
Video Upload Options
Nanomaterials are defined as materials that contain particles measuring between 1.0 and 100 nm in at least one dimension.
1. Introduction
During the past few decades, numerous treatment methods have been developed to deal with heavy metal contamination, including physical methods, such as adsorption, coagulation, evaporation, and filtration; chemical methods, such as chemical precipitation, oxidation, ion exchange, and electrochemical processes; and biological methods, such as biodegradation and phytoremediation [1][2][3][4]. However, most of these treatment methods have significant drawbacks, such as high costs, complexity of operation, and secondary pollution [5][6][7]. For instance, despite the great removal efficiency of chemical precipitation, its installation cost is quite high [8]. Of all of the known methods, adsorption is widely used because of its low cost, high removal efficiency, strong practicality, high applicability, and good operability [9][10].
Absorbency is a key factor of the adsorption method. Therefore, it is crucial to select the most suitable adsorption material. A good adsorbent should have the advantages of a large specific surface, great sorption sites, diverse surface interactions, fast adsorption rates, and low costs [11][12][13]. Currently, the most commonly used adsorbents are biochar, activated carbon, carbon film, biopolymers, clay materials, and nanomaterials [14][15][16].
Nanomaterials are defined as materials that contain particles measuring between 1.0 and 100 nm in at least one dimension [17][18]. Since the emergence of nanomaterials in the 1970s, an increasing number of researchers have focused on the application of nanomaterials in removing pollutants, such as heavy metals, organic pollutants, and pathogens, from contaminated surface waters, groundwater, sediments, and soil [16][19][20]. Nanomaterials are promising adsorbents and catalysts for the application of environmental remediation because of their great chemical reactivity, large adsorption surface, low temperature modification, and active atomicity [21][22]. The small size of nanoparticles makes it easier for the atoms at the surface to adsorb and have reactions with other atoms in order to achieve charge stabilization [23]. The large specific surface area can greatly improve the adsorption capacities of adsorbents [18][24]. In addition, because of the reduced size, nanomaterials have surfaces that are very reactive [25]. Not only can they efficiently adsorb pollutants, but they also have unique redox properties that are beneficial for the removal of redox-sensitive pollutants via degradation [17][26]. Studies on the removal of heavy metals using nanomaterials are of increasing importance and academic interest, as can be seen from the number of papers published every year, as shown in Figure 1. However, some of the commonly used nanomaterials do have limitations, including high costs, potential toxicity, difficulty in recycling, and an easy interaction with other media [5]. Even though nanomaterials have been widely studied in the field of heavy metal remediation, a comprehensive and systematic review of the application of nanomaterials for the removal of heavy metal ions is relatively lacking.
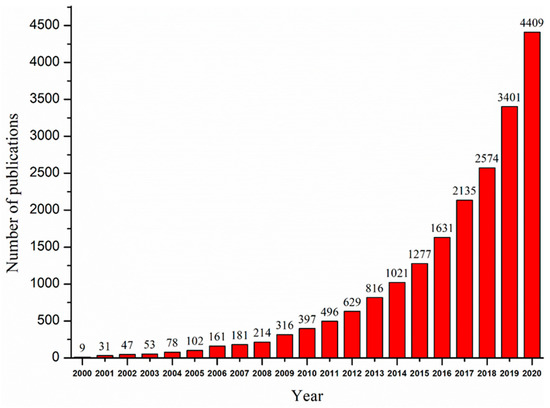
Figure 1. Trend of published papers on nanomaterials for removing heavy metals. Obtained from ScienceDirect. Search words (nanomaterials; heavy metal).
2. Types of Nanomaterials to Remove Heavy Metals
Nanomaterials are classified into carbon-based nanomaterials and inorganic nanomaterials [27]. They have been widely applied in the field of environmental remediation. Among them, nano zero-valent iron (NZVI), carbon nanotubes (CNTs), and titanium dioxide nanoparticles (TiO2 NPs) are the most frequently used and studied nanomaterials [28][29]. Table 1 summarizes the applications and the performance highlights of nanomaterials for removing heavy metals from water and soil environments.
