RDI of lenvatinib (>67%) at eight weeks had significantly better OS in comparison to those with lower RDI (≤67%) [3]. However, care is required in the interpretation of the results of OS, because the observation period in many previous reports was insufficient. Accordingly, further research with an adequate follow-up period is warranted for investigating predictors of OS (Table 1).
The results of these real-world studies were mainly reported from Japan. According to the Japanese subpopulation analysis of the REFLECT trial [23], the Japanese patients were older, had a lower body weight, better PS, lower serum level of AFP, and, more frequently, had BCLC intermediate stage and underlying liver diseases of HCV in comparison to the overall population of the REFELCT trial. The OS of Japanese patients was better than that in the overall population of the REFLECT trial (17.6 months vs. 13.6 months), which may be explained by a low HCC stage, low serum level of AFP, and high percentage of patients receiving anticancer therapy after lenvatinib [23]. Hence, these tendencies might be found in these real-world studies and it might be necessary to pay attention to the interpretation of the results of the real-world studies.
2. Adverse events
While physicians were accustomed to sorafenib toxic profile based on ten-year experience, the experience in the management of lenvatinib toxicity was limited [24]. Therefore, we described not only the common adverse events (AEs), but also those requiring special attention in this paragraph.
2.1. The fatigue, appetite loss and treatment discontinuation
Adverse events that were frequently reported in many previous studies included fatigue, appetite loss, hand-foot skin reaction (HFSR), diarrhea, hypertension, hypothyroidism, and proteinuria [19][2][5][6][7][8][25]. The rate of discontinuation due to AEs was 8.6–43.5% [2][6][25]. Thus, the management of AEs plays a central role in lenvatinib treatment. The median onset of fatigue, appetite loss, HFSR and diarrhea was approximately 1 months after the start of lenvatinib while the median onset of hypertension was on day 4 [25]. Among these frequent AEs, the occurrence of appetite loss was strongly correlated with the therapeutic effect [2][7][8]. In addition, fatigue and appetite loss were risk factors for treatment discontinuation [40]. Some studies showed that these AEs [2] and the incidence of discontinuation due AEs [1][25] were less frequently found in patients with ALBI grade 1 in comparison to those with ALBI grade ≥2, indicating that AEs of lenvatinib were manageable in the patients with a good baseline liver function. In contrast, other studies reported that the frequency of appetite loss and fatigue was not significantly associated with the mALBI score [6][8]. One of the reasons was presumed to be the small number of the patients with a relatively poor liver function. Another reason was considered to be the shorter period of lenvatinib administration in patients with a poorer liver function in comparison to those with a better liver function. Hence, the liver function will play a central role in the management of AEs.
Dose reduction or dose interruption are performed as countermeasures against fatigue and appetite loss. However, this decreases the efficacy of lenvatinib and possibly causes tumor regrowth. The weekend-off protocol, which was defined as a scheduled protocol with administration for a period of 5 days on and 2 days off, was the one of the methods for improving tolerability and sustaining efficacy [26]. Although no medical agents relieving these symptoms have been established, one study showed that lenvatinib-related fatigue was associated with carnitine insufficiency [27]. Thus, carnitine supplementation may be effective for improving fatigue in patients receiving lenvatinib. The efficacy of other medical agents, including dexamethasone and Chinese herbal medicines, remains unknown.
While older patients seem to experience more AEs, a retrospective study showed that there were no significant differences in the incidence or severity of AEs between elderly (≥75 years old) and non-elderly patients [28]. Lenvatinib may be safe for elderly patients; however, the study population was relatively small. Incidentally, few reports have investigated whether sex differences or the presence of comorbidity have an impact on AEs.