Table 1. Applications of nanomaterials in removing heavy metals from the environment.
| Types of Nanomaterials | Environment | Target Heavy Metals | Performance Highlights |
|---|---|---|---|
| NZVI-HCS | Water | Pb(II), Cu(II), and Zn(II) | The maximum adsorption capacities were 195.1, 161.9, and 109.7 mg·g−1 for Pb(II), Cu(II), and Zn(II), respectively |
| NZVI | Sediment | Cd(II) | The maximum adsorption capacity of for Cd(II) was 769.2 mg g−1 at 297 K |
| BC-NZVI | Water | Cr(VI) | The performance of BC-NZVI was pH dependent, with a maximum Cr(VI) removal efficiency of 98.71% at pH 2 |
| BC-NZVI | Soil | Cr(VI) | The immobilization efficiency of Cr(VI) and total Cr reached 100% and 92.9%, respectively, when 8 g kg−1 of BC-NZVI was applied for 15 d |
| NZVI | Water | Pb(II) | The maximum adsorption capacity of NZVI was 807.23mg·g−1 at pH 6 |
| OA-NZVI | Soil | Cd(II), Pb(II), and Zn(II) | The highest Cd, Pb, and Zn removal efficiencies were 46.66%, 48.88% and 47.01%, respectively, for farmland soil at the NZVI concentration of 0.4 g L−1 |
| MWCNTs | Water | Zn(II) | The maximum adsorption efficiency was 96.27% at pH 5 for 6 h |
| MWCNTs-COOH | Water | Hg(II) and As(III) | The maximum removal efficiencies for Hg(II) and As(III) were 80.5% and 72.4% at the adsorbent dose of 20 mg L−1 and pH 7.6–7.9, respectively |
| CNTs | Water | Zn(II) | The maximum adsorption capacities of Zn(II) were 43.66 and 32.68 mg g−1 by SWCNTs and MWCNTs, respectively |
| TiO2-NCH | Water | Cd(II) and Cu(II) | The maximum adsorption efficiency of Cu(II) and Cd(II) from wastewater samples were 88.01% and 70.67%, respectively |
| Mesoporous carbonated TiO2 NPs |
Water | Sr(II) | The maximum adsorption capacity of Sr(II) 204.4 mg g−1 at the natural pH by 4C-TiO2 |
| TiO2 NPs | Soil | Cd(II) | The greatest Cd accumulation capacity of Trifolium repens reached 1235 µg pot−1 with PGPR + 500 mg kg−1 TiO2 NPs treatment |
2.1. Nano Zero-Valent Iron (NZVI)
NZVI is the most widely studied and applied nanomaterial in environmental remediation and has been proven to be an effective adsorbent, reductant, and catalyst for a variety of contaminants, such as heavy metal ions, halogenated organic compounds, organic dyes, and pharmaceuticals [35][36][37]. NZVI has a typical core shell structure generated during the synthesis process that contains a shell of Fe(II), Fe(III), and zero-valent iron [42]. As a result of the unique structure, NZVI has the abilities of reduction, surface sorption, stabilization, and precipitation of various contaminants [54,55,56]. Several studies have reported that NZVI exhibited excellent performance for removing heavy metal(loid) ions from contaminated environments [30][31][32][33]. For instance, Yang et al. [30] applied a corn stalk-derived, biochar-supported NZVI for the removal of heavy metal ions from water. The results showed that the equilibrium adsorption capacities reached 195.1, 161.9, and 109.7 mg·g−1 for Pb(II), Cu(II), and Zn(II) after 6 h, respectively. Boparai et al. [31] reported that NZVI could be applied as an efficient adsorbent to remove cadmium from contaminated water. The maximum adsorption capacity of NZVI for Cd(II) was 769.2 mg g−1, which was achieved at a temperature of 297 K. Su et al. [33] found that the immobilization efficiency of Cr(VI) reached 100% when 8 g kg−1 of biochar-supported NZVI was applied in hexavalent chromium-contaminated soil for 15 days. Acid mine water was treated using NZVI, and this resulted in a significant decrease in the concentrations of microcontaminants, such as U, V, As, Cr, Cu, Cd, Ni, and Zn [46]. Huang et al. [47] investigated the effects of different dosages of NZVI on plant growth and the Pb accumulation of Lolium perenne. The Pb accumulation and plant biomass were significantly enhanced when the NZVI and Pb accumulation in L. perenne reached a maximum of 1175.40μg per pot with the treatment of 100 mg kg−1 NZVI. Vítková et al. [48] reported that NZVI application significantly stabilized the As and Zn in As-rich and Zn-rich soils by the formation of Fe (hydr)oxides. Han et al. [49] investigated the removal efficiency of permeable reactive barriers (PRBs) filled with zero-valent iron (ZVI) and zero-valent aluminum (ZVAl) as a reactive medium and discussed the reaction mechanism of Cr(VI), Cd(II), Ni(II), Cu(II), and Zn(II) with ZVI/ZVAl. The main possible mechanisms were adsorption, formation of metal hydroxide precipitates, and reduction, which are shown in Figure 2.
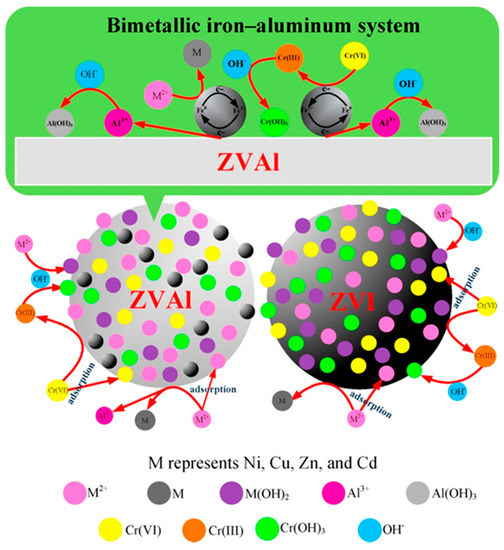
Figure 2. Removal mechanisms of five heavy metal ions by zero-valent iron/zero-valent aluminum (ZVI/ZVAl). Reproduced with permission from ref 60 published by Elsevier, 2016.
2.2. Carbon Nanotubes (CNTs)
CNTs, which were first discovered in 1991, have a unique chemical structure that consists of a graphitic sheet rolled up in a cylindrical shape [50][51]. CNTs are very strong materials that are over 100 times more resistant and six times lighter than steel [52]. Depending on the number of cylindrical shells, CNTs are classified into two categories: single wall CNTs (SWCNTs) and multi-wall CNTs (MWCNTs). Because of their extraordinary characteristics, such as a large specific surface area, unique morphology, and high reactivity, CNTs are considered to be an excellent nanomaterial for the removal of various organic and inorganic pollutants [53][54]. CNTs can be produced via methods such as chemical vapor deposition, laser ablation, and arc discharge. The adsorption capacity of CNTs is greatly affected by the methods by which they are synthesized with different reactants and catalysts [55]. For instance, Mubarak et al. [56] studied the effect of microwave-assisted MWCNTs on the removal of Zn(II) from wastewater. The results showed that the highest removal rate (99.9%) was achieved at pH 10 and a CNTs dosage of 0.05 g. Sun et al. [57] found that the removal efficiency of Cd(II) by CNTs increased at pH 3. Osman et al. [58] reported that CNTs synthesized from potato peel-waste material removed up to 84% of Pb(II) within 1 h of the CNTs’ application. Yaghmaeian et al. [59] used MWCNTs as a sorbent to remove Hg(II) from wastewater. The results showed that an adsorption capacity of 25.64 mg g−1 and a removal rate of greater than 85% were achieved when operated at 25 °C, pH 7, with a contact time of 120 min. Sobhanardakani et al. [60] prepared oxidized MWCNTs and used it as an adsorbent for the removal of Cu(II) from an aqueous solution. The maximum removal rate for Cu(II) was 99.5% at the optimum temperature (25 °C) and the most suitable pH value (6.0). There may be various pathways for heavy metal removal by CNTs, including adsorption, electrostatic interaction, reduction, and ion exchange, depending on the novel properties provided by functionalization and the heavy metal ions (Figure 3).
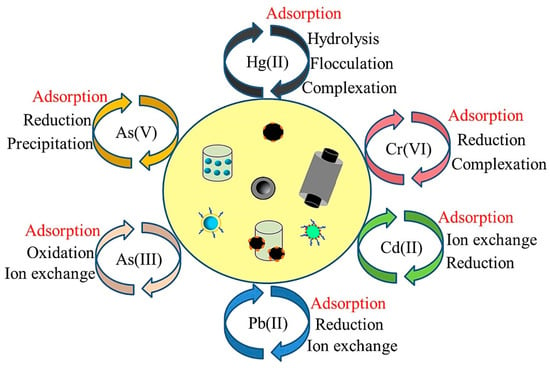
Figure 3. The mechanisms of removing heavy metal from an aqueous environment by carbon nanotubes (CNTs). Reproduced with permission from ref [61] published by Elsevier, 2018.