2.2. The hemorrhage events
Vascular endothelial growth factor (VEGF) inhibitor treatment was found to be associated with an increased incidence of hemorrhage events according to a meta-analysis [29][30]; the major frequent hemorrhage events were reported to be upper gastrointestinal hemorrhage and intracranial hemorrhage [31]. Indeed, intracranial hemorrhage was found in 3 patients treated with lenvatinib in the REFLECT trial [19]. Moreover, intraperitoneal or intratumoral hemorrhage is a rare but life-threatening complication. As the original nature, HCC tends to cause intraperitoneal or intratumoral hemorrhage spontaneously. Although there is geographic variation in the incidence of spontaneous HCC rupture, it is reported to be 2.6% in Western countries and 10-26% in Asian countries [32]. Spontaneous tumor rupture was found in approximately 2.6% patients according to the latest nationwide survey of Japan [33]. Accordingly, lenvatinib treatment was deemed to increase the risk of tumor hemorrhage. While the incidence of tumor hemorrhage during lenvatinib treatment remains unknown, Uchida-Kobayashi et al. [34] reported that the lenvatinib-induced tumor hemorrhage was found in 5 patients with a median onset of 3 days after the initiation of lenvatinib. The median maximum tumor diameter was 10 cm in these 5 patients, in agreement with the risk factors for spontaneous HCC bleeding [35]. Hence, careful monitoring is required when administering lenvatinib to patients with huge HCC and further research is warranted to clarify the clinical features of tumor hemorrhage during lenvatinib treatment.
2.3. Hepatic encephalopathy and ascites
Hepatic encephalopathy rarely occurred during lenvatinib treatment. The risk factors for hepatic encephalopathy were shown to be an elevated blood concentration of ammonia at baseline and the presence of portosystemic shunt [6]. The mechanism has remained unclear. One hypothesis is that VEGF inhibitors, including lenvatinib, increase intrahepatic vascular resistance and reduce intrahepatic blood flow via a reduction in nitric oxide production [36]. In fact, the portal venous flow velocity assessed by Doppler ultrasonography during lenvatinib treatment was significantly reduced in comparison to baseline [37][38]. Accordingly, the administration of lenvatinib aggravated the portal hypertension and increased the blood flow in portosystemic shunt, resulting in the occurrence of hepatic encephalopathy. In this connection, a retrospective study revealed that hepatic ascites was found in approximately 29% patients during lenvatinib treatment and that Child-Pugh score 6 and a low platelet count (<12×104/μL) were significant risk factors [20]. Because the patients with a low platelet count tend to have portal hypertension, lenvatinib was presumed to have an impact on the further increase in portal hypertension, resulting in the appearance of hepatic ascites.
2.4. Thyroid dysfunction
Thyroid dysfunction is a frequent complication in patients treated with tyrosine kinase inhibitors (TKIs). According to the phase 3 trial in renal cell carcinoma, sunitinib and pazopanib, which are oral multi-TKIs, caused treatment-related hypothyroidism in approximately 24% and 12% patients, respectively [39]. In the REFLECT trial, hypothyroidism was found in 78 (16%) and 8 (2%) patients who received lenvatinib and sorafenib, respectively [19]. While the clear mechanism underlying the development of thyroid dysfunction due to TKIs remains uncertain, several possible mechanisms have been proposed. One plausible mechanism is that the TKIs cause the destructive thyroiditis [40]. Another mechanism is that TKIs inhibited the binding of VEGF to normal thyroid cells and/or reduce the thyroid flow, resulting in thyroiditis [40].
According to a retrospective study that included 50 lenvatinib-treated patients, subclinical hypothyroidism, overt hypothyroidism, and thyrotoxicosis occurred in 7 (14.0%), 26 (52.0%), and 5 (10.0%) patients, respectively [41]. PFS in patients with hypothyroidism was longer than that in those without hypothyroidism. However, one retrospective study showed that grade 1 and 2 thyroid dysfunction occurred in 6 (7.8%) and 14 (18.2%) patients, respectively, and grade 2 thyroid dysfunction was found to be a significantly unfavorable factor for PFS in a multivariate analysis [7]. On the other hand, another study showed that lenvatinib-induced hypothyroidism of grade 1, 2 and 3 was found in 6 (13%), 12 (26%) and 1 (2%) patients, respectively, and grade 2/3 hypothyroidism was found to be a favorable factor that affected OS in a multivariate analysis [42]. Thus, based on the obtained results of these studies, the relevance of thyroid dysfunction to the clinical outcomes remains controversial.