2.3. Titanium Dioxide Nanoparticles (TiO2 NPs)
Among the nanomaterials used for environmental remediation, TiO2 NPs have been extensively studied [62]. TiO2 NPs show good abilities for photocatalysis, high reactivity, and chemical stability, and they have been successfully applied for modifying the mobility and toxicity of heavy metals in water, soil, and sediment [63][64]. In addition, another advantage of TiO2 NPs is their ease of synthesis. Goutam et al. [65] synthesized TiO2 NPs using a leaf extract and used it to treat tannery wastewater. The results showed that 76.48% of the Cr was removed from the wastewater using green-synthesized TiO2 NPs. Mahmoud et al. [66] reported that the microwave-synthesized TiO2 NPs bonded with the chitosan nanolayer and removed 88.01% of the Cu (II) and 70.67% of the Cd (II) from wastewater when the pH value was 7.0. Gebru et al. [67] synthesized cellulose acetate (CA)/TiO2 NPs using a new electrospinning technique and tested its adsorption capacity for removing Pb(II) and Cu(II) ions from water. The CA/TiO2 adsorbent removed 99.7% and 98.9% of Pb(II) and Cu(II) ions under the most optimized conditions. Fan et al. [68] reported that the concentrations of exchangeable, carbonate, and iron-manganese oxide of As and Pb in the sediments decreased with an increasing amount of TiO2 NPs. Singh and Lee [69] investigated the effect of TiO2 NPs on Cd phytoremediation in Glycine max. The results showed that the Cd accumulation in the aerial portions of the plants increased by approximately 2.6 times when 300 mg kg−1 TiO2 NPs were added to the soil. Zhao et al. [70] proposed the possible removal mechanisms of Cr(VI) by reduced graphene oxide decorated with TiO2 NPs (TiO2-RGO), which is shown in Figure 4. It was speculated that the negatively charged Cr(VI) was bound to the surface of TiO2-RGO, which had a positive charge and was reduced to Cr(III). Then, the Cr(III) species was released into the solution due to electrostatic repulsion with the surface of TiO2-RGO.
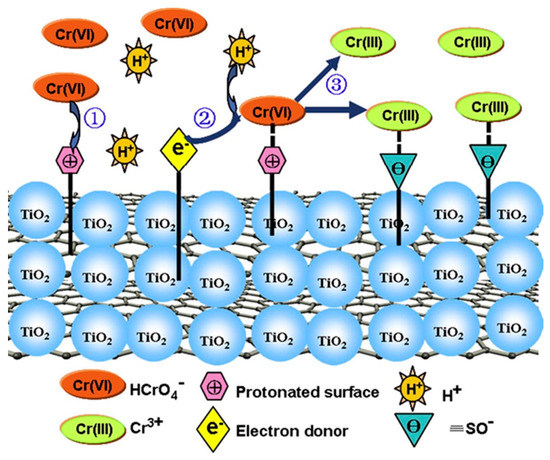
Figure 4. The possible mechanism of Cr(VI) reduction by TiO2-RGO. Reproduced with permission from ref 81 published by Elsevier, 2013.
References
- Zhai, T.; Fang, X.; Liao, M.; Xu, X.; Zeng, H.; Yoshio, B.; Golberg, D. A Comprehensive Review of One-Dimensional Met-al-Oxide Nanostructure Photodetectors. Sensors 2009, 9, 6504–6529, doi:10.3390/s90806504.
- Meunier, N.; Drogui, P.; Montane, C.; Hausler, R.; Mercier, G.; Blais, J.F. Comparison between electrocoagulation and chem-ical precipitation for metals removal from acidic soil leachate. J. Hazard. Mater. 2006, 137, 581−590, doi:10.1016/j.jhazmat.2006.02.050.
- Minju, N.; Swaroop, K.; Haribabu, K.; Sivasubramanian, V.; Kumar, P. Removal of fluoride from aqueous media by magne-sium oxide-coated nanoparticles. Desalin. Water Treat. 2015, 53, 2905−2914, doi:10.1080/19443994.2013.868837.
- Liu, J.; Shang, W.; Zhang, X.; Zhu, Y.; Yu, K. Mn accumulation and tolerance in Celosia argentea Linn.: A new Mn-hyperaccumulating plant species. J. Hazard. Mater. 2014, 267, 136–41, doi:10.1016/j.jhazmat.2013.12.051.