3. Lenvatinib for patients with Barcelona Clinic Liver Cancer intermediate stage
3.1 Transcatheter arterial chemoembolization and heterogeneity
Transcatheter arterial chemoembolization significantly improved OS in patients with unresectable HCC in comparison to a placebo group [43][44]. It has become the standard treatment for patients with BCLC intermediate stage and is widely used in the clinical setting [45]. However, various molecular targeted agents (MTAs) have been available for advanced HCC and the treatment strategy for BCLC intermediate stage will be reconsidered.
According to a systemic review of 10,108 patients treated with lipiodol-based TACE, the survival rate at 1-, 2-, 3- and 5-year was reported to be 70.3%, 51.8%, 40.4%, and 32.4%, respectively, with median OS of 19.4 months [46]. However, not all patients can obtain benefits from TACE [47]. The heterogeneity in BCLC intermediate stage has led to the development of several prognostic scores based on liver function and tumor burden that attempt to determine who might benefit most [48]. To date, Bolondi’s subclassification [49], hepatoma arterial embolization prognostic (HAP) score [50], the Kinki criteria [51] were proposed. Based on these subclassification, TACE was recommended in either patients with low tumor burden or those with good liver function or both.
A retrospective study analyzed the clinical outcomes in treatment-naïve patients who initially received TACE treatment, and indicated that patients who achieved an objective response with initial TACE showed the longest survival, followed by patients who subsequently obtained an objective response after at least 2 sessions and those who did not achieve an objective response during the course of treatment [52]. This study also showed that large (>5 cm) and multiple (>4) tumors were independently associated with failure to achieve a complete response [52]. The response rate of additional TACE reduced in comparison to that of initial TACE [53]. Moreover, additional TACE increased the risk of deterioration of the liver function. Therefore, repeated TACE was not recommended based on its association with the deterioration of the liver function and a poor therapeutic response.
3.2 Transcatheter arterial chemoembolization refractoriness
During the sorafenib era, TACE failure/refractoriness was proposed as a criterion for switching from TACE to systemic therapy [54]. The TACE failure/refractoriness was defined as the following characteristics: (1) two or more consecutive insufficient responses of the treated tumor (viable lesion >50%); (2) two or more consecutive progressions in the liver (tumor number increases as compared to tumor number before the previous TACE procedure) [54]. Two retrospective studies [55][56] demonstrated that switching to sorafenib treatment was associated with better survival in comparison to repeated TACE. A prospective international observational trial (OPTIMIS trial) showed that, in TACE-refractory patients, survival time was better in those who received sorafenib in comparison to those who continued TACE [57]. In addition, a recent study showed that the median PFS in TACE-refractory patients treated with lenvatinib, sorafenib and TACE was 5.8, 3.2 and 2.4 months, respectively [58]. Our retrospective studies reported that lenvatinib treatment was associated with a high ORR and good PFS in patients with intermediate stage HCC [2]. Therefore, lenvatinib treatment could result in a good outcome in TACE-refractory intermediate stage HCC patients.
3.3 Exceeding the up-to seven criteria, transcatheter arterial chemoembolization unsuitability and upfront systemic therapies
The up-to seven criteria were defined as the sum of the maximum tumor diameter in the liver (cm) and the number of tumors and were originally developed for liver transplantation [59]. In patients exceeding the up-to seven criteria TACE treatment was reported to be likely to worsen the liver function, which may result in missing the chance to receive MTA treatment [60][61]. The proof of concept study demonstrated that among intermediate stage HCC patients who exceeded the up-to seven criteria, the lenvatinib group had a higher ORR, longer PFS and OS than the TACE group [62]. When the ALBI scores at baseline and at the end of treatment were compared, the ALBI score was sustained in the lenvatinib group while it worsened in the TACE group [62]. The results of this study demonstrated that lenvatinib is superior to TACE in intermediate stage HCC patients exceeding the up-to seven criteria. The Asia-Pacific Primary Liver Cancer Expert (APPLE) Consensus Statement proposed the TACE unsuitability should be defined by the following characteristics: (1) unlikely to respond to TACE (confluent multinodular type, massive or infiltrative type, simple nodular type with extranodular growth, poorly differentiated type, intrahepatic multiple disseminated nodules, or sarcomatous changes after TACE); (2) likely to develop TACE failure/refractoriness (Exceeding the up-to seven criteria); and (3) likely to become Child-Pugh B or C after TACE (Exceeding the up-to seven criteria and mALBI grade 2b) [63]. The AASLD Consensus Conference showed that locoregional TACE may still be best approach when patients have a low tumor burden and nodules accessible super-selectively [64]. In contrast, upfront systemic therapy may be better for the patients exceeding the up-to seven criteria [64].
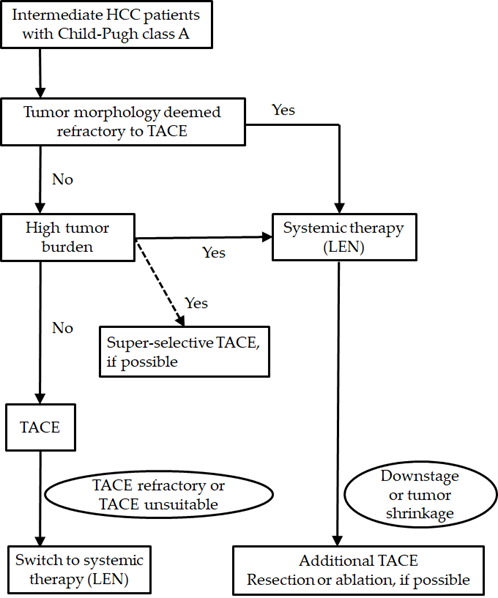
Considering these previous reports, when patients have a low tumor burden, selective TACE will be recommended to obtain a curative response. Non-selective TACE was not recommended due to the low rate of curative response and the risk of deterioration of the liver function. When patients meet the definitions of TACE refractoriness or TACE unsuitable, switching to systemic therapy, including lenvatinib, may be better in order to avoid impairment of the liver function. Upfront lenvatinib may be better in patients with a high tumor burden or who are deemed to be refractory to TACE. When a tumor responds well, additional TACE (or surgical resection or ablation) will be considered. Because lenvatinib rapidly reduced the tumor enhancement on radiological imaging and achieved the high ORR in BCLC intermediate stage, upfront lenvatinib strategy is promising treatment. However, the efficacy and safety of this strategy has not been validated. In addition, the optimal timing of additional TACE treatment has not been established. Further research to investigate the efficacy and safety of this strategy will be necessary in the future (Figure 1).
Recently, an RCT comparing the efficacy and safety of TACE plus sorafenib to TACE alone (TACTICS trial), indicated that PFS in a TACE plus sorafenib group was significantly longer than that in a TACE alone group; however, a benefit in OS was not proved [65]. Systemic therapy improves the clinical outcomes of TACE, presumably by promoting vascular normalization and contributing to the denser deposition of lipiodol [63]. Hence, lenvatinib-TACE sequential therapy might be a promising treatment for patients with intermediate HCC.
References
- Ueshima, K.; Nishida, N.; Hagiwara, S.; Aoki, T.; Minami, T.; Chishina, H.; Takita, M.; Minami, Y.; Ida, H.; Takenaka, M.; et al. Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: A multicenter study. Cancers 2019, 11, 952.
- Hatanaka, T.; Kakizaki, S.; Nagashima, T.; Namikawa, M.; Tojima, H.; Shimada, Y.; Takizawa, D.; Naganuma, A.; Arai, H.; Sato, K.; et al. Analyses of objective response rate, progression-free survival, and adverse events in hepatocellular carcinoma patients treated with lenvatinib: A multicenter retrospective study. Hepatol. Res. 2020, 50, 382–395.
- Sasaki, R.; Fukushima, M.; Haraguchi, M.; Miuma, S.; Miyaaki, H.; Hidaka, M.; Eguchi, S.; Matsuo, S.; Tajima, K.; Matsuzaki, T.; et al. Response to lenvatinib is associated with optimal relativedose intensity in hepatocellular carcinoma: Experience in clinical settings. Cancers 2019, 11, 1769.