- Cai, C.; Zhao, M.; Yu, Z.; Rong, H.; Zhang, C. Utilization of nanomaterials for in-situ remediation of heavy metal (loid) con-taminated sediments: A review. Sci. Total Environ. 2019, 662, 205–217, doi:10.1016/j.scitotenv.2019.01.180.
- Yu, S.; Wang, X.; Pang, H.; Zhang, R.; Song, W.; Fu, D.; Hayat, T.; Wang, X. Boron nitride-based materials for the removal of pollutants from aqueous solutions: A review. Chem. Eng. J. 2018, 333, 343–360, doi:10.1016/j.cej.2017.09.163.
- Phenrat, T.; Saleh, N.; Sirk, K.; Kim, H.-J.; Tilton, R.D.; Lowry, G.V. Stabilization of aqueous nanoscale zerovalent iron dis-persions by anionic polyelectrolytes: Adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation. J. Nanopart. Res. 2008, 10, 795–814, doi:10.1007/s11051-007-9315-6.
- Zhang, Y.; Wang, L.; Zhang, N.; Zhou, Z. Adsorptive environmental applications of MXene nanomaterials: A review. RSC Adv. 2018, 8, 19895–19905, doi:10.1039/c8ra03077d.
- Hu, R.; Wang, X.; Dai, S.; Shao, D.; Hayat, T.; Alsaedi, A. Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solutions. Chem. Eng. J. 2015, 260, 469–477, doi:10.1016/j.cej.2014.09.013.
- Ihsanullah, A.A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161, doi:10.1016/j.seppur.2015.11.039.
- Thines, R.K.; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C.; Ganesan, P. Application potential of carbon na-nomaterials in water and wastewater treatment: A review. J. Taiwan Inst. Chem. Eng. 2017, 72, 116–133, doi:10.1016/j.jtice.2017.01.018.
- Ibrahim, R.K.; Hayyan, M.; AlSaadi, M.A.; Hayyan, A.; Ibrahim, S. Environmental application of nanotechnology: Air, soil, and water. Environ. Sci. Pollut. Res. 2016, 23, 13754–13788, doi:10.1007/s11356-016-6457-z.
- Qiao, Y.; Wu, J.; Xu, Y.; Fang, Z.; Zheng, L.; Cheng, W.; Tsang, E.P.; Fang, J.; Zhao, D. Remediation of cadmium in soil by biochar-supported iron phosphate nanoparticles. Ecol. Eng. 2017, 106, 515–522, doi:10.1016/j.ecoleng.2017.06.023.
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.H.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental remediation and applica-tion of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review. Environ. Sci. Technol. 2016, 50, 7290–7304, doi:10.1021/acs.est.6b01897.
- Chaithawiwat, K.; Vangnai, A.; McEvoy, J.M.; Pruess, B.; Krajangpan, S.; Khan, E. Impact of nanoscale zero valent iron on bacteria is growth phase dependent. Chemosphere 2016, 144, 352–359, doi:10.1016/j.chemosphere.2015.09.025.
- Lefevre, E.; Bossa, N.; Wiesner, M.R.; Gunsch, C.K. A review of the environmental implications of in situ remediation by na-noscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities. Sci. Total Environ. 2016, 565, 889–901, doi:10.1016/j.scitotenv.2016.02.003.
- Abbas, Q.; Yousaf, B.; Amina Ali, M.U.; Munir, M.A.M.; El-Naggar, A.; Rinklebe, J.; Naushad, M.; Naushad, M. Transfor-mation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review. Environ. Int. 2020, 138, 105646, doi:10.1016/j.envint.2020.105646.
- Bhowmick, S.; Chakraborty, S.; Mondal, P.; Renterghem, W.V.; Berghe, S.V.D.; Roman-Ross, G.; Chatterjee, D.; Iglesias, M. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: Kinetics and mecha-nism. Chem. Eng. J. 2014, 243, 14–23, doi:10.1016/j.cej.2013.12.049.
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109, doi:10.1039/c2ee21818f.
- Jiang, Z.; Lv, L.; Zhang, W.; Du, Q.; Pan, B.; Yang, L.; Zhang, Q. Nitrate reduction using nanosized zero-valent iron sup-ported by polystyrene resins: Role of surface functional groups. Water Res. 2011, 45, 2191–2198, doi:10.1016/j.watres.2011.01.005.
- Fu, R.; Yang, Y.; Xu, Z.; Zhang, X.; Guo, X.; Bi, D. The removal of chromium (VI) and lead (II) from groundwater using sepio-lite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015,138, 726–734, doi:10.1016/j.chemosphere.2015.07.051.
- Liang, S.; Jin, Y.; Liu, W.; Li, X.; Shen, S.; Ding, L. Feasibility of Pb phytoextraction using nano-materials assisted ryegrass: Results of a one-year field-scale experiment. J. Environ. Manag. 2017, 190, 170–175, doi:10.1016/j.watres.2011.01.005.
- Tosco, T.; Papini, M.P.; Viggi, C.C.; Sethi, R. Nanoscale zerovalent iron particles for groundwater remediation: A review. J. Clean. Prod. 2014, 77, 10–21, doi:10.1016/j.jclepro.2013.12.026.
- Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Hayat, T.; Wang, X. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018, 240, 493–505, doi:10.1016/j.envpol.2018.04.136.
- Geary, S.M.; Morris, A.S.; Salem, A.K. Assessing the effect of engineered nanomaterials on the environment and human health. J. Allergy Clin. Immunol. 2016, 138, 405–408, doi:10.1016/j.envpol.2018.04.136.
- Liu, W.; Tian, S.; Zhao, X.; Xie, W.; Gong, Y.; Zhao, D. Application of stabilized nanoparticles for in situ remediation of met-al-contaminated soil and groundwater: A critical review. Curr. Pollut. Rep. 2015, 1, 280–291, doi:10.1007/s40726-015-0017-x.
- Stone, V.; Nowack, B.; Baun, A.; van den Brink, N.; Kammer, F.; Dusinska, M.; Handy, R.; Hankin, S.; Hassellov, M.; Joner, E.; et al. Nanomaterials for environmental studies: Classification, reference material issues, and strategies for physicochemical characterisation. Sci. Total Environ. 2010, 408, 1745–1754, doi:10.1016/j.scitotenv.2009.10.035.
- Ren, X.M.; Chen, C.L.; Nagatsu, M.; Wang, X.K. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410, doi:10.1016/j.cej.2010.08.045.
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A re-view. J. Hazard. Mater. 2014, 267, 194–205, doi:10.1016/j.jhazmat.2013.12.062.
- Yang, F.; Zhang, S.; Sun, Y.; Cheng, K.; Li, J.; Tsang, D.C.W. Fabrication and characterization of hydrophilic corn stalk bio-char-supported nanoscale zero-valent iron composites for efficient metal removal. Bioresour. Technol. 2018, 265, 490–497, doi:10.1016/j.biortech.2018.06.029.
- Boparai, H.K.; Meera Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465, doi:10.1016/j.jhazmat.2010.11.029.
- Shang, J.; Zong, M.; Yu, Y.; Kong, X.; Du, Q.; Liao, Q. Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar. J. Environ. Manag. 2017, 197, 331–337, doi:10.1016/j.jenvman.2017.03.085.
- Su, H.; Fang, Z.; Tsang, P.E.; Fang, J.; Zhao, D. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environ. Pollut. 2016, 214, 94–100, doi:10.1016/j.envpol.2016.03.072.
- Zhang, D.; Gao, W.; Chang, G.; Luo, S.; Jiao, W.; Liu, Y. Removal of heavy metal lead(II) using nanoscale zero-valent iron with different preservation methods. Adv. Powder Technol. 2019, 30, 581–589, doi:10.1016/j.apt.2018.12.013.
- Cao, Y.; Zhang, S.; Zhong, Q.; Wang, G.; Xu, X.; Li, T.; Wang, L.; Jia, Y.; Li, Y. Feasibility of nanoscale zero-valent iron to en-hance the removal efficiencies of heavy metals from polluted soils by organic acids. Ecotoxicol. Environ. Saf. 2018, 162, 464–473, doi:10.1016/j.ecoenv.2018.07.036.
- Ahmed, A.M.; Ali, M.; Noor, A.H. Removal of zinc ions from aqueous solution by bioadsorbents and CNTs. Am. J. Mater. Sci. 2016, 6, 105–114, doi:10.5923/j.materials.20160604.04.
- Alimohammady, M.; Jahangiri, M.; Kiani, F.; Tahermansouri, H. Design and evaluation of functionalized multi-walled car-bon nanotubes by 3-aminopyrazole for the removal of Hg(II) and As(III) ions from aqueous solution. Res. Chem. Intermed. 2018, 44, 69–92, doi:10.1007/s11164-017-3091-4.