- Sho, T.; Suda, G.; Ogawa, K.; Shigesawa, T.; Suzuki, K.; Nakamura, A.; Ohara, M.; Umemura, M.; Kawagishi, N.; Natsuizaka, M.; et al. Lenvatinib in patients with unresectable hepatocellular carcinoma who do not meet the REFLECT trial eligibility criteria. Hepatol. Res. 2020, 50, 966–977.
- Ogushi, K.; Chuma, M.; Uojima, H.; Hidaka, H.; Numata, K.; Kobayashi, S.; Hirose, S.; Hattori, N.; Fujikawa, T.; Nakazawa, T.; et al. Safety and efficacy of lenvatinib treatment in Child-Pugh A and B patients with unresectable hepatocellular carcinoma in clinical practice: A multicenter analysis. Clin. Exp. Gastroenterol. 2020, 13, 385–396.
- Maruta, S.; Ogasawara, S.; Ooka, Y.; Obu, M.; Inoue, M.; Itokawa, N.; Haga, Y.; Seki, A.; Okabe, S.; Azemoto, R.; et al. Potential of lenvatinib for an expanded indication from the REFLECT trial in patients with advanced hepatocellular carcinoma. Liver Cancer 2020, 9, 382–396.
- Ohki, T.; Sato, K.; Kondo, M.; Goto, E.; Sato, T.; Kondo, Y.; Akamatsu, M.; Sato, S.; Yoshida, H.; Koike, Y.; et al. Impact of adverse events on the progression-free survival of patients with advanced hepatocellular carcinoma treated with lenvatinib: A multicenter retrospective study. Drugs Real World Outcomes 2020, 7, 141–149.
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-Multicenter analysis. Cancer Med. 2019, 8, 3719–3728.
- Wang, D.X.; Yang, X.; Lin, J.Z.; Bai, Y.; Long, J.Y.; Yang, X.B.; Seery, S.; Zhao, H.T. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: A retrospective, real-world study conducted in China. World J. Gastroenterol. 2020, 26, 4465–4478.
- Cheon, J.; Chon, H.J.; Bang, Y.; Park, N.H.; Shin, J.W.; Kim, K.M.; Lee, H.C.; Lee, J.; Yoo, C.; Ryoo, B.Y. Real-World efficacy and safety of lenvatinib in Korean patients with advanced hepatocellular carcinoma: A multicenter retrospective analysis. Liver Cancer 2020, 9, 613–624.
- Kudo, M. Extremely high objective response rate of lenvatinib: Its clinical relevance and changing the treatment paradigm in hepatocellular carcinoma. Liver Cancer 2018, 7, 215–224.
- Kirino, S.; Tsuchiya, K.; Kurosaki, M.; Kaneko, S.; Inada, K.; Yamashita, K.; Osawa, L.; Hayakawa, Y.; Sekiguchi, S.; Okada, M.; et al. Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS ONE 2020, 15, e0231828.
- Ono, A.; Aikata, H.; Yamauchi, M.; Kodama, K.; Ohishi, W.; Kishi, T.; Ohya, K.; Teraoka, Y.; Osawa, M.; Fujino, H.; et al. Circulating cytokines and angiogenic factors based signature associated with the relative dose intensity during treatment in patients with advanced hepatocellular carcinoma receiving lenvatinib. Ther. Adv. Med. Oncol. 2020, 12, 1758835920922051.
- Takahashi, A.; Moriguchi, M.; Seko, Y.; Ishikawa, H.; Yo, T.; Kimura, H.; Fujii, H.; Shima, T.; Mitsumoto, Y.; Ishiba, H.; et al. Impact of relative dose intensity of early-phase lenvatinib treatment on therapeutic response in hepatocellular carcinoma. Anticancer Res. 2019, 39, 5149–5156.
- Eso, Y.; Nakano, S.; Mishima, M.; Arasawa, S.; Iguchi, E.; Nakamura, F.; Takeda, H.; Takai, A.; Takahashi, K.; Taura, K.; et al. Dose intensity/body surface area ratio is a novel marker useful for predicting response to lenvatinib against hepatocellular carcinoma. Cancers 2019, 12, 49.
- Kuorda, H.; Abe, T.; Fujiwara, Y.; Okamoto, T.; Yonezawa, M.; Sato, H.; Endo, K.; Oikawa, T.; Sawara, K.; Takikawa, Y. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 2365–2372.
- Kodama, K.; Kawaoka, T.; Namba, M.; Uchikawa, S.; Ohya, K.; Morio, K.; Nakahara, T.; Murakami, E.; Yamauchi, M.; Hiramatsu, A.; et al. Correlation between early tumor marker response and imaging response in patients with advanced hepatocellular carcinoma treated with lenvatinib. Oncology 2019, 97, 75–81.
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Fujishiro, M. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol. Res. 2020, 50, 374–381.
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173.
- Hatanaka, T.; Kakizaki, S.; Nagashima, T.; Namikawa, M.; Ueno, T.; Tojima, H.; Takizawa, D.; Naganuma, A.; Arai, H.; Sato, K.; et al. Liver function changes in patients with hepatocellular carcinoma treated with lenvatinib: Predictive factors of progression to Child-pugh class B, the formation of ascites and the candidates for the post-progression treatment. Cancers 2020, 12, 2906.
- Hayashi, T.; Shibata, M.; Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. C-reactive protein can predict dose intensity, time to treatment failure and overall survival in HCC treated with lenvatinib. PLoS ONE 2020, 15, e0244370.
- Reiss, K.A.; Yu, S.; Mamtani, R.; Mehta, R.; D’Addeo, K.; Wileyto, E.P.; Taddei, T.H.; Kaplan, D.E. Starting dose of sorafenib for the treatment of hepatocellular carcinoma: A retrospective, multi-institutional study. J. Clin. Oncol. 2017, 35, 3575–3581.
- Yamashita, T.; Kudo, M.; Ikeda, K.; Izumi, N.; Tateishi, R.; Ikeda, M.; Aikata, H.; Kawaguchi, Y.; Wada, Y.; Numata, K.; et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: An analysis of Japanese subset. J. Gastroenterol. 2020, 55, 113–122.
- Spallanzani, A.; Orsi, G.; Andrikou, K.; Gelsomino, F.; Rimini, M.; Riggi, L.; Cascinu, S. Lenvatinib as a therapy for unresectable hepatocellular carcinoma. Expert Rev. Anticancer Ther. 2018, 18, 1069–1076.
- Shimose, S.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; Suga, H.; Kuromatsu, R.; et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: A multicenter retrospective study. Cancers 2020, 12, 1867.
- Iwamoto, H.; Suzuki, H.; Shimose, S.; Niizeki, T.; Nakano, M.; Shirono, T.; Okamura, S.; Noda, Y.; Kamachi, N.; Nakamura, T.; et al. Weekends-off lenvatinib for unresectable hepatocellular carcinoma improves therapeutic response and tolerability toward adverse events. Cancers 2020, 12, 1010.
- Okubo, H.; Ando, H.; Ishizuka, K.; Kitagawa, R.; Okubo, S.; Saito, H.; Kokubu, S.; Miyazaki, A.; Ikejima, K.; Shiina, S.; et al. Carnitine insufficiency is associated with fatigue during lenvatinib treatment in patients with hepatocellular carcinoma. PLoS ONE 2020, 15, e0229772.
- Tada, T.; Kumada, T.; Hiraoka, A.; Michitaka, K.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; et al. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: A multicenter analysis with propensity score matching. Hepatol. Res. 2020, 50, 75–83.
- Je, Y.; Schutz, F.A.; Choueiri, T.K. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: A systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009, 10, 967–974.
- Schutz, F.A.; Je, Y.; Richards, C.J.; Choueiri, T.K. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J. Clin. Oncol. 2012, 30, 871–877.