- Lu, C.; Chiu, H. Adsorption of zinc(II) from water with purified carbon nanotubes. Chem. Eng. Sci. 2006, 61, 1138–1145, doi:10.1016/j.ces.2005.08.007.
- Mohammad, A.M.; Salah Eldin, T.A.; Hassan, M.A.; El-Anadouli, B.E. Efficient treatment of lead-containing wastewater by hydroxyapatite/chitosan nanostructures. Arab. J. Chem. 2017, 10, 683–690, doi:10.1016/j.arabjc.2014.12.016.
- Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly efficient adsorption of strontium ions by car-bonated mesoporous TiO2. J. Mol. Liq. 2019, 285, 742−753, doi:10.1016/j.molliq.2019.04.111.
- Zand, A.D.; Tabrizi, A.M.; Heir, A.V. Application of titanium dioxide nanoparticles to promote phytoremediation of Cd-polluted soil: Contribution of PGPR inoculation. Bioremediat. J. 2020, 24, 171–189, doi:10.1080/10889868.2020.1799929.
- Crane, R.; Scott, T. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Ma-ter. 2012, 211, 112–125, doi:10.1016/j.jhazmat.2011.11.073.
- Elsner, M.; Chartrand, M.; VanStone, N.; Lacrampe Couloume, G.; Sherwood Lollar, B. Identifying abiotic chlorinated ethene degradation: Characteristic isotope patterns in reaction products with nanoscale zero-valent iron. Environ. Sci. Technol. 2008, 42, 5963–5970, doi:10.1021/es8001986.
- Luo, S.; Lu, T.; Peng, L.; Shao, J.; Zeng, Q.; Gu, J. Synthesis of nanoscale zerovalent iron immobilized in alginate microcap-sules for removal of Pb(II) from aqueous solution. J. Mater. Chem. 2014, 2, 15463–15472, doi:10.1039/C4TA02920H.
- Mueller, N.; Braun, J.; Bruns, J.; Cernik, M.; Rissing, P.; Rickerby, D.; Nowack, B. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ. Sci. Pollut. Res. 2012, 19, 550−558, doi:10.1007/s11356-011-0576-3.
- Klimkova, S.; Cernik, M.; Lacinova, L.; Filip, J.; Jancik, D.; Zboril, R. Zero-valent iron nanoparticles in treatment of acid mine water from in situ uranium leaching. Chemosphere 2011, 82, 1178–1184, doi:10.1016/j.chemosphere.2010.11.075.
- Huang, D.; Qin, X.; Peng, Z.; Liu, Y.; Gong, X.; Zeng, G.; Huang, C.; Cheng, M.; Xue, W.; Wang, X.; et al. Nanoscale ze-ro-valent iron assisted phytoremediation of Pb in sediment: Impacts on metal accumulation and antioxidative system of Lo-lium perenne. Ecotoxicol. Environ. Saf. 2018, 153, 229–237, doi:10.1016/j.ecoenv.2018.01.060.
- Vítková, M.; Puschenreite, M.; Komárek, M. Effect of nano zero-valent iron application on As, Cd, Pb, and Zn availability in the rhizosphere of metal(loid) contaminated soils. Chemosphere 2018, 200, 217–226, doi:10.1016/j.chemosphere.2018.02.118.
- Han, W.; Fu, F.; Cheng, Z.; Wu, S. Studies on the optimum conditions using acid-washed zero-valent iron/aluminum mix-tures in permeable reactive barriers for the removal of different heavy metal ions from wastewater. J. Hazard. Mater. 2016, 302, 437–446, doi:10.1016/j.jhazmat.2015.09.041.
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673, doi:10.1021/la701067r.
- Li, J.; Chen, C.; Zhang, S.; Wang, X. Surface functional groups and defects on carbon nanotubes affect adsorption–desorption hysteresis of metal cations and oxoanions in water. Environ. Sci. Nano 2014, 1, 488–495, doi:10.1039/c4en00044g.
- Trojanowicz, M. Analytical applications of carbon nanotubes: A review. Trac-Trends Anal. Chem. 2006, 25, 480–489, doi:10.1016/j.trac.2005.11.008.
- Lv, X.; Xu, J.; Jiang, G.; Xu, X. Removal of chromium (VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere 2011, 85, 1204–1209, doi:10.1016/j.chemosphere.2011.09.005.