- Li, X.; Wan, J.; Wu, Z.; Tu, J.; Hu, Y.; Wu, S.; Lou, L. Fatal adverse events with molecular targeted agents in the treatment of advanced hepatocellular carcinoma: A meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2018, 12, 3043–3049.
- Sahu, S.K.; Chawla, Y.K.; Dhiman, R.K.; Singh, V.; Duseja, A.; Taneja, S.; Kalra, N.; Gorsi, U. Rupture of hepatocellular carcinoma: A review of literature. J. Clin. Exp. Hepatol. 2019, 9, 245–256.
- Kudo, M.; Izumi, N.; Kubo, S.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Tateishi, R.; Nakashima, O.; Murakami, T.; Matsuyama, Y.; et al. Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan. Hepatol. Res. 2020, 50, 15–46.
- Uchida-Kobayashi, S.; Kageyama, K.; Yamamoto, A.; Ikenaga, H.; Yoshida, K.; Kotani, K.; Kimura, K.; Odagiri, N.; Hagihara, A.; Fujii, H.; et al. Lenvatinib-induced tumor-related hemorrhages in patients with large hepatocellular carcinomas. Oncology 2020, 8, 1–6.
- Aoki, T.; Kokudo, N.; Matsuyama, Y.; Izumi, N.; Ichida, T.; Kudo, M.; Ku, Y.; Sakamoto, M.; Nakashima, O.; Matsui, O.; et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: An analysis of 1160 cases from a nationwide survey. Ann. Surg. 2014, 259, 532–542.
- Ohya, K.; Kawaoka, T.; Namba, M.; Uchikawa, S.; Kodama, K.; Morio, K.; Nakahara, T.; Murakami, E.; Hiramatsu, A.; Tsuge, M.; et al. Early changes in ammonia levels and liver function in patients with advanced hepatocellular carcinoma treated by lenvatinib therapy. Sci. Rep. 2019, 9, 12101.
- Narita, R.; Kotoh, K.; Yoneda, A.; Motomura, M.; Harada, M. Factors raising serum ammonia level during lenvatinib treatment of patients with hepatocellular carcinoma. Anticancer Res. 2020, 40, 5271–5276.
- Hidaka, H.; Uojima, H.; Nakazawa, T.; Shao, X.; Hara, Y.; Iwasaki, S.; Wada, N.; Kubota, K.; Tanaka, Y.; Shibuya, A.; et al. Portal hemodynamic effects of lenvatinib in patients with advanced hepatocellular carcinoma: A prospective cohort study. Hepatol. Res. 2020, 50, 1083–1090.
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731.
- Torino, F.; Corsello, S.M.; Longo, R.; Barnabei, A.; Gasparini, G. Hypothyroidism related to tyrosine kinase inhibitors: An emerging toxic effect of targeted therapy. Nat. Rev. Clin. Oncol. 2009, 6, 219–228.
- Koizumi, Y.; Hirooka, M.; Hiraoka, A.; Ochi, H.; Tanaka, T.; Yukimoto, A.; Imai, Y.; Watanabe, T.; Yoshida, O.; Miyake, T.; et al. Lenvatinib-induced thyroid abnormalities in unresectable hepatocellular carcinoma. Endocr. J. 2019, 66, 787–792.
- Shomura, M.; Okabe, H.; Sato, E.; Fukai, K.; Shiraishi, K.; Hirose, S.; Tsuruya, K.; Arase, Y.; Anzai, K.; Kagawa, T. Hypothyroidism is a predictive factor for better clinical outcomes in patients with advanced hepatocellular carcinoma undergoing lenvatinib therapy. Cancers 2020, 12, 3078.
- Llovet, J.M.; Real, M.I.; Montana, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Sola, R.; et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739.
- Lo, C.M.; Ngan, H.; Tso, W.K.; Liu, C.L.; Lam, C.M.; Poon, R.T.; Fan, S.T.; Wong, J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236.
- Lencioni, R.; de Baere, T.; Soulen, M.C.; Rilling, W.S.; Geschwind, J.F. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016, 64, 106–116.
- Bruix, J.; Reig, M.; Sherman, M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016, 150, 835–853.