- Helland, A.; Wick, P.; Koehler, A.; Schmid, K.; Som, C. Reviewing the environmental and human health knowledge base of carbon nanotubes. Environ. Health Perspect. 2007, 115, 1125–1131, doi:10.1289/ehp.9652.
- Sheng, G.; Alsaedi, A.; Shammakh, W.; Monaquel, S.; Sheng, J.; Wang, X.; Li, H.; Huang, Y. Enhanced sequestration of sele-nite in water by nanoscale zero valent iron immobilization on carbon nanotubes by a combined batch, XPS and XAFS inves-tigation. Carbon 2016, 99, 123–130, doi:10.1016/j.carbon.2015.12.013.
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Microwave-assisted synthesis of multi-walled car-bon nanotubes for enhanced removal of Zn(II) from wastewater. Res. Chem. Intermed. 2015, 42, 1–25, doi:10.1007/s11164-015-2209-9.
- Sun, W.L.; Jiang, B.F.; Wang, F.; Xu, N. Effect of carbon nanotubes on Cd(II) adsorption by sediments. Chem. Eng. J. 2015, 264, 645–653, doi:10.1016/j.cej.2014.11.137.
- Osman, A.I.; Blewitt, J.; Abu-Dahrieh, J.K.; Farrell, C.; Ala’a, H.; Harrison, J.; Rooney, D.W. Production and characterisation of activated carbon and carbon nanotubes from potato peel waste and their application in heavy metal removal. Environ. Sci. Pollut. Res. 2019, 26, 37228–37241, doi:10.1007/s11356-019-06594-w.
- Yaghmaeian, K.; Mashizi, R.K.; Nasseri, S.; Mahvi, A.H.; Ali-mohammadi, M.; Nazmara, S. Removal of inorganic mercury from aquatic environments by multi-walled carbon nanotubes. J. Environ. Health Sci. Eng. 2015, 13, 55, doi:10.1186/s40201-015-0209-8.
- Sobhanardakani, S.; Zandipak, R.; Cheraghi, M. Adsorption of Cu2+ ions from aqueous solutions using oxidized multi-walled carbon nanotubes. Avicenna J. Environ. Health Eng. 2015, 2, e790, doi:10.17795/ajehe790.
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364, doi:10.1016/j.chemosphere.2017.12.061.
- Taghipour, S.; Hosseini, S.M.; Ataie-Ashtiani, B. Engineering nanomaterials for water and wastewater treatment: Review of classifications, properties and applications. New J. Chem. 2019, 43, 7902–7927, doi:10.1039/C9NJ00157C.
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for pho-tocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146, doi:10.1016/j.watres.2015.04.038.
- Nguyen, V.N.H.; Amal, R.; Beydoun, D. Effect of formate and methanol on photoreduction/removal of toxic cadmium ions using TiO2 semiconductor as photocatalyst. Chem. Eng. Sci. 2003, 58, 4429–4439, doi:10.1016/s0009-2509(03)00336-1.
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. For photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396, doi:10.1016/j.cej.2017.12.029.
- Mahmoud, M.E.; Ali, S.A.A.; Elweshahy, S.M. Microwave functionalization of titanium oxide nanoparticles with chitosan nanolayer for instantaneous microwave sorption of Cu (II) and Cd (II) from water. Int. J. Biol. Macromol. 2018, 111, 393–399, doi:10.1016/j.ijbiomac.2018.01.014.
- Gebru, K.A.; Das, C. Removal of Pb (II) and Cu (II) ions from wastewater using composite electrospun cellulose ace-tate/titanium oxide (TiO2) adsorbent. J. Water Process. Eng. 2017, 16, 1–13, doi:10.1016/j.jwpe.2016.11.008.
- Fan, X.; Wang, C.; Wang, P.; Hu, B.; Wang, X. TiO2 nanoparticles in sediments: Effect on the bioavailability of heavy metals in the freshwater bivalve Corbicula fluminea. J. Hazard. Mater. 2018, 342, 41–50, doi:10.1016/j.jhazmat.2017.07.041.
- Singh, J.; Lee, B.K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016, 170, 88–96, doi:10.1016/j.jenvman.2016.01.015.
- Zhao, Y.; Zhao, D.; Chen, C.; Wang, X. Enhanced photo-reduction and removal of Cr(VI) on reduced graphene oxide deco-rated with TiO2 nanoparticles. J. Colloid Interface Sci. 2013, 405, 211–217, doi:10.1016/j.jcis.2013.05.004.