- Perera, S.; Kelly, D.; O’Kane, G.M. Non-immunotherapy options for the first-line management of hepatocellular carcinoma: Exploring the evolving role of sorafenib and lenvatinib in advanced disease. Curr. Oncol. 2020, 27, S165–S172.
- Bolondi, L.; Burroughs, A.; Dufour, J.F.; Galle, P.R.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Sangro, B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012, 32, 348–359.
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann. Oncol. 2013, 24, 2565–2570.
- Kudo, M.; Arizumi, T.; Ueshima, K.; Sakurai, T.; Kitano, M.; Nishida, N. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: Proposal of modified Bolondi’s subclassification (Kinki Criteria). Dig. Dis. 2015, 33, 751–758.
- Kim, B.K.; Kim, S.U.; Kim, K.A.; Chung, Y.E.; Kim, M.J.; Park, M.S.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, M.D.; et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J. Hepatol. 2015, 62, 1304–1310.
- Golfieri, R.; Renzulli, M.; Mosconi, C.; Forlani, L.; Giampalma, E.; Piscaglia, F.; Trevisani, F.; Bolondi, L. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: An issue of nodule dimension? J. Vasc. Interv. Radiol. 2013, 24, 509–517.
- Kudo, M.; Matsui, O.; Izumi, N.; Kadoya, M.; Okusaka, T.; Miyayama, S.; Yamakado, K.; Tsuchiya, K.; Ueshima, K.; Hiraoka, A.; et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014, 87 (Suppl. 1), 22–31.
- Ogasawara, S.; Chiba, T.; Ooka, Y.; Kanogawa, N.; Motoyama, T.; Suzuki, E.; Tawada, A.; Kanai, F.; Yoshikawa, M.; Yokosuka, O. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology 2014, 87, 330–341.
- Arizumi, T.; Ueshima, K.; Minami, T.; Kono, M.; Chishina, H.; Takita, M.; Kitai, S.; Inoue, T.; Yada, N.; Hagiwara, S.; et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer 2015, 4, 253–262.
- Kudo, M.; Raoul, J.-L.; Lee, H.C.; Cheng, A.-L.; Nakajima, K.; Peck-Radosavljevic, M. Deterioration of liver function after transarterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): An exploratory analysis of OPTIMIS—An international observational study assessing the use of sorafenib after TACE. J. Clin. Oncol. 2018, 36, 368.
- Shimose, S.; Kawaguchi, T.; Tanaka, M.; Iwamoto, H.; Miyazaki, K.; Moriyama, E.; Suzuki, H.; Niizeki, T.; Shirono, T.; Nakano, M.; et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: A multicenter cohort study using data mining analysis. Oncol. Lett. 2020, 20, 2257–2265.
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43.
- Eso, Y.; Takai, A.; Takahashi, K.; Ueda, Y.; Taura, K.; Marusawa, H.; Seno, H. Combination of Mac-2 binding protein glycosylation isomer and up-to-seven criteria as a useful predictor for Child-Pugh grade deterioration after transarterial chemoembolization for hepatocellular carcinoma. Cancers 2019, 11, 405.
- Yasui, Y.; Tsuchiya, K.; Kurosaki, M.; Takeguchi, T.; Takeguchi, Y.; Okada, M.; Wang, W.; Kubota, Y.; Goto, T.; Komiyama, Y.; et al. Up-to-seven criteria as a useful predictor for tumor downstaging to within Milan criteria and Child-Pugh grade deterioration after initial conventional transarterial chemoembolization. Hepatol. Res. 2018, 48, 442–450.
- Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child-Pugh A liver function: A proof-of-concept study. Cancers 2019, 11, 1084.
- Kudo, M.; Han, K.H.; Ye, S.L.; Zhou, J.; Huang, Y.H.; Lin, S.M.; Wang, C.K.; Ikeda, M.; Chan, S.L.; Choo, S.P.; et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020, 9, 245–260.
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J.; et al. Trial design and endpoints in hepatocellular carcinoma: AASLD Consensus Conference. Hepatology 2020.
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020, 69, 1492–1501.